From Wikipedia, the free encyclopedia
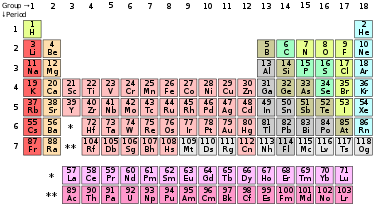
The periodic table is a tabular arrangement of the chemical elements, organized on the basis of their atomic number (number of protons in the nucleus), electron configurations, and recurring chemical properties. Elements are presented in order of increasing atomic number, which is typically listed with the chemical symbol in each box. The standard form of the table consists of a grid of elements laid out in 18 columns and 7 rows, with a double row of elements below that. The table can also be deconstructed into four rectangular blocks: the s-block to the left, the p-block to the right, the d-block in the middle, and the f-block below that.
The rows of the table are called periods; the columns are called groups, with some of these having names such as halogens or noble gases. Since, by definition, a periodic table incorporates recurring trends, the table can be used to derive relationships between the properties of the elements and predict the properties of new, yet to be discovered or synthesized, elements. As a result, a periodic table provides a useful framework for analyzing chemical behavior, and so the tables, in various forms, are widely used in chemistry and other sciences.
Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table. He developed his table to illustrate periodic trends in the properties of the then-known elements. Mendeleev also predicted some properties of then-unknown elements that would be expected to fill gaps in this table. Most of his predictions were proved correct when the elements in question were subsequently discovered. Mendeleev's periodic table has since been expanded and refined with the discovery or synthesis of further new elements and the development of new theoretical models to explain chemical behavior.
All elements from atomic numbers 1 (hydrogen) to 118 (ununoctium) have been discovered or reportedly synthesized, with elements 113, 115, 117, and 118 having yet to be confirmed. The first 98 elements exist naturally although some are found only in trace amounts and were synthesized in laboratories before being found in nature.[n 1] Elements with atomic numbers from 99 to 118 have only been synthesized, or claimed to be so, in laboratories. Production of elements having higher atomic numbers is being pursued, with the question of how the periodic table may need to be modified to accommodate any such additions being a matter of ongoing debate. Numerous synthetic radionuclides of naturally occurring elements have also been produced in laboratories.
Overview
| Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkali metals | Alkaline earth metals | Pnictogens | Chalcogens | Halogens | Noble gases | |||||||||||||||||||||||||
| Period 1 |
||||||||||||||||||||||||||||||
| 2 | ||||||||||||||||||||||||||||||
| 3 | ||||||||||||||||||||||||||||||
| 4 | ||||||||||||||||||||||||||||||
| 5 | ||||||||||||||||||||||||||||||
| 6 | ||||||||||||||||||||||||||||||
| 7 | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
All versions of the periodic table include only chemical elements, not mixtures, compounds, or subatomic particles.[n 2] Each chemical element has a unique atomic number representing the number of protons in its nucleus. Most elements have differing numbers of neutrons among different atoms, with these variants being referred to as isotopes. For example, carbon has three naturally occurring isotopes: all of its atoms have six protons and most have six neutrons as well, but about one per cent have seven neutrons, and a very small fraction have eight neutrons. Isotopes are never separated in the periodic table; they are always grouped together under a single element. Elements with no stable isotopes have the atomic masses of their most stable isotopes, where such masses are shown, listed in parentheses.[1]
In the standard periodic table, the elements are listed in order of increasing atomic number (the number of protons in the nucleus of an atom). A new row (period) is started when a new electron shell has its first electron. Columns (groups) are determined by the electron configuration of the atom; elements with the same number of electrons in a particular subshell fall into the same columns (e.g. oxygen and selenium are in the same column because they both have four electrons in the outermost p-subshell). Elements with similar chemical properties generally fall into the same group in the periodic table, although in the f-block, and to some respect in the d-block, the elements in the same period tend to have similar properties, as well. Thus, it is relatively easy to predict the chemical properties of an element if one knows the properties of the elements around it.[2]
As of 2014, the periodic table has 114 confirmed elements, comprising elements 1 (hydrogen) to 112 (copernicium), 114 (flerovium) and 116 (livermorium). Elements 113, 115, 117 and 118 have reportedly been synthesised in laboratories however none of these claims have been officially confirmed by the International Union of Pure and Applied Chemistry (IUPAC), nor are they named. As such these elements are currently identified by their atomic number (e.g., "element 113"), or by their provisional systematic name ("ununtrium", symbol "Uut").[3]
A total of 98 elements occur naturally; the remaining 16 elements, from einsteinium to copernicium, and flerovium and livermorium, occur only when synthesised in laboratories. Of the 98 elements that occur naturally, 84 are primordial. The other 14 naturally occurring elements occur only in decay chains of primordial elements.[4] No element heavier than einsteinium (element 99) has ever been observed in macroscopic quantities in its pure form.[5]
Layout variants
| Periodic table layouts | |
|---|---|
| Lanthanides and actinides separated (left; 18 columns) and in the main table (right; 32 columns) | |
This convention is entirely a matter of formatting practicality. The same table structure can be shown in a 32-column format, with the lanthanides and actinides in the main table's row 6 and 7.
However, based on the chemical and physical properties of elements, many alternative table structures have been constructed.
Grouping methods
Groups
A group or family is a vertical column in the periodic table. Groups usually have more significant periodic trends than periods and blocks, explained below. Modern quantum mechanical theories of atomic structure explain group trends by proposing that elements within the same group generally have the same electron configurations in their valence shell.[7] Consequently, elements in the same group tend to have a shared chemistry and exhibit a clear trend in properties with increasing atomic number.[8] However in some parts of the periodic table, such as the d-block and the f-block, horizontal similarities can be as important as, or more pronounced than, vertical similarities.[9][10][11]Under an international naming convention, the groups are numbered numerically from 1 to 18 from the leftmost column (the alkali metals) to the rightmost column (the noble gases).[12] Previously, they were known by roman numerals. In America, the roman numerals were followed by either an "A" if the group was in the s- or p-block, or a "B" if the group was in the d-block. The roman numerals used correspond to the last digit of today's naming convention (e.g. the group 4 elements were group IVB, and the group 14 elements was group IVA). In Europe, the lettering was similar, except that "A" was used if the group was before group 10, and "B" was used for groups including and after group 10. In addition, groups 8, 9 and 10 used to be treated as one triple-sized group, known collectively in both notations as group VIII. In 1988, the new IUPAC naming system was put into use, and the old group names were deprecated.[13]
Some of these groups have been given trivial (unsystematic) names, as seen in the table below, although some are rarely used. Groups 3–10 have no trivial names and are referred to simply by their group numbers or by the name of the first member of their group (such as 'the scandium group' for Group 3), since they display fewer similarities and/or vertical trends.[12]
Elements in the same group tend to show patterns in atomic radius, ionization energy, and electronegativity. From top to bottom in a group, the atomic radii of the elements increase. Since there are more filled energy levels, valence electrons are found farther from the nucleus. From the top, each successive element has a lower ionization energy because it is easier to remove an electron since the atoms are less tightly bound. Similarly, a group has a top to bottom decrease in electronegativity due to an increasing distance between valence electrons and the nucleus.[14] There are exceptions to these trends, however, an example of which occurs in group 11 where electronegativity increases farther down the group.[15]
Periods
A period is a horizontal row in the periodic table. Although groups generally have more significant periodic trends, there are regions where horizontal trends are more significant than vertical group trends, such as the f-block, where the lanthanides and actinides form two substantial horizontal series of elements.[16]Elements in the same period show trends in atomic radius, ionization energy, electron affinity, and electronegativity. Moving left to right across a period, atomic radius usually decreases. This occurs because each successive element has an added proton and electron which causes the electron to be drawn closer to the nucleus.[17] This decrease in atomic radius also causes the ionization energy to increase when moving from left to right across a period. The more tightly bound an element is, the more energy is required to remove an electron. Electronegativity increases in the same manner as ionization energy because of the pull exerted on the electrons by the nucleus.[14] Electron affinity also shows a slight trend across a period. Metals (left side of a period) generally have a lower electron affinity than nonmetals (right side of a period), with the exception of the noble gases.[18]
Blocks

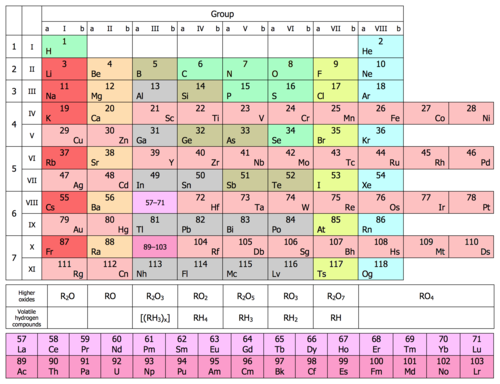
Specific regions of the periodic table can be referred to as blocks in recognition of the sequence in which the electron shells of the elements are filled. Each block is named according to the subshell in which the "last" electron notionally resides.[19][n 3] The s-block comprises the first two groups (alkali metals and alkaline earth metals) as well as hydrogen and helium. The p-block comprises the last six groups which are groups 13 to 18 in IUPAC (3A to 8A in American) and contains, among other elements, all of the metalloids. The d-block comprises groups 3 to 12 (or 3B to 2B in American group numbering) and contains all of the transition metals. The f-block, often offset below the rest of the periodic table, has no group numbers and comprises lanthanides and actinides.[20]
Metals, metalloids and nonmetals
According to their shared physical and chemical properties, the elements can be classified into the major categories of metals, metalloids and nonmetals. Metals are generally shiny, highly conducting solids which form alloys with one another and salt-like ionic compounds with nonmetals (other than the noble gases). The majority of nonmetals are coloured or colourless insulating gases; nonmetals that form compounds with other nonmetals feature covalent bonding. In between metals and nonmetals are metalloids, which have intermediate or mixed properties.[21]
Metal and nonmetals can be further classified into subcategories that show a gradation from metallic to non-metallic properties, when going left to right in the rows. The metals are subdivided into the highly reactive alkali metals, through the less reactive alkaline earth metals, lanthanides and actinides, via the archetypal transition metals, and ending in the physically and chemically weak post-transition metals. The nonmetals are simply subdivided into the polyatomic nonmetals which, being nearest to the metalloids, show some incipient metallic character; the diatomic nonmetals, which are essentially nonmetallic; and the monatomic noble gases, which are nonmetallic and almost completely inert. Specialized groupings such as the refractory metals and the noble metals, which are subsets (in this example) of the transition metals, are also known[22] and occasionally denoted.[23]
Placing the elements into categories and subcategories based on shared properties is imperfect. There is a spectrum of properties within each category and it is not hard to find overlaps at the boundaries, as is the case with most classification schemes.[24] Beryllium, for example, is classified as an alkaline earth metal although its amphoteric chemistry and tendency to mostly form covalent compounds are both attributes of a chemically weak or post transition metal. Radon is classified as a nonmetal and a noble gas yet has some cationic chemistry that is more characteristic of a metal. Other classification schemes are possible such as the division of the elements into mineralogical occurrence categories, or crystalline structures. Categorising the elements in this fashion dates back to at least 1869 when Hinrichs[25] wrote that simple boundary lines could be drawn on the periodic table to show elements having like properties, such as the metals and the nonmetals, or the gaseous elements.
Periodic trends
Electron configuration

Approximate order in which shells and subshells are arranged by increasing energy according to the Madelung rule
The electron configuration or organisation of electrons orbiting neutral atoms shows a recurring pattern or periodicity. The electrons occupy a series of electron shells (numbered shell 1, shell 2, and so on). Each shell consists of one or more subshells (named s, p, d, f and g). As atomic number increases, electrons progressively fill these shells and subshells more or less according to the Madelung rule or energy ordering rule, as shown in the diagram. The electron configuration for neon, for example, is 1s2 2s2 2p6. With an atomic number of ten, neon has two electrons in the first shell, and eight electrons in the second shell—two in the s subshell and six in the p subshell. In periodic table terms, the first time an electron occupies a new shell corresponds to the start of each new period, these positions being occupied by hydrogen and the alkali metals.[26][27]
Since the properties of an element are mostly determined by its electron configuration, the properties of the elements likewise show recurring patterns or periodic behaviour, some examples of which are shown in the diagrams below for atomic radii, ionization energy and electron affinity. It is this periodicity of properties, manifestations of which were noticed well before the underlying theory was developed, that led to the establishment of the periodic law (the properties of the elements recur at varying intervals) and the formulation of the first periodic tables.[26][27]
Atomic radii

Atomic number plotted against atomic radius[n 4]
Atomic radii vary in a predictable and explainable manner across the periodic table. For instance, the radii generally decrease along each period of the table, from the alkali metals to the noble gases; and increase down each group. The radius increases sharply between the noble gas at the end of each period and the alkali metal at the beginning of the next period. These trends of the atomic radii (and of various other chemical and physical properties of the elements) can be explained by the electron shell theory of the atom; they provided important evidence for the development and confirmation of quantum theory.[28]
The electrons in the 4f-subshell, which is progressively filled from cerium (element 58) to ytterbium (element 70), are not particularly effective at shielding the increasing nuclear charge from the sub-shells further out. The elements immediately following the lanthanides have atomic radii which are smaller than would be expected and which are almost identical to the atomic radii of the elements immediately above them.[29] Hence hafnium has virtually the same atomic radius (and chemistry) as zirconium, and tantalum has an atomic radius similar to niobium, and so forth. This is known as the lanthanide contraction. The effect of the lanthanide contraction is noticeable up to platinum (element 78), after which it is masked by a relativistic effect known as the inert pair effect.[30] The d-block contraction, which is a similar effect between the d-block and p-block, is less pronounced than the lanthanide contraction but arises from a similar cause.[29]
Ionization energy
Large jumps in the successive molar ionization energies occur when removing an electron from a noble gas (complete electron shell) configuration. For magnesium again, the first two molar ionization energies of magnesium given above correspond to removing the two 3s electrons, and the third ionization energy is a much larger 7730 kJ/mol, for the removal of a 2p electron from the very stable neon-like configuration of Mg2+. Similar jumps occur in the ionization energies of other third-row atoms.[30]
Electronegativity

Electronegativity is the tendency of an atom to attract electrons.[31] An atom's electronegativity is affected by both its atomic number and the distance between the valence electrons and the nucleus. The higher its electronegativity, the more an element attracts electrons. It was first proposed by Linus Pauling in 1932.[32] In general, electronegativity increases on passing from left to right along a period, and decreases on descending a group. Hence, fluorine is the most electronegative of the elements,[n 5] while caesium is the least, at least of those elements for which substantial data is available.[15]
There are some exceptions to this general rule. Gallium and germanium have higher electronegativities than aluminium and silicon respectively because of the d-block contraction. Elements of the fourth period immediately after the first row of the transition metals have unusually small atomic radii because the 3d-electrons are not effective at shielding the increased nuclear charge, and smaller atomic size correlates with higher electronegativity.[15] The anomalously high electronegativity of lead, particularly when compared to thallium and bismuth, appears to be an artifact of data selection (and data availability)—methods of calculation other than the Pauling method show the normal periodic trends for these elements.[33]
Electron affinity

Dependence of electron affinity on atomic number.[34] Values generally increase across each period, culminating with the halogens before decreasing precipitously with the noble gases. Examples of localized peaks seen in hydrogen, the alkali metals and the group 11 elements are caused by a tendency to complete the s-shell (with the 6s shell of gold being further stabilized by relativistic effects and the presence of a filled 4f sub shell). Examples of localized troughs seen in the alkaline earth metals, and nitrogen, phosphorus, manganese and rhenium are caused by filled s-shells, or half-filled p- or d-shells.[35]
The electron affinity of an atom is the amount of energy released when an electron is added to a neutral atom to form a negative ion. Although electron affinity varies greatly, some patterns emerge. Generally, nonmetals have more positive electron affinity values than metals. Chlorine most strongly attracts an extra electron. The electron affinities of the noble gases have not been measured conclusively, so they may or may not have slightly negative values.[36]
Electron affinity generally increases across a period. This is caused by the filling of the valence shell of the atom; a group 17 atom releases more energy than a group 1 atom on gaining an electron because it obtains a filled valence shell and is therefore more stable.[36]
A trend of decreasing electron affinity going down groups would be expected. The additional electron will be entering an orbital farther away from the nucleus. As such this electron would be less attracted to the nucleus and would release less energy when added. However, in going down a group, around one-third of elements are anomalous, with heavier elements having higher electron affinities than their next lighter congenors. Largely, this is due to the poor shielding by d and f electrons. A uniform decrease in electron affinity only applies to group 1 atoms.[37]
Metallic character
The lower the values of ionization energy, electronegativity and electron affinity, the more metallic character the element has. Conversely, nonmetallic character increases with higher values of these properties.[38] Given the periodic trends of these three properties, metallic character tends to decrease going across a period (or row) and, with some irregularities (mostly) due to poor screening of the nucleus by d and f electrons, and relativistic effects,[39] tends to increase going down a group (or column or family). Thus, the most metallic elements (such as caesium and francium) are found at the bottom left of traditional periodic tables and the most nonmetallic elements (oxygen, fluorine, chlorine) at the top right. The combination of horizontal and vertical trends in metallic character explains the stair-shaped dividing line between metals and nonmetals found on some periodic tables, and the practice of sometimes categorizing several elements adjacent to that line, or elements adjacent to those elements, as metalloids.[40][41]History
First systemization attempts
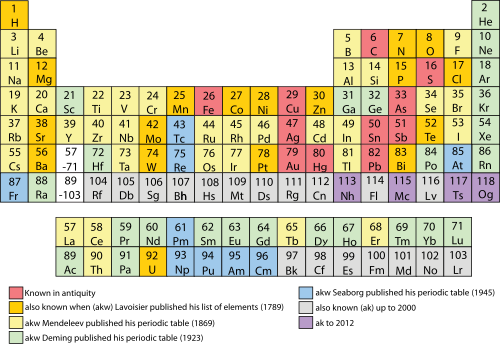
The discovery of the elements mapped to significant periodic table development dates (pre-, per- and post-)
In 1789, Antoine Lavoisier published a list of 33 chemical elements, grouping them into gases, metals, nonmetals, and earths.[42] Chemists spent the following century searching for a more precise classification scheme. In 1829, Johann Wolfgang Döbereiner observed that many of the elements could be grouped into triads based on their chemical properties. Lithium, sodium, and potassium, for example, were grouped together in a triad as soft, reactive metals. Döbereiner also observed that, when arranged by atomic weight, the second member of each triad was roughly the average of the first and the third;[43] this became known as the Law of Triads.[44] German chemist Leopold Gmelin worked with this system, and by 1843 he had identified ten triads, three groups of four, and one group of five. Jean-Baptiste Dumas published work in 1857 describing relationships between various groups of metals. Although various chemists were able to identify relationships between small groups of elements, they had yet to build one scheme that encompassed them all.[43]
In 1858, German chemist August Kekulé observed that carbon often has four other atoms bonded to it. Methane, for example, has one carbon atom and four hydrogen atoms. This concept eventually became known as valency; different elements bond with different numbers of atoms.[45]
In 1862, Alexandre-Emile Béguyer de Chancourtois, a French geologist, published an early form of periodic table, which he called the telluric helix or screw. He was the first person to notice the periodicity of the elements. With the elements arranged in a spiral on a cylinder by order of increasing atomic weight, de Chancourtois showed that elements with similar properties seemed to occur at regular intervals. His chart included some ions and compounds in addition to elements. His paper also used geological rather than chemical terms and did not include a diagram; as a result, it received little attention until the work of Dmitri Mendeleev.[46]
In 1864, Julius Lothar Meyer, a German chemist, published a table with 44 elements arranged by valency. The table showed that elements with similar properties often shared the same valency.[47] Concurrently, William Odling (an English chemist) published an arrangement of 57 elements, ordered on the basis of their atomic weights. With some irregularities and gaps, he noticed what appeared to be a periodicity of atomic weights amongst the elements and that this accorded with 'their usually received groupings.' [48] Odling alluded to the idea of a periodic law but did not pursue it.[49] He subsequently proposed (in 1870) a valence-based classification of the elements.[50]
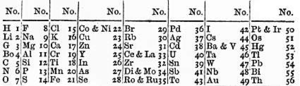
Newlands's periodic table, as presented to the Chemical Society in 1866, and based on the law of octaves
English chemist John Newlands produced a series of papers from 1863 to 1866 noting that when the elements were listed in order of increasing atomic weight, similar physical and chemical properties recurred at intervals of eight; he likened such periodicity to the octaves of music.[51][52] This so termed Law of Octaves, however, was ridiculed by Newlands' contemporaries, and the Chemical Society refused to publish his work.[53] Newlands was nonetheless able to draft a table of the elements and used it to predict the existence of missing elements, such as germanium.[54] The Chemical Society only acknowledged the significance of his discoveries five years after they credited Mendeleev.[55]
In 1867, Gustavus Hinrichs, a Danish born academic chemist based in America, published a spiral periodic system based on atomic spectra and weights, and chemical similarities. His work was regarded as idiosyncratic, ostentatious and labyrinthine and this may have militated against its recognition and acceptance.[56][57]
Mendeleev's table
Russian chemistry professor Dmitri Mendeleev and German chemist Julius Lothar Meyer independently published their periodic tables in 1869 and 1870, respectively.[58] Mendeleev's table was his first published version; that of Meyer was an expanded version of his (Meyer's) table of 1864.[59] They both constructed their tables by listing the elements in rows or columns in order of atomic weight and starting a new row or column when the characteristics of the elements began to repeat.[60]
The recognition and acceptance afforded to Mendeleev's table came from two decisions he made. The first was to leave gaps in the table when it seemed that the corresponding element had not yet been discovered.[61] Mendeleev was not the first chemist to do so, but he was the first to be recognized as using the trends in his periodic table to predict the properties of those missing elements, such as gallium and germanium.[62] The second decision was to occasionally ignore the order suggested by the atomic weights and switch adjacent elements, such as tellurium and iodine, to better classify them into chemical families. With the development of theories of atomic structure, it became apparent that Mendeleev had unintentionally listed the elements in order of increasing atomic number or nuclear charge.[63]
The significance of atomic numbers to the organization of the periodic table was not appreciated until the existence and properties of protons and neutrons became understood. Mendeleev's periodic tables used atomic weight instead of atomic number to organize the elements, information determinable to fair precision in his time. Atomic weight worked well enough in most cases to (as noted) give a presentation that was able to predict the properties of missing elements more accurately than any other method then known. Substitution of atomic numbers, once understood, gave a definitive, integer-based sequence for the elements, still used today even as new synthetic elements are being produced and studied.[64]
Further development
In 1871, Mendeleev published a form of periodic table, with groups of similar elements arranged in columns from I to VIII (as shown). He also gave detailed predictions for the properties of elements he had earlier noted were missing, but should exist.[65] These gaps were subsequently filled as chemists discovered additional naturally occurring elements.[66] It is often stated that the last naturally occurring element to be discovered was francium (referred to by Mendeleev as eka-caesium) in 1939.[67] However, plutonium, produced synthetically in 1940, was identified in trace quantities as a naturally occurring primordial element in 1971,[68] and by 2011 it was known that all the elements up to californium can occur naturally as trace amounts in uranium ores by neutron capture and beta decay.[4]
The popular[69] periodic table layout, also known as the common or standard form (as shown at various other points in this article), is attributable to Horace Groves Deming. In 1923, Deming, an American chemist, published short (Mendeleev style) and medium (18-column) form periodic tables.[70][n 6] Merck and Company prepared a handout form of Deming's 18-column medium table, in 1928, which was widely circulated in American schools. By the 1930s Deming's table was appearing in handbooks and encyclopaedias of chemistry. It was also distributed for many years by the Sargent-Welch Scientific Company.[71][72][73]
With the development of modern quantum mechanical theories of electron configurations within atoms, it became apparent that each period (row) in the table corresponded to the filling of a quantum shell of electrons. Larger atoms have more electron sub-shells, so later tables have required progressively longer periods.[74]

Glenn T. Seaborg who, in 1945, suggested a new periodic table showing the actinides as belonging to a second f-block series
In 1945, Glenn Seaborg, an American scientist, made the suggestion that the actinide elements, like the lanthanides were filling an f sub-level. Before this time the actinides were thought to be forming a fourth d-block row. Seaborg's colleagues advised him not to publish such a radical suggestion as it would most likely ruin his career. As Seaborg considered he did not then have a career to bring into disrepute, he published anyway. Seaborg's suggestion was found to be correct and he subsequently went on to win the 1951 Nobel prize in chemistry for his work in synthesizing actinide elements.[75][76][n 7]
Although minute quantities of some transuranic elements occur naturally,[4] they were all first discovered in laboratories. Their production has expanded the periodic table significantly, the first of these being neptunium, synthesized in 1939.[77] Because many of the transuranic elements are highly unstable and decay quickly, they are challenging to detect and characterize when produced. There have been controversies concerning the acceptance of competing discovery claims for some elements, requiring independent review to determine which party has priority, and hence naming rights. The most recently accepted and named elements are flerovium (element 114) and livermorium (element 116), both named on 31 May 2012.[78] In 2010, a joint Russia–US collaboration at Dubna, Moscow Oblast, Russia, claimed to have synthesized six atoms of ununseptium (element 117), making it the most recently claimed discovery.[79]
Alternative structures

There are many periodic tables with structures other than that of the standard form. Within 100 years of the appearance of Mendeleev's table in 1869 it has been estimated that around 700 different periodic table versions were published.[80] As well as numerous rectangular variations, other periodic table formats have included, for example,[n 8] circular, cubic, cylindrical, edificial (building-like), helical, lemniscate, octagonal prismatic, pyramidal, separated, spherical, spiral, and triangular forms. Such alternatives are often developed to highlight or emphasize chemical or physical properties of the elements that are not as apparent in traditional periodic tables.[80]
A popular[81] alternative structure is that of Theodor Benfey (1960). The elements are arranged in a continuous spiral, with hydrogen at the center and the transition metals, lanthanides, and actinides occupying peninsulas.[82]
Most periodic tables are two-dimensional[4] however three-dimensional tables are known to as far back as at least 1862 (pre-dating Mendeleev's two-dimensional table of 1869). More recent examples include Courtines' Periodic Classification (1925),[83] Wringley's Lamina System (1949),[84] Giguère's Periodic helix (1965)[85][n 9] and Dufour's Periodic Tree (1996).[86] Going one better, Stowe's Physicist's Periodic Table (1989)[87] has been described as being four-dimensional (having three spatial dimensions and one colour dimension).[88]
The various forms of periodic tables can be thought of as lying on a chemistry–physics continuum.[89] Towards the chemistry end of the continuum can be found, as an example, Rayner-Canham's 'unruly'[90] Inorganic Chemist's Periodic Table (2002),[91] which emphasizes trends and patterns, and unusual chemical relationships and properties. Near the physics end of the continuum is Janet's Left-Step Periodic Table (1928). This has a structure which shows a closer connection to the order of electron-shell filling and, by association, quantum mechanics.[92] Somewhere in the middle of the continuum is the ubiquitous common or standard form of periodic table. This is regarded as better expressing empirical trends in physical state, electrical and thermal conductivity, and oxidation numbers, and other properties easily inferred from traditional techniques of the chemical laboratory.[93]
| Janet left-step periodic table | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1s | H | He | ||||||||||||||||||||||||||||||
| 2s | Li | Be | ||||||||||||||||||||||||||||||
| 2p 3s | B | C | N | O | F | Ne | Na | Mg | ||||||||||||||||||||||||
| 3p 4s | Al | Si | P | S | Cl | Ar | K | Ca | ||||||||||||||||||||||||
| 3d 4p 5s | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | Rb | Sr | ||||||||||||||
| 4d 5p 6s | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | Cs | Ba | ||||||||||||||
| 4f 5d 6p 7s | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | Fr | Ra |
| 5f 6d 7p 8s | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | 113 | Fl | 115 | Lv | 117 | 118 | 119 | 120 |
| f-block | d-block | p-block | s-block | |||||||||||||||||||||||||||||
| This form of periodic table is more congruent with the order in which electron shells are filled, as shown in the accompanying sequence in the left margin (read from top to bottom, left to right). | ||||||||||||||||||||||||||||||||
Open questions and controversies
Elements with unknown chemical properties
Although all elements up to ununoctium have been discovered, of the elements above hassium (element 108), only copernicium (element 112) and flerovium (element 114) have known chemical properties. The other elements may behave differently from what would be predicted by extrapolation, due to relativistic effects; for example, flerovium has been predicted to possibly exhibit some noble-gas-like properties, even though it is currently placed in the carbon group.[94] More recent experiments have suggested, however, that flerovium behaves chemically like lead, as expected from its periodic table position.[95]Further periodic table extensions
It is unclear whether new elements will continue the pattern of the current periodic table as period 8, or require further adaptations or adjustments. Seaborg expected the eighth period to follow the previously established pattern exactly, so that it would include a two-element s-block for elements 119 and 120, a new g-block for the next 18 elements, and 30 additional elements continuing the current f-, d-, and p-blocks.[96] More recently, physicists such as Pekka Pyykkö have theorized that these additional elements do not follow the Madelung rule, which predicts how electron shells are filled and thus affects the appearance of the present periodic table.[97]Element with the highest possible atomic number
The number of possible elements is not known. A very early suggestion made by Elliot Adams in 1911, and based on the arrangement of elements in each horizontal periodic table row, was that elements of atomic weight greater than 256± (which would equate to between elements 99 and 100 in modern-day terms) did not exist.[98] A higher—more recent—estimate is that the periodic table may end soon after the island of stability,[99] which is expected to center around element 126, as the extension of the periodic and nuclides tables is restricted by proton and neutron drip lines.[100] Other predictions of an end to the periodic table include at element 128 by John Emsley,[4] at element 137 by Richard Feynman,[101] and at element 155 by Albert Khazan.[4][n 10]- Bohr model
- Relativistic Dirac equation
Placement of hydrogen and helium
Hydrogen and helium are often placed in different places than their electron configurations would indicate; hydrogen is usually placed above lithium, in accordance with its electron configuration, but is sometimes placed above fluorine,[106] or even carbon,[106] as it also behaves somewhat similarly to them. Hydrogen is also sometimes placed in its own group, as it does not behave similarly enough to any element to be placed in a group with another.[107] Helium is almost always placed above neon, as they are very similar chemically, although it is occasionally placed above beryllium on account of having a comparable electron shell configuration (helium: 1s2; beryllium: [He] 2s2).[19]Groups included in the transition metals
The definition of a transition metal, as given by IUPAC, is an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell.[108] By this definition all of the elements in groups 3–11 are transition metals. The IUPAC definition therefore excludes group 12, comprising zinc, cadmium and mercury, from the transition metals category.Some chemists treat the categories "d-block elements" and "transition metals" interchangeably, thereby including groups 3–12 among the transition metals. In this instance the group 12 elements are treated as a special case of transition metal in which the d electrons are not ordinarily involved in chemical bonding. The recent discovery that mercury can use its d electrons in the formation of mercury(IV) fluoride (HgF4) has prompted some commentators to suggest that mercury can be regarded as a transition metal.[109] Other commentators, such as Jensen,[110] have argued that the formation of a compound like HgF4 can occur only under highly abnormal conditions. As such, mercury could not be regarded as a transition metal by any reasonable interpretation of the ordinary meaning of the term.[110]
Still other chemists further exclude the group 3 elements from the definition of a transition metal. They do so on the basis that the group 3 elements do not form any ions having a partially occupied d shell and do not therefore exhibit any properties characteristic of transition metal chemistry.[111] In this case, only groups 4–11 are regarded as transition metals.
Period 6 and 7 elements in group 3
Although scandium and yttrium are always the first two group 3 elements, the identity of the next two elements is not agreed upon; they are either lanthanum and actinium, or lutetium and lawrencium. Although there are some strong physical and chemical arguments supporting the latter arrangement not all authors are convinced.[112] The current IUPAC definition of the term "lanthanoid" includes fifteen elements including both lanthanum and lutetium, and that of "transition element"[108] applies to lanthanum and actinium, as well as lutetium but not lawrencium, since it does not correctly follow the Aufbau principle. Normally, the 103rd electron would enter the d-subshell, but quantum mechanical research has found that the configuration is actually [Rn] 5f14 7s2 7p1[n 11] due to relativistic effects.[113][114] IUPAC thus has not recommended a specific format for the in-line-f-block periodic table, leaving the dispute open.- Lanthanum and actinium are sometimes considered the remaining members of group 3.[115] In their most commonly encountered tripositive ion forms, these elements do not possess any partially filled f-orbitals, thus continuing the scandium—yttrium—lanthanum—actinium trend, in which all the elements have relationship similar to that of elements of the calcium—strontium—barium—radium series, the elements' left neighbors in s-block. However, different behavior is observed in other d-block groups, especially in group 4, in which zirconium, hafnium and rutherfordium share similar chemical properties lacking a clear trend.
- In other tables, lutetium and lawrencium are classified as the remaining members of group 3.[116] In these tables, lutetium and lawrencium end (or sometimes follow) the lanthanide and actinide series, respectively. Since the f-shell is nominally full in the ground state electron configuration for both of these metals, they behave most similarly to other period 6 and period 7 transition metals compared to the other lanthanides and actinides, and thus logically exhibit properties similar to those of scandium and yttrium. (This behavior is expected for lawrencium, but has not been observed because sufficient quantities of lawrencium have not yet been synthesized.)
- Some tables, including the IUPAC table[117][n 12] refer to all lanthanides and actinides by a marker in group 3. This sometimes is believed to be the inclusion of all 30 lanthanide and actinide elements as included in group 3. Lanthanides, as electropositive trivalent metals, all have a closely related chemistry, and all show many similarities to scandium and yttrium, but they also show additional properties characteristic of their partially filled f-orbitals which are not common to scandium and yttrium.
- Exclusion of all elements is based on properties of earlier actinides, which show a much wider variety of chemistry (for instance, in range of oxidation states) within their series than the lanthanides, and comparisons to scandium and yttrium are even less useful.[118] However, these elements are destabilized,[119] and if they were stabilized to more closely match chemistry laws, they would be similar to lanthanides as well. Also, the later actinides from berkelium onwards behave more like the corresponding lanthanides, with only the valence +3 (and sometimes +2 and +4) shown.[118]