From Wikipedia, the free encyclopedia

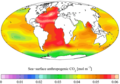
Estimated change in sea water pH caused by human created CO
2 between the 1700s and the 1990s, from the Global Ocean Data Analysis Project (GLODAP) and the World Ocean Atlas
2 between the 1700s and the 1990s, from the Global Ocean Data Analysis Project (GLODAP) and the World Ocean Atlas

NOAA provides evidence for upwelling of corrosive "acidified" water onto the Continental Shelf. In the figure above, note the vertical sections of (A) temperature, (B) aragonite saturation, (C) pH, (D) DIC, and (E) pCO2 on transect line 5 off Pt. St. George, California. The potential density surfaces are superimposed on the temperature section. The 26.2 potential density surface delineates the location of the first instance in which the undersaturated water is upwelled from depths of 150 to 200 m onto the shelf and outcropping at the surface near the coast. The red dots represent sample locations.[1]
Ocean acidification is the ongoing decrease in the pH of the Earth's oceans, caused by the uptake of carbon dioxide (CO
2) from the atmosphere.[2] An estimated 30–40% of the carbon dioxide released by humans into the atmosphere dissolves into oceans, rivers and lakes.[3][4] To achieve chemical equilibrium, some of it reacts with the water to form carbonic acid. Some of these extra carbonic acid molecules react with a water molecule to give a bicarbonate ion and a hydronium ion, thus increasing ocean "acidity" (H+ ion concentration). Between 1751 and 1994 surface ocean pH is estimated to have decreased from approximately 8.25 to 8.14,[5] representing an increase of almost 30% in H+ ion concentration in the world's oceans.[6][7] Earth System Models project that within the last decade ocean acidity exceeded historical analogs[8] and in combination with other ocean biogeochemical changes could undermine the functioning of marine ecosystems and disrupt the provision of many goods and services associated with the ocean.[9]
Increasing acidity is thought to have a range of possibly harmful consequences, such as depressing metabolic rates and immune responses in some organisms, and causing coral bleaching. This also causes decreasing oxygen levels as it kills off algae.
Other chemical reactions are triggered which result in a net decrease in the amount of carbonate ions available. This makes it more difficult for marine calcifying organisms, such as coral and some plankton, to form biogenic calcium carbonate, and such structures become vulnerable to dissolution.[10] Ongoing acidification of the oceans threatens food chains connected with the oceans.[11][12] As members of the InterAcademy Panel, 105 science academies have issued a statement on ocean acidification recommending that by 2050, global CO
2 emissions be reduced by at least 50% compared to the 1990 level.[13]
Ocean acidification has been called the "evil twin of global warming"[14][15][16][17][18] and "the other CO
2 problem".[15][17][19]
Ocean acidification has occurred previously in Earth's history. The most notable example is the Paleocene-Eocene Thermal Maximum (PETM),[20] which occurred approximately 56 million years ago. For reasons that are currently uncertain, massive amounts of carbon entered the ocean and atmosphere, and led to the dissolution of carbonate sediments in all ocean basins.
Carbon cycle
The carbon cycle describes the fluxes of carbon dioxide (CO
2) between the oceans, terrestrial biosphere, lithosphere,[21] and the atmosphere. Human activities such as the combustion of fossil fuels and land use changes have led to a new flux of CO
2 into the atmosphere. About 45% has remained in the atmosphere; most of the rest has been taken up by the oceans,[22] with some taken up by terrestrial plants.[23]
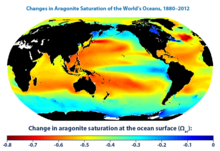
The map was created by the National Oceanic and Atmospheric Administration and the Woods Hole Oceanographic Institution using Community Earth System Model data. This map was created by comparing average conditions during the 1880s with average conditions during the most recent 10 years (2003–2012). Aragonite saturation has only been measured at selected locations during the last few decades, but it can be calculated reliably for different times and locations based on the relationships scientists have observed among aragonite saturation, pH, dissolved carbon, water temperature, concentrations of carbon dioxide in the atmosphere, and other factors that can be measured. This map shows changes in the amount of aragonite dissolved in ocean surface waters between the 1880s and the most recent decade (2003–2012). Aragonite saturation is a ratio that compares the amount of aragonite that is actually present with the total amount of aragonite that the water could hold if it were completely saturated. The more negative the change in aragonite saturation, the larger the decrease in aragonite available in the water, and the harder it is for marine creatures to produce their skeletons and shells. The global map shows changes over time in the amount of aragonite dissolved in ocean water, which is called aragonite saturation.[citation needed]
The carbon cycle involves both organic compounds such as cellulose and inorganic carbon compounds such as carbon dioxide and the carbonates. The inorganic compounds are particularly relevant when discussing ocean acidification for it includes many forms of dissolved CO
2 present in the Earth's oceans.[25]
When CO
2 dissolves, it reacts with water to form a balance of ionic and non-ionic chemical species: dissolved free carbon dioxide (CO
2(aq)), carbonic acid (H
2CO
3), bicarbonate (HCO−
3) and carbonate (CO2−
3). The ratio of these species depends on factors such as seawater temperature and alkalinity (as shown in a Bjerrum plot). These different forms of dissolved inorganic carbon are transferred from an ocean's surface to its interior by the ocean's solubility pump.
The resistance of an area of ocean to absorbing atmospheric CO
2 is known as the Revelle factor.
Acidification
Dissolving CO2 in seawater increases the hydrogen ion (H+) concentration in the ocean, and thus decreases ocean pH, as follows:[26]
Caldeira and Wickett (2003)[2] placed the rate and magnitude of modern ocean acidification changes in the context of probable historical changes during the last 300 million years.
Since the industrial revolution began, it is estimated that surface ocean pH has dropped by slightly more than 0.1 units on the logarithmic scale of pH, representing about a 29% increase in H+. It is expected to drop by a further 0.3 to 0.5 pH units[9] (an additional doubling to tripling of today's post-industrial acid concentrations) by 2100 as the oceans absorb more anthropogenic CO
2, the impacts being most severe for coral reefs and the Southern Ocean.[2][10][27] These changes are predicted to continue rapidly as the oceans take up more anthropogenic CO
2 from the atmosphere. The degree of change to ocean chemistry, including ocean pH, will depend on the mitigation and emissions pathways[28] society takes.[29]
Although the largest changes are expected in the future,[10] a report from NOAA scientists found large quantities of water undersaturated in aragonite are already upwelling close to the Pacific continental shelf area of North America.[30] Continental shelves play an important role in marine ecosystems since most marine organisms live or are spawned there, and though the study only dealt with the area from Vancouver to Northern California, the authors suggest that other shelf areas may be experiencing similar effects.[30]
| Time | pH | pH change relative to pre-industrial |
Source | H+ concentration change relative to pre-industrial |
|---|---|---|---|---|
| Pre-industrial (18th century) | 8.179 | analysed field[31][not in citation given] | ||
| Recent past (1990s) | 8.104 | −0.075 | field[31] | + 18.9% |
| Present levels | ~8.069 | −0.11 | field[6][7][32][33] | + 28.8% |
| 2050 (2×CO 2 = 560 ppm) |
7.949 | −0.230 | model[10] | + 69.8% |
| 2100 (IS92a)[34] | 7.824 | −0.355 | model[10] | + 126.5% |
Rate
One of the first detailed datasets to examine how pH varied over a period of time at a temperate coastal location found that acidification was occurring much faster than previously predicted, with consequences for near-shore benthic ecosystems.[35][36] Thomas Lovejoy, former chief biodiversity advisor to the World Bank, has suggested that "the acidity of the oceans will more than double in the next 40 years. This rate is 100 times faster than any changes in ocean acidity in the last 20 million years, making it unlikely that marine life can somehow adapt to the changes."[37] It is predicted that, by the year 2100, the level of acidity in the ocean will reach the levels experienced by the earth 20 million years ago.[9][38]Current rates of ocean acidification have been compared with the greenhouse event at the Paleocene–Eocene boundary (about 55 million years ago) when surface ocean temperatures rose by 5–6 degrees Celsius. No catastrophe was seen in surface ecosystems, yet bottom-dwelling organisms in the deep ocean experienced a major extinction. The current acidification is on a path to reach levels higher than any seen in the last 65 million years,[39] and the rate of increase is about ten times the rate that preceded the Paleocene–Eocene mass extinction. The current and projected acidification has been described as an almost unprecedented geological event.[40] A National Research Council study released in April 2010 likewise concluded that "the level of acid in the oceans is increasing at an unprecedented rate."[41][42] A 2012 paper in the journal Science examined the geological record in an attempt to find a historical analog for current global conditions as well as those of the future. The researchers determined that the current rate of ocean acidification is faster than at any time in the past 300 million years.[43][44]
A review by climate scientists at the RealClimate blog, of a 2005 report by the Royal Society of the UK similarly highlighted the centrality of the rates of change in the present anthropogenic acidification process, writing:[45]
"The natural pH of the ocean is determined by a need to balance the deposition and burial of CaCOIn the 15-year period 1995–2010 alone, acidity has increased 6 percent in the upper 100 meters of the Pacific Ocean from Hawaii to Alaska.[46] According to a statement in July 2012 by Jane Lubchenco, head of the U.S. National Oceanic and Atmospheric Administration "surface waters are changing much more rapidly than initial calculations have suggested. It's yet another reason to be very seriously concerned about the amount of carbon dioxide that is in the atmosphere now and the additional amount we continue to put out."[14]
3 on the sea floor against the influx of Ca2+ and CO2−
3 into the ocean from dissolving rocks on land, called weathering. These processes stabilize the pH of the ocean, by a mechanism called CaCO
3 compensation...The point of bringing it up again is to note that if the CO
2 concentration of the atmosphere changes more slowly than this, as it always has throughout the Vostok record, the pH of the ocean will be relatively unaffected because CaCO
3 compensation can keep up. The [present] fossil fuel acidification is much faster than natural changes, and so the acid spike will be more intense than the earth has seen in at least 800,000 years."
A 2013 study claimed acidity was increasing at a rate 10 times faster than in any of the evolutionary crises in the earth's history.[47]
Calcification
Overview
Changes in ocean chemistry can have extensive direct and indirect effects on organisms and their habitats. One of the most important repercussions of increasing ocean acidity relates to the production of shells and plates out of calcium carbonate (CaCO3).[27] This process is called calcification and is important to the biology and survival of a wide range of marine organisms. Calcification involves the precipitation of dissolved ions into solid CaCO
3 structures, such as coccoliths. After they are formed, such structures are vulnerable to dissolution unless the surrounding seawater contains saturating concentrations of carbonate ions (CO32−).
Mechanism
Of the extra carbon dioxide added into the oceans, some remains as dissolved carbon dioxide, while the rest contributes towards making additional bicarbonate (and additional carbonic acid). This also increases the concentration of hydrogen ions, and the percentage increase in hydrogen is larger than the percentage increase in bicarbonate,[48] creating an imbalance in the reaction HCO3− $ \leftrightarrow $ CO32− + H+. To maintain chemical equilibrium, some of the carbonate ions already in the ocean combine with some of the hydrogen ions to make further bicarbonate. Thus the ocean's concentration of carbonate ions is reduced, creating an imbalance in the reaction Ca2+ + CO32− $ \leftrightarrow $ CaCO3, and making the dissolution of formed CaCO
3 structures more likely.
These increases in concentrations of dissolved carbon dioxide and bicarbonate, and reduction in carbonate, are shown in a Bjerrum plot.
Saturation state
The saturation state of seawater for a mineral (known as Ω) is a measure of the thermodynamic potential for the mineral to form or to dissolve, and is described by the following equation:$ {\Omega }={\frac {\left[{\textrm {Ca}}^{{2+}}\right]\left[{\textrm {CO}}_{{3}}^{{2-}}\right]}{K_{{sp}}}} $
Here Ω is the product of the concentrations (or activities) of the reacting ions that form the mineral (Ca2+ and CO2−
3), divided by the product of the concentrations of those ions when the mineral is at equilibrium (K
sp), that is, when the mineral is neither forming nor dissolving.[49] In seawater, a natural horizontal boundary is formed as a result of temperature, pressure, and depth, and is known as the saturation horizon, or lysocline.[27] Above this saturation horizon, Ω has a value greater than 1, and CaCO
3 does not readily dissolve. Most calcifying organisms live in such waters.[27] Below this depth, Ω has a value less than 1, and CaCO
3 will dissolve. However, if its production rate is high enough to offset dissolution, CaCO
3 can still occur where Ω is less than 1. The carbonate compensation depth occurs at the depth in the ocean where production is exceeded by dissolution.[50]
The decrease in the concentration of CO32− decreases Ω, and hence makes CaCO
3 dissolution more likely.
Calcium carbonate occurs in two common polymorphs (crystalline forms): aragonite and calcite. Aragonite is much more soluble than calcite, so the aragonite saturation horizon is always nearer to the surface than the calcite saturation horizon.[27] This also means that those organisms that produce aragonite may be more vulnerable to changes in ocean acidity than those that produce calcite.[10] Increasing CO
2 levels and the resulting lower pH of seawater decreases the saturation state of CaCO
3 and raises the saturation horizons of both forms closer to the surface.[51] This decrease in saturation state is believed to be one of the main factors leading to decreased calcification in marine organisms, as the inorganic precipitation of CaCO
3 is directly proportional to its saturation state.[52]
Possible impacts
Video summarizing the impacts of ocean acidification. Source: NOAA Environmental Visualization Laboratory.
Increasing acidity has possibly harmful consequences, such as depressing metabolic rates in jumbo squid,[53] depressing the immune responses of blue mussels,[54] and coral bleaching. However it may benefit some species, for example increasing the growth rate of the sea star, Pisaster ochraceus,[55] while shelled plankton species may flourish in altered oceans.[56]
The report "Ocean Acidification Summary for Policymakers 2013" describes research findings and possible impacts.[57]
Impacts on oceanic calcifying organisms
Although the natural absorption of CO2 by the world's oceans helps mitigate the climatic effects of anthropogenic emissions of CO
2, it is believed that the resulting decrease in pH will have negative consequences, primarily for oceanic calcifying organisms. These span the food chain from autotrophs to heterotrophs and include organisms such as coccolithophores, corals, foraminifera, echinoderms, crustaceans and molluscs.[9][58] As described above, under normal conditions, calcite and aragonite are stable in surface waters since the carbonate ion is at supersaturating concentrations. However, as ocean pH falls, the concentration of carbonate ions required for saturation to occur increases, and when carbonate becomes undersaturated, structures made of calcium carbonate are vulnerable to dissolution. Therefore, even if there is no change in the rate of calcification, the rate of dissolution of calcareous material increases.[59]
Corals,[60][61][62] coccolithophore algae,[63][64][65][66] coralline algae,[67] foraminifera,[68] shellfish[69] and pteropods[10][70] experience reduced calcification or enhanced dissolution when exposed to elevated CO
2.
The Royal Society published a comprehensive overview of ocean acidification, and its potential consequences, in June 2005.[27] However, some studies have found different response to ocean acidification, with coccolithophore calcification and photosynthesis both increasing under elevated atmospheric pCO
2,[71][72][73] an equal decline in primary production and calcification in response to elevated CO
2[74] or the direction of the response varying between species.[75] A study in 2008 examining a sediment core from the North Atlantic found that while the species composition of coccolithophorids has remained unchanged for the industrial period 1780 to 2004, the calcification of coccoliths has increased by up to 40% during the same time.[73] A 2010 study from Stony Brook University suggested that while some areas are overharvested and other fishing grounds are being restored, because of ocean acidification it may be impossible to bring back many previous shellfish populations.[76] While the full ecological consequences of these changes in calcification are still uncertain, it appears likely that many calcifying species will be adversely affected.
When exposed in experiments to pH reduced by 0.2 to 0.4, larvae of a temperate brittlestar, a relative of the common sea star, fewer than 0.1 percent survived more than eight days.[46] There is also a suggestion that a decline in the coccolithophores may have secondary effects on climate, contributing to global warming by decreasing the Earth's albedo via their effects on oceanic cloud cover.[77] All marine ecosystems on Earth will be exposed to changes in acidification and several other ocean biogeochemical changes.[9]
The fluid in the internal compartments where corals grow their exoskeleton is also extremely important for calcification growth. When the saturation rate of aragonite in the external seawater is at ambient levels, the corals will grow their aragonite crystals rapidly in their internal compartments, hence their exoskeleton grows rapidly. If the level of aragonite in the external seawater is lower than the ambient level, the corals have to work harder to maintain the right balance in the internal compartment. When that happens, the process of growing the crystals slows down, and this slows down the rate of how much their exoskeleton is growing. Depending on how much aragonite is in the surrounding water, the corals may even stop growing because the levels of aragonite are too low to pump in to the internal compartment. They could even dissolve faster than they can make the crystals to their skeleton, depending on the aragonite levels in the surrounding water.[78]
Ocean acidification may force some organisms to reallocate resources away from productive endpoints such as growth in order to maintain calcification.[79]
Other biological impacts
Aside from the slowing and/or reversing of calcification, organisms may suffer other adverse effects, either indirectly through negative impacts on food resources,[27] or directly as reproductive or physiological effects. For example, the elevated oceanic levels of CO2 may produce CO
2-induced acidification of body fluids, known as hypercapnia. Also, increasing ocean acidity is believed to have a range of direct consequences. For example, increasing acidity has been observed to: reduce metabolic rates in jumbo squid;[53] depress the immune responses of blue mussels;[54] and make it harder for juvenile clownfish to tell apart the smells of non-predators and predators,[80] or hear the sounds of their predators.[81] This is possibly because ocean acidification may alter the acoustic properties of seawater, allowing sound to propagate further, and increasing ocean noise.[82] This impacts all animals that use sound for echolocation or communication.[83] Atlantic longfin squid eggs took longer to hatch in acidified water, and the squid's statolith was smaller and malformed in animals placed in sea water with a lower pH.[84] However, as with calcification, as yet there is not a full understanding of these processes in marine organisms or ecosystems.[85]
Nonbiological impacts
Leaving aside direct biological effects, it is expected that ocean acidification in the future will lead to a significant decrease in the burial of carbonate sediments for several centuries, and even the dissolution of existing carbonate sediments.[86] This will cause an elevation of ocean alkalinity, leading to the enhancement of the ocean as a reservoir for CO2 with implications for climate change as more CO
2 leaves the atmosphere for the ocean.[87]
Impact on human industry
The threat of acidification includes a decline in commercial fisheries and in the Arctic tourism industry and economy. Commercial fisheries are threatened because acidification harms calcifying organisms which form the base of the Arctic food webs. pteropods and brittle stars both form the base of the Arctic food webs and are both seriously damaged from acidification. Pteropods shells dissolve with increasing acidification and the brittle stars lose muscle mass when re-growing appendages.[88]For pteropods to create shells they require aragonite which is produced through carbonate ions and dissolved calcium. Pteropods are severely affected because increasing acidification levels have steadily deceased the amount of water supersaturated with carbonate which is needed for aragonite creation.[89] Arctic waters are changing so rapidly that they will become undersaturated with aragonite as early as 2016.[89] Additionally the brittle star's eggs die within a few days when exposed to expected conditions resulting from Arctic acidification.[90] Acidification threatens to destroy Arctic food webs from the base up. Arctic food webs are considered simple, meaning there are few steps in the food chain from small organisms to larger predators. For example pteropods are “a key prey item of a number of higher predators - larger plankton, fish, seabirds, whales"[91] Both pteropods and sea stars serve as a substantial food source and their removal from the simple food web would pose a serious threat to the whole ecosystem. The effects on the calcifying organisms at the base of the food webs could potentially destroy fisheries. The value of fish caught from US commercial fisheries in 2007 was valued at $3.8 billion and of that 73% was derived from calcifiers and their direct predators.[92] Other organisms are directly harmed as a result of acidification. For example decrease in the growth of marine calcifiers such as the American Lobster, Ocean Quahog, and scallops means there is less shellfish meat available for sale and consumption.[93] Red king crab fisheries are also at a serious threat because crabs are calcifiers and rely on carbonate ions for shell development. Baby red king crab when exposed to increased acidification levels experienced 100% mortality after 95 days.[94] In 2006 Red King Cab accounted for 23% of the total guideline harvest levels and a serious decline in red crab population would threaten the crab harvesting industry.[95] Several ocean goods and services are likely to be undermined by future ocean acidification potentially affecting the livelihoods of some 400 to 800 million people depending upon the emission scenario.[9]
Impact on indigenous peoples
Acidification could damage the Arctic tourism economy and affect the way of life of indigenous peoples. A major pillar of Arctic tourism is the sport fishing and hunting industry. The sport fishing industry is threatened by collapsing food webs which provide food for the prized fish. A decline in tourism lowers revenue input in the area, and threatens the economies that are increasingly dependent on tourism.[96] Acidification is not merely a threat but has significantly declined whole fish populations. For example, In Scandinavia studies conducted on acidic water revealed that 15% of species populations had disappeared and that many more populations were limited in numbers or declining.[97] The rapid decrease or disappearance of marine life could also affect the diet of Indigenous peoples.Possible responses
Reducing CO
2 emissions
Members of the InterAcademy Panel recommended that by 2050, global anthropogenic CO2 emissions be reduced less than 50% of the 1990 level.[13] The 2009[13] statement also called on world leaders to:
Stabilizing atmospheric CO
- Acknowledge that ocean acidification is a direct and real consequence of increasing atmospheric CO
2 concentrations, is already having an effect at current concentrations, and is likely to cause grave harm to important marine ecosystems as CO
2 concentrations reach 450 [parts-per-million (ppm)] and above;- [...] Recognise that reducing the build up of CO
2 in the atmosphere is the only practicable solution to mitigating ocean acidification;- [...] Reinvigorate action to reduce stressors, such as overfishing and pollution, on marine ecosystems to increase resilience to ocean acidification.
2 concentrations at 450 ppm would require near-term emissions reductions, with steeper reductions over time.[98]
The German Advisory Council on Global Change[99] stated:
In order to prevent disruption of the calcification of marine organisms and the resultant risk of fundamentally altering marine food webs, the following guard rail should be obeyed: the pH of near surface waters should not drop more than 0.2 units below the pre-industrial average value in any larger ocean region (nor in the global mean).One policy target related to ocean acidity is the magnitude of future global warming. Parties to the United Nations Framework Convention on Climate Change (UNFCCC) adopted a target of limiting warming to below 2 °C, relative to the pre-industrial level.[100] Meeting this target would require substantial reductions in anthropogenic CO
2 emissions.[101]
Limiting global warming to below 2 °C would imply a reduction in surface ocean pH of 0.16 from pre-industrial levels. This would represent a substantial decline in surface ocean pH.[102]
Climate engineering
Climate engineering (mitigating temperature or pH effects of emissions) has been proposed as a possible response to ocean acidification. The IAP (2009)[13] statement cautioned against climate engineering as a policy response:Mitigation approaches such as adding chemicals to counter the effects of acidification are likely to be expensive, only partly effective and only at a very local scale, and may pose additional unanticipated risks to the marine environment. There has been very little research on the feasibility and impacts of these approaches. Substantial research is needed before these techniques could be applied.Reports by the WGBU (2006),[99] the UK's Royal Society (2009),[103] and the US National Research Council (2011)[104] warned of the potential risks and difficulties associated with climate engineering.
Iron fertilization
Iron fertilization of the ocean could stimulate photosynthesis in phytoplankton (see Iron Hypothesis). The phytoplankton would convert the ocean's dissolved carbon dioxide into carbohydrate and oxygen gas, some of which would sink into the deeper ocean before oxidizing. More than a dozen open-sea experiments confirmed that adding iron to the ocean increases photosynthesis in phytoplankton by up to 30 times.[105] While this approach has been proposed as a potential solution to the ocean acidification problem, mitigation of surface ocean acidification might increase acidification in the less-inhabited deep ocean.[106]A report by the UK's Royal Society (2009)[107] reviewed the approach for effectiveness, affordability, timeliness and safety. The rating for affordability was "medium", or "not expected to be very cost-effective." For the other three criteria, the ratings ranged from "low" to "very low" (i.e., not good). For example, in regards to safety, the report found a "[high] potential for undesirable ecological side effects," and that ocean fertilization "may increase anoxic regions of ocean ('dead zones')."[108]