From Wikipedia, the free encyclopedia
The Rare Earth Hypothesis argues that planets with complex life, like
Earth, are exceptionally rare
In
planetary astronomy and
astrobiology, the
Rare Earth Hypothesis argues that the
origin of life and the
evolution of biological complexity such as
sexually reproducing,
multicellular organisms on
Earth (and, subsequently,
human intelligence) required an improbable combination of
astrophysical and
geological events and circumstances. According to the hypothesis, complex
extraterrestrial life is a very improbable phenomenon and likely to be extremely rare. The term "Rare Earth" originates from
Rare Earth: Why Complex Life Is Uncommon in the Universe (2000), a book by
Peter Ward, a geologist and paleontologist, and
Donald E. Brownlee, an astronomer and astrobiologist, both faculty members at the University of Washington.
A contrary view was argued in the 1970s and 1980s by
Carl Sagan and
Frank Drake, among others. It holds that Earth is a typical rocky
planet in a typical
planetary system, located in a non-exceptional region of a common
barred-spiral galaxy. Given the
principle of mediocrity (in the same vein as the
Copernican principle),
it is probable that we are typical, and the universe teems with complex
life. However, Ward and Brownlee argue that planets, planetary systems,
and galactic regions that are as friendly to complex life as the Earth,
the
Solar System, and our
galactic region are very rare.
Requirements for complex life
The Rare Earth hypothesis argues that the
evolution of biological complexity requires a host of fortuitous circumstances, such as a
galactic habitable zone, a central star and planetary system having the requisite character, the
circumstellar habitable zone, a right-sized terrestrial planet, the advantage of a gas giant guardian like Jupiter and a large
natural satellite, conditions needed to ensure the planet has a
magnetosphere and
plate tectonics, the chemistry of the
lithosphere,
atmosphere, and oceans, the role of "evolutionary pumps" such as massive
glaciation and rare
bolide impacts, and whatever led to the appearance of the
eukaryote cell,
sexual reproduction and the
Cambrian explosion of
animal,
plant, and
fungi phyla. The
evolution of human intelligence may have required yet further events, which are extremely unlikely to have happened were it not for the
Cretaceous–Paleogene extinction event 66 million years ago removing
dinosaurs as the dominant terrestrial
vertebrates.
In order for a small rocky planet to support complex life, Ward and
Brownlee argue, the values of several variables must fall within narrow
ranges. The
universe
is so vast that it could contain many Earth-like planets. But if such
planets exist, they are likely to be separated from each other by many
thousands of
light years. Such distances may preclude communication among any intelligent species evolving on such planets, which would solve the
Fermi paradox: "If extraterrestrial aliens are common, why aren't they obvious?"
[1]
The right location in the right kind of galaxy
Rare Earth
suggests that much of the known universe, including large parts of our
galaxy, are "dead zones" unable to support complex life. Those parts of a
galaxy where complex life is possible make up the
galactic habitable zone, primarily characterized by distance from the
Galactic Center. As that distance increases:
- Star metallicity declines. Metals (which in astronomy means all elements other than hydrogen and helium) are necessary to the formation of terrestrial planets.
- The X-ray and gamma ray radiation from the black hole at the galactic center, and from nearby neutron stars, becomes less intense. Thus the early universe, and present-day galactic regions where stellar density is high and supernovae are common, will be dead zones.[2]
- Gravitational perturbation of planets and planetesimals
by nearby stars becomes less likely as the density of stars decreases.
Hence the further a planet lies from the Galactic Center or a spiral
arm, the less likely it is to be struck by a large bolide which could extinguish all complex life on a planet.
Dense center of galaxies such as
NGC 7331 (often referred to as a "twin" of the
Milky Way[3]) have high radiation levels toxic to complex life.
According to Rare Earth, globular clusters are unlikely to support life.
Item #1 rules out the outer reaches of a galaxy; #2 and #3 rule out
galactic inner regions. Hence a galaxy's habitable zone may be a ring
sandwiched between its uninhabitable center and outer reaches.
Also, a habitable planetary system must maintain its favorable location long enough for complex life to evolve. A star with an
eccentric
(elliptic or hyperbolic) galactic orbit will pass through some spiral
arms, unfavorable regions of high star density; thus a life-bearing star
must have a galactic orbit that is nearly circular, with a close
synchronization between the orbital velocity of the star and of the
spiral arms. This further restricts the galactic habitable zone within a
fairly narrow range of distances from the Galactic Center. Lineweaver
et al.
[4] calculate this zone to be a ring 7 to 9
kiloparsecs in radius, including no more than 10% of the stars in the
Milky Way,
[5] about 20 to 40 billion stars. Gonzalez, et al.
[6] would halve these numbers; they estimate that at most 5% of stars in the Milky Way fall in the galactic habitable zone.
Approximately 77% of observed galaxies are spiral,
[7] two-thirds of all spiral galaxies are barred, and more than half, like the Milky Way, exhibit multiple arms.
[8] According to Rare Earth, our own galaxy is unusually quiet and dim (see below), representing just 7% of its kind.
[9] Even so, this would still represent more than 200 billion galaxies in the known universe.
Our galaxy also appears unusually favorable in suffering fewer
collisions with other galaxies over the last 10 billion years, which can
cause more supernovae and other disturbances.
[10] Also, the Milky Way's central
black hole seems to have neither too much nor too little activity.
[11]
The orbit of the Sun around the center of the Milky Way is indeed almost perfectly circular, with
a period of 226 Ma
(million years), closely matching the rotational period of the galaxy.
However, the majority of stars in barred spiral galaxies populate the
spiral arms rather than the halo and tend to move in
gravitationally aligned orbits,
so there is little that is unusual about the Sun's orbit. While the
Rare Earth hypothesis predicts that the Sun should rarely, if ever, have
passed through a spiral arm since its formation, astronomer Karen
Masters has calculated that the orbit of the Sun takes it through a
major spiral arm approximately every 100 million years.
[12] Some researchers have suggested that several mass extinctions do correspond with previous crossings of the spiral arms.
[13]
Orbiting at the right distance from the right type of star
According to the hypothesis, Earth has an improbable orbit in the very narrow habitable zone (dark green) around the Sun.
The terrestrial example suggests that complex life requires liquid
water, requiring an orbital distance neither too close nor too far from
the central star, another scale of
habitable zone or
Goldilocks Principle:
[14] The habitable zone varies with the star's type and age.
For advanced life, the star must also be highly stable, which is
typical of middle star life, about 4.6 billion years old. Proper
metallicity and size are also very important to stability. The Sun has a low 0.1%
luminosity variation. To date no
solar twin
star twin, with an exact match of the sun's luminosity variation, has
been found, though some come close. The star must have no stellar
companions, as in
binary systems, which would disrupt the orbits of planets. Estimates suggest 50% or more of all star systems are binary.
[15][16][17][18]
The habitable zone for a main sequence star very gradually moves out
over its lifespan until it becomes a white dwarf and the habitable zone
vanishes.
The liquid water and other gases available in the habitable zone bring the benefit of
greenhouse warming. Even though the
Earth's atmosphere
contains a water vapor concentration from 0% (in arid regions) to 4%
(in rain forest and ocean regions) and – as of February 2018 – only
408.05
[citation needed] parts per million of CO
2, these small amounts suffice to raise the average surface temperature by about 40 °C,
[19]
with the dominant contribution being due to water vapor, which together
with clouds makes up between 66% and 85% of Earth's greenhouse effect,
with CO
2 contributing between 9% and 26% of the effect.
[20]
Rocky planets must orbit within the habitable zone for life to form. Although the habitable zone of such hot stars as
Sirius or
Vega is wide, hot stars also emit much more
ultraviolet radiation that
ionizes any planetary
atmosphere. They may become
red giants before advanced life
evolves on their planets. These considerations rule out the massive and powerful stars of type F6 to O (see
stellar classification) as homes to evolved
metazoan life.
Small
red dwarf stars conversely have small
habitable zones wherein planets are in
tidal lock,
with one very hot side always facing the star and another very cold
side; and they are also at increased risk of solar flares (see
Aurelia).
Life therefore cannot arise in such systems. Rare Earth proponents
claim that only stars from F7 to K1 types are hospitable. Such stars are
rare: G type stars such as the Sun (between the hotter F and cooler K)
comprise only 9%
[21] of the hydrogen-burning stars in the Milky Way.
Such aged stars as
red giants and
white dwarfs are also unlikely to support life. Red giants are common in globular clusters and
elliptical galaxies.
White dwarfs are mostly dying stars that have already completed their
red giant phase. Stars that become red giants expand into or overheat
the habitable zones of their youth and middle age (though theoretically
planets at a much greater distance
may become habitable).
An energy output that varies with the lifetime of the star will very likely prevent life (e.g., as
Cepheid variables).
A sudden decrease, even if brief, may freeze the water of orbiting
planets, and a significant increase may evaporate it and cause a
greenhouse effect that prevents the oceans from reforming.
All known life requires the complex chemistry of
metallic elements. The
absorption spectrum
of a star reveals the presence of metals within, and studies of stellar
spectra reveal that many, perhaps most, stars are poor in metals.
Because heavy metals originate in
supernova
explosions, metallicity increases in the universe over time. Low
metallicity characterizes the early universe: globular clusters and
other stars that formed when the universe was young, stars in most
galaxies other than large
spirals,
and stars in the outer regions of all galaxies. Metal-rich central
stars capable of supporting complex life are therefore believed to be
most common in the quiet suburbs
[vague] of the larger spiral galaxies—where radiation also happens to be weak.
[22]
With the right arrangement of planets
Depiction of the Sun and planets of the Solar System and the sequence of
planets. Rare Earth argues that without such an arrangement, in
particular the presence of the massive gas giant Jupiter (fifth planet
from the Sun and the largest), complex life on Earth would not have
arisen.
Rare Earth proponents argue that a planetary system capable of
sustaining complex life must be structured more or less like the Solar
System, with small and rocky inner planets and outer gas giants.
[23]
Without the protection of 'celestial vacuum cleaner' planets with
strong gravitational pull, a planet would be subject to more
catastrophic asteroid collisions.
Observations of exo-planets have shown that arrangements of planets
similar to our Solar System are rare. Most planetary systems have super
Earths, several times larger than Earth, close to their star, whereas
our Solar System's inner region has only a few small rocky planets and
none inside Mercury's orbit. Only 10% of stars have giant planets
similar to Jupiter and Saturn, and those few rarely have stable nearly
circular orbits distant from their star.
Konstantin Batygin
and colleagues argue that these features can be explained if, early in
the history of the Solar System, Jupiter and Saturn drifted towards the
Sun, sending showers of planetesimals towards the super-Earths which
sent them spiralling into the Sun, and ferrying icy building blocks into
the terrestrial region of the Solar System which provided the building
blocks for the rocky planets. The two giant planets then drifted out
again to their present position. However, in the view of Batygin and his
colleagues: "The concatenation of chance events required for this
delicate choreography suggest that small, Earth-like rocky planets – and
perhaps life itself – could be rare throughout the cosmos."
[24]
A continuously stable orbit
Rare
Earth argues that a gas giant must not be too close to a body where
life is developing. Close placement of gas giant(s) could disrupt the
orbit of a potential life-bearing planet, either directly or by drifting
into the habitable zone.
Newtonian dynamics can produce
chaotic planetary orbits, especially in a system having
large planets at high
orbital eccentricity.
[25]
The need for stable orbits rules out
stars with systems of planets that contain large planets with orbits close to the host star (called "
hot Jupiters").
It is believed that hot Jupiters have migrated inwards to their current
orbits. In the process, they would have catastrophically disrupted the
orbits of any planets in the habitable zone.
[26] To exacerbate matters, hot Jupiters are much more common orbiting F and G class stars.
[27]
A terrestrial planet of the right size
Planets of the Solar System to scale. Rare Earth argues that complex
life cannot exist on large gaseous planets like Jupiter and Saturn (top
row) or Uranus and Neptune (top middle) or smaller planets such as Mars
and Mercury
It is argued that life requires terrestrial planets like Earth and as
gas giants lack such a surface, that complex life cannot arise there.
[28]
A planet that is too small cannot hold much atmosphere, making
surface temperature low and variable and oceans impossible. A small
planet will also tend to have a rough surface, with large mountains and
deep canyons. The core will cool faster, and
plate tectonics may be brief or entirely absent. A planet that is too large will retain too dense atmosphere like
Venus.
Although Venus is similar in size and mass to Earth, its surface
atmospheric pressure is 92 times that of Earth, and surface temperature
of 735 K (462 °C; 863 °F). Earth had a similar early atmosphere to
Venus, but may have lost it in the
giant impact event.
[29]
With plate tectonics
The
Great American Interchange on Earth, around ~ 3.5 to 3 Ma, an example of species competition, resulting from continental plate interaction.
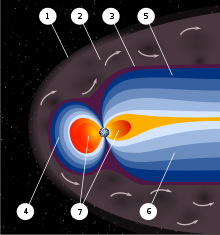
An artist's rendering of the structure of Earth's magnetic field-magnetosphere that protects Earth's life from
solar radiation.
1) Bow shock. 2) Magnetosheath. 3) Magnetopause. 4) Magnetosphere.
5) Northern tail lobe. 6) Southern tail lobe. 7) Plasmasphere.
Rare Earth proponents argue that
plate tectonics and a strong
magnetic field are essential for
biodiversity,
global temperature regulation, and the
carbon cycle.
[30] The lack of
mountain chains
elsewhere in the Solar System is direct evidence that Earth is the only
body with plate tectonics, and thus the only nearby body capable of
supporting life.
[31]
Plate tectonics depend on the right chemical composition and a long-lasting source of heat from
radioactive decay. Continents must be made of less dense
felsic rocks that "float" on underlying denser
mafic rock. Taylor
[32] emphasizes that tectonic
subduction zones require the lubrication of oceans of water. Plate tectonics also provides a means of
biochemical cycling.
[33]
Plate tectonics and as a result
continental drift and the creation of separate land masses would create diversified
ecosystems and
biodiversity, one of the strongest defences against extinction.
[34] An example of species diversification and later competition on Earth's continents is the
Great American Interchange. North and Middle America drifted into
South America at around 3.5 to 3 Ma. The
fauna of South America evolved separately for about 30 million years, since
Antarctica separated. Many species were subsequently wiped out in mainly South America by competing
Northern American animals.
A large moon
Tide pools resulting from tidal interaction of the Moon are said to have promoted the evolution of complex life.
The Moon is unusual because the other rocky planets in the Solar System either have no satellites (
Mercury and
Venus), or only tiny satellites which are probably captured asteroids (
Mars).
The
Giant-impact theory hypothesizes that the Moon resulted from the impact of a
Mars-sized body, dubbed
Theia, with the very young Earth. This giant impact also gave the Earth its
axial tilt (inclination) and velocity of rotation.
[32] Rapid rotation reduces the daily variation in temperature and makes
photosynthesis viable.
[35] The
Rare Earth hypothesis further argues that the axial tilt cannot be too large or too small (relative to the
orbital plane).
A planet with a large tilt will experience extreme seasonal variations
in climate. A planet with little or no tilt will lack the stimulus to
evolution that climate variation provides.
[citation needed]
In this view, the Earth's tilt is "just right". The gravity of a large
satellite also stabilizes the planet's tilt; without this effect the
variation in tilt would be
chaotic, probably making complex life forms on land impossible.
[36]
If the Earth had no Moon, the ocean
tides resulting solely from the Sun's gravity would be only half that of the lunar tides. A large satellite gives rise to
tidal pools, which may be essential for the formation of
complex life, though this is far from certain.
[37]
A large satellite also increases the likelihood of
plate tectonics through the effect of
tidal forces on the planet's crust.
[citation needed] The impact that formed the Moon may also have initiated plate tectonics, without which the
continental crust would cover the entire planet, leaving no room for
oceanic crust.
[citation needed] It is possible that the large scale
mantle convection needed to drive plate tectonics could not have emerged in the absence of crustal inhomogeneity.
Atmosphere
A
terrestrial planet of the right size is needed to retain an atmosphere,
like Earth and Venus. On Earth, once the giant impact of
Theia thinned
Earth's atmosphere, other events were needed to make the atmosphere capable of sustaining life. The
Late Heavy Bombardment reseeded Earth with water lost after the impact of Theia.
[38] The development of an
ozone layer formed protection from
ultraviolet (UV) sunlight.
[39][40] Nitrogen and
carbon dioxide are needed in a correct ratio for life to form.
[41] Lightning is needed for
nitrogen fixation.
[42][42] The carbon dioxide
gas needed for life comes from sources such as
volcanoes and
geysers. Carbon dioxide is only needed at low levels
[citation needed] (currently at 400
ppm); at high levels it is poisonous.
[43][44] Precipitation is needed to have a stable water cycle.
[45] A proper atmosphere must reduce
diurnal temperature variation.
[46][47]
One or more evolutionary triggers for complex life
This diagram illustrates the twofold cost of sex. If each individual were to contribute to the same number of offspring (two), (a) the sexual population remains the same size each generation, where the (b) asexual population doubles in size each generation
Regardless of whether planets with similar physical attributes to the
Earth are rare or not, some argue that life usually remains simple
bacteria. Biochemist
Nick Lane argues that simple cells (
prokaryotes)
emerged soon after Earth's formation, but since almost half the
planet's life had passed before they evolved into complex ones (
eukaryotes) all of whom share a
common ancestor, this event can only have happened once. In some views,
prokaryotes
lack the cellular architecture to evolve into eukaryotes because a
bacterium expanded up to eukaryotic proportions would have tens of
thousands of times less energy available; two billion years ago, one
simple cell incorporated itself into another, multiplied, and evolved
into
mitochondria
that supplied the vast increase in available energy that enabled the
evolution of complex life. If this incorporation occurred only once in
four billion years or is otherwise unlikely, then life on most planets
remains simple.
[48]
An alternative view is that mitochondria evolution was environmentally
triggered, and that mitochondria-containing organisms appeared very soon
after the first traces of atmospheric oxygen.
[49]
The evolution and persistence of
sexual reproduction is another mystery in biology. The purpose of
sexual reproduction is unclear, as in many organisms it has a 50% cost (fitness disadvantage) in relation to
asexual reproduction.
[50] Mating types (types of
gametes, according to their compatibility) may have arisen as a result of
anisogamy (gamete dimorphism), or the male and female genders may have evolved before anisogamy.
[51][52] It is also unknown why most sexual organisms use a binary
mating system,
[53] and why some organisms have gamete dimorphism.
Charles Darwin was the first to suggest that
sexual selection drives
speciation; without it, complex life would probably not have evolved.
The right time in evolution
Timeline of evolution;
human writings exists for only 0.000218% of Earth's history.
While life on Earth is regarded to have spawned relatively early in
the planet's history, the evolution from multicellular to intelligent
organisms took around 800 million years.
[54]
Civilizations on Earth have existed for about 12,000 years and radio
communication reaching space has existed for less than 100 years.
Relative to the age of the Solar System (~4.57 Ga) this is a short time,
in which extreme climatic variations, super volcanoes, and large
meteorite impacts were absent. These events would severely harm
intelligent life, as well as life in general. For example, the
Permian-Triassic mass extinction,
caused by widespread and continuous volcanic eruptions in an area the
size of Western Europe, led to the extinction of 95% of known species
around 251.2
Ma ago. About 65 million years ago, the
Chicxulub impact at the
Cretaceous–Paleogene boundary (~65.5 Ma) on the
Yucatán peninsula in
Mexico led to a mass extinction of the most advanced species at that time.
If there were intelligent extraterrestrial civilizations able to make
contact with distant Earth, they would have to live in the same 12Ka
period of the 800Ma evolution of life.
Rare Earth equation
The following discussion is adapted from Cramer.
[55] The Rare Earth equation is Ward and Brownlee's
riposte to the
Drake equation. It calculates

, the number of Earth-like planets in the Milky Way having complex life forms, as:
According to Rare Earth, the Cambrian explosion that saw extreme diversification of
chordata from simple forms like Pikaia (pictured) was an improbable event
 [56]
[56]
where:
- N* is the number of stars in the Milky Way.
This number is not well-estimated, because the Milky Way's mass is not
well estimated, with little information about the number of very small
stars. N* is at least 100 billion, and may be as high as 500 billion, if there are many low visibility stars.
 is the average number of planets in a star's habitable zone. This zone
is fairly narrow, because constrained by the requirement that the
average planetary temperature be consistent with water remaining liquid
throughout the time required for complex life to evolve. Thus
is the average number of planets in a star's habitable zone. This zone
is fairly narrow, because constrained by the requirement that the
average planetary temperature be consistent with water remaining liquid
throughout the time required for complex life to evolve. Thus  =1 is a likely upper bound.
=1 is a likely upper bound.
We assume

.
The Rare Earth hypothesis can then be viewed as asserting that the
product of the other nine Rare Earth equation factors listed below,
which are all fractions, is no greater than 10
−10 and could plausibly be as small as 10
−12. In the latter case,

could be as small as 0 or 1. Ward and Brownlee do not actually calculate the value of

,
because the numerical values of quite a few of the factors below can
only be conjectured. They cannot be estimated simply because
we have but one data point: the Earth, a rocky planet orbiting a
G2 star in a quiet suburb of a large
barred spiral galaxy, and the home of the only intelligent species we know, namely ourselves.
 is the fraction of stars in the galactic habitable zone (Ward, Brownlee, and Gonzalez estimate this factor as 0.1[6]).
is the fraction of stars in the galactic habitable zone (Ward, Brownlee, and Gonzalez estimate this factor as 0.1[6]). is the fraction of stars in the Milky Way with planets.
is the fraction of stars in the Milky Way with planets. is the fraction of planets that are rocky ("metallic") rather than gaseous.
is the fraction of planets that are rocky ("metallic") rather than gaseous. is the fraction of habitable planets where microbial life arises. Ward
and Brownlee believe this fraction is unlikely to be small.
is the fraction of habitable planets where microbial life arises. Ward
and Brownlee believe this fraction is unlikely to be small. is the fraction of planets where complex life evolves. For 80% of the
time since microbial life first appeared on the Earth, there was only
bacterial life. Hence Ward and Brownlee argue that this fraction may be
very small.
is the fraction of planets where complex life evolves. For 80% of the
time since microbial life first appeared on the Earth, there was only
bacterial life. Hence Ward and Brownlee argue that this fraction may be
very small. is the fraction of the total lifespan of a planet during which complex
life is present. Complex life cannot endure indefinitely, because the
energy put out by the sort of star that allows complex life to emerge
gradually rises, and the central star eventually becomes a red giant,
engulfing all planets in the planetary habitable zone. Also, given
enough time, a catastrophic extinction of all complex life becomes ever
more likely.
is the fraction of the total lifespan of a planet during which complex
life is present. Complex life cannot endure indefinitely, because the
energy put out by the sort of star that allows complex life to emerge
gradually rises, and the central star eventually becomes a red giant,
engulfing all planets in the planetary habitable zone. Also, given
enough time, a catastrophic extinction of all complex life becomes ever
more likely. is the fraction of habitable planets with a large moon. If the giant impact theory of the Moon's origin is correct, this fraction is small.
is the fraction of habitable planets with a large moon. If the giant impact theory of the Moon's origin is correct, this fraction is small. is the fraction of planetary systems with large Jovian planets. This fraction could be large.
is the fraction of planetary systems with large Jovian planets. This fraction could be large. is the fraction of planets with a sufficiently low number of extinction
events. Ward and Brownlee argue that the low number of such events the
Earth has experienced since the Cambrian explosion may be unusual, in which case this fraction would be small.
is the fraction of planets with a sufficiently low number of extinction
events. Ward and Brownlee argue that the low number of such events the
Earth has experienced since the Cambrian explosion may be unusual, in which case this fraction would be small.
The Rare Earth equation, unlike the
Drake equation, does not factor the probability that complex life evolves into
intelligent life that discovers technology (Ward and Brownlee are not
evolutionary biologists). Barrow and Tipler
[57] review the consensus among such biologists that the evolutionary path from primitive Cambrian
chordates, e.g.,
Pikaia to
Homo sapiens, was a highly improbable event. For example, the large
brains of humans have marked adaptive disadvantages, requiring as they do an expensive
metabolism, a long
gestation period, and a childhood lasting more than 25% of the average total life span. Other improbable features of humans include:
- Being one of a handful of extant bipedal land (non-avian) vertebrate. Combined with an unusual eye–hand coordination, this permits dextrous manipulations of the physical environment with the hands;
- A vocal apparatus far more expressive[citation needed] than that of any other mammal, enabling speech. Speech makes it possible for humans to interact cooperatively, to share knowledge, and to acquire a culture;
- The capability of formulating abstractions to a degree permitting the invention of mathematics, and the discovery of science and technology. Only recently did humans acquire anything like their current scientific and technological sophistication.
Advocates
Writers who support the Rare Earth hypothesis:
- Stuart Ross Taylor,[32]
a specialist on the Solar System, firmly believes in the hypothesis.
Taylor concludes that the Solar System is probably very unusual, because
it resulted from so many chance factors and events.
- Stephen Webb,[1] a physicist, mainly presents and rejects candidate solutions for the Fermi paradox. The Rare Earth hypothesis emerges as one of the few solutions left standing by the end of the book.
- Simon Conway Morris, a paleontologist, endorses the Rare Earth hypothesis in chapter 5 of his Life's Solution: Inevitable Humans in a Lonely Universe,[58] and cites Ward and Brownlee's book with approval.[59]
- John D. Barrow and Frank J. Tipler (1986. 3.2, 8.7, 9), cosmologists, vigorously defend the hypothesis that humans are likely to be the only intelligent life in the Milky Way, and perhaps the entire universe. But this hypothesis is not central to their book The Anthropic Cosmological Principle, a thorough study of the anthropic principle and of how the laws of physics are peculiarly suited to enable the emergence of complexity in nature.
- Ray Kurzweil, a computer pioneer and self-proclaimed Singularitarian, argues in The Singularity Is Near that the coming Singularity
requires that Earth be the first planet on which sentient,
technology-using life evolved. Although other Earth-like planets could
exist, Earth must be the most evolutionarily advanced, because otherwise
we would have seen evidence that another culture had experienced the Singularity and expanded to harness the full computational capacity of the physical universe.
- John Gribbin, a prolific science writer, defends the hypothesis in Alone in the Universe: Why our planet is unique.[60]
- Guillermo Gonzalez, astrophysicist who coined the term galactic habitable zone uses the hypothesis in his book The Privileged Planet to promote the concept of intelligent design.[61]
- Michael H. Hart, astrophysicist
who proposed a very narrow habitable zone based on climate studies,
edited the influential book "Extraterrestrials: Where are They" and
authored one of its chapters "Atmospheric Evolution, the Drake Equation
and DNA: Sparse Life in an Infinite Universe".[62]
- Howard Alan Smith,
astrophysicist and author of 'Let there be light: modern cosmology and
Kabbalah: a new conversation between science and religion'.[63]
- Marc J. Defant, professor of geochemistry and volcanology,
elaborated on several aspects of the rare earth hypothesis in his TEDx
talk entitled: Why We are Alone in the Galaxy.[64]
Criticism
Cases against the Rare Earth Hypothesis take various forms.
Anthropic reasoning
The
hypothesis concludes, more or less, that complex life is rare because
it can evolve only on the surface of an Earth-like planet or on a
suitable satellite of a planet. Some biologists, such as
Jack Cohen, believe this assumption too restrictive and unimaginative; they see it as a form of
circular reasoning.
According to
David Darling, the Rare Earth hypothesis is neither
hypothesis nor
prediction, but merely a description of how life arose on Earth.
[65] In his view Ward and Brownlee have done nothing more than select the factors that best suit their case.
What matters is not whether there's anything unusual about the Earth; there's going to be something idiosyncratic
about every planet in space. What matters is whether any of Earth's
circumstances are not only unusual but also essential for complex life.
So far we've seen nothing to suggest there is.[66]
Critics also argue that there is a link between the Rare Earth Hypothesis and the
creationist ideas of
intelligent design.
[67]
Exoplanets around main sequence stars are being discovered in large numbers
An increasing number of
extrasolar planet discoveries are being made with 3,786 planets in 2,834 planetary systems known as of 2 June 2018.
[68] Rare Earth proponents argue life cannot arise outside Sun-like systems. However,
some exobiologists have suggested that stars outside this range may give
rise to life
under the right circumstances; this possibility is a central point of
contention to the theory because these late-K and M category stars make
up about 82% of all hydrogen-burning stars.
[21]
Current technology limits the testing of important Rare Earth criteria: surface water, tectonic plates, a large moon and
biosignatures
are currently undetectable. Though planets the size of Earth are
difficult to detect and classify, scientists now think that rocky
planets are common around Sun-like stars.
[69] The
Earth Similarity Index (ESI) of mass, radius and temperature provides a means of measurement, but falls short of the full Rare Earth criteria.
[70][71]
Rocky planets orbiting within habitable zones may not be rare
Some argue that Rare Earth's estimates of rocky planets in habitable zones (

in the Rare Earth equation) are too restrictive.
James Kasting cites the
Titius-Bode law
to contend that it is a misnomer to describe habitable zones as narrow
when there is a 50% chance of at least one planet orbiting within one.
[73]
In 2013 a study that was published in the journal Proceedings of the
National Academy of Sciences calculated that about "one in five" of all
sun-like
stars are expected to have earthlike planets "within the
habitable zones of their stars"; 8.8 billion of them therefore exist in the Milky Way galaxy alone.
[74] On 4 November 2013, astronomers reported, based on
Kepler space mission data, that there could be as many as 40 billion
Earth-sized planets orbiting in the
habitable zones of
sun-like stars and
red dwarf stars within the
Milky Way Galaxy.
[75][76] 11 billion of these estimated planets may be orbiting sun-like stars.
[77]
Uncertainty over Jupiter's role
The requirement for a system to have a Jovian planet as protector (Rare Earth equation factor

) has been challenged, affecting the number of proposed extinction events (Rare Earth equation factor

). Kasting's 2001 review of Rare Earth questions whether a Jupiter protector has any bearing on the incidence of complex life.
[78] Computer modelling including the 2005
Nice model and 2007
Nice 2 model yield inconclusive results in relation to Jupiter's gravitational influence and impacts on the inner planets.
[79]
A study by Horner and Jones (2008) using computer simulation found that
while the total effect on all orbital bodies within the Solar System is
unclear, Jupiter has caused more impacts on Earth than it has
prevented.
[80] Lexell's Comet,
a 1770 near miss that passed closer to Earth than any other comet in
recorded history, was known to be caused by the gravitational influence
of Jupiter.
[81] Grazier (2017) claims that the idea of Jupiter as a shield is a misinterpretation of a 1996 study by
George Wetherill,
and using computer models Grazier was able to demonstrate that Saturn
protects Earth from more asteroids and comets than does Jupiter.
[82]
Plate tectonics may not be unique to Earth or a requirement for complex life
Geological discoveries like the active features of Pluto's
Tombaugh Regio appear to contradict the argument that geologically active worlds like Earth are rare.
[83]
Ward and Brownlee argue that
tectonics is necessary to support
biogeochemical cycles
required for complex life, and predicted that such geological features
would not be found outside of Earth, pointing to a lack of observable
mountain ranges and
subduction.
[84]
There is, however, no scientific consensus on the evolution of plate
tectonics on Earth. Though it is believed that tectonic motion first
began around three billion years ago,
[85]
by this time photosynthesis and oxygenation had already begun.
Furthermore, recent studies point to plate tectonics as an episodic
planetary phenomenon, and that life may evolve during periods of
"stagnant-lid" rather than plate tectonic states.
[86]
Recent evidence also points to similar activity either having occurred or continuing to occur elsewhere. The
geology of Pluto, for example, described by Ward and Brownlee as "without mountains or volcanoes ... devoid of volcanic activity",
[22] has since been found to be quite the contrary, with a geologically active surface possessing organic molecules
[87] and mountain ranges
[88] like
Tenzing Montes and
Hillary Montes comparable in relative size to those of Earth, and observations suggest the involvement of endogenic processes.
[89] Plate tectonics has been suggested as a hypothesis for the
Martian dichotomy, and in 2012 geologist An Yin put forward evidence for active plate tectonics on Mars.
[90] Europa has long been suspected to have plate tectonics
[91] and in 2014 NASA announced evidence of active subduction.
[92] In 2017, scientists studying the
Geology of Charon confirmed that icy plate tectonics also operated on Pluto's largest moon.
[93]
Kasting suggests that there is nothing unusual about the occurrence
of plate tectonics in large rocky planets and liquid water on the
surface as most should generate internal heat even without the
assistance of radioactive elements.
[78] Studies by Valencia
[94] and Cowan
[95] suggest that plate tectonics may be inevitable for terrestrial planets Earth sized or larger, that is,
Super-Earths, which are now known to be more common in planetary systems.
[96]
Free oxygen may neither be rare nor a prerequisite for multicellular life
Animals like
Spinoloricus nov. sp. appear to defy the premise that animal life would not exist without oxygen
The hypothesis that
molecular oxygen, necessary for
animal life, is rare and that a
Great Oxygenation Event (Rare Earth equation factor

) could only have been triggered and sustained by tectonics, appears to have been invalidated by more recent discoveries.
Ward and Brownlee ask "whether oxygenation, and hence the rise of
animals, would ever have occurred on a world where there were no
continents to erode".
[97] Extraterrestrial free oxygen has recently been detected around other solid objects, including Mercury,
[98] Venus,
[99] Mars
[100] Jupiter's four
Galilean moons,
[101] Saturn's moons Enceladus,
[102] Dione
[103][104] and Rhea
[105] and even the atmosphere of a comet.
[106]
This has led scientists to speculate whether processes other than
photosynthesis could be capable of generating an environment rich in
free oxygen. Wordsworth (2014) concludes that oxygen generated other
than through
photodissociation may be likely on Earth-like exoplanets, and could actually lead to false positive detections of life.
[107] Narita (2015) suggests
photocatalysis by
titanium dioxide as a geochemical mechanism for producing oxygen atmospheres.
[108]
Since Ward & Brownlee's assertion that "there is irrefutable
evidence that oxygen is a necessary ingredient for animal life",
[97] anaerobic metazoa have been found that indeed do metabolise without oxygen.
Spinoloricus nov. sp., for example, a species discovered in the
hypersaline anoxic L'Atalante basin at the bottom of the
Mediterranean Sea in 2010, appears to metabolise with hydrogen, lacking
mitochondria and instead using
hydrogenosomes.
[109][110] Stevenson (2015) has proposed other membrane alternatives for complex life in worlds without oxygen.
[111] In 2017, scientists from the
NASA Astrobiology Institute discovered the necessary chemical preconditions for the formation of
azotosomes on Saturn's moon Titan, a world that lacks atmospheric oxygen.
[112]
Independent studies by Schirrmeister and by Mills concluded that
Earth's multicellular life existed prior to the Great Oxygenation Event,
not as a consequence of it.
[113][114]
NASA scientists Hartman and McKay argue that plate tectonics may in
fact slow the rise of oxygenation (and thus stymie complex life rather
than promote it).
[115]
Computer modelling by Tilman Spohn in 2014 found that plate tectonics
on Earth may have arisen from the effects of complex life's emergence,
rather than the other way around as the Rare Earth might suggest. The
action of lichens on rock may have contributed to the formation of
subduction zones in the presence of water.
[116]
Kasting argues that if oxygenation caused the Cambrian explosion then
any planet with oxygen producing photosynthesis should have complex
life.
[117]
A magnetic field may not be a requirement
The
importance of Earth's magnetic field to the development of complex life
has been disputed. Kasting argues that the atmosphere provides
sufficient protection against cosmic rays even during times of magnetic
pole reversal and atmosphere loss by sputtering.
[78] Kasting also dismisses the role of the magnetic field in the evolution of eukaryotes, citing the age of the oldest known
magnetofossils.
[118]
A large moon may neither be rare nor necessary
The requirement of a large moon (Rare Earth equation factor

)
has also been challenged. Even if it were required, such an occurrence
may not be as unique as predicted by the Rare Earth Hypothesis. Recent
work by
Edward Belbruno and
J. Richard Gott of Princeton University suggests that giant impacts such as those that may have formed the
Moon can indeed form in planetary
trojan points (
L4 or
L5 Lagrangian point) which means that similar circumstances may occur in other planetary systems.
[119]
Collision between two planetary bodies (artist concept).
Rare Earth's assertion that the Moon's stabilization of Earth's
obliquity and spin is a requirement for complex life has been
questioned. Kasting argues that a moonless Earth would still possess
habitats with climates suitable for complex life and questions whether
the spin rate of a moonless Earth can be predicted.
[78] Although the
giant impact theory
posits that the impact forming the Moon increased Earth's rotational
speed to make a day about 5 hours long, the Moon has slowly "
stolen"
much of this speed to reduce Earth's solar day since then to about 24
hours and continues to do so: in 100 million years Earth's solar day
will be roughly 24 hours 38 minutes (the same as Mars's solar day); in 1
billion years, 30 hours 23 minutes. Larger secondary bodies would exert
proportionally larger tidal forces that would in turn decelerate their
primaries faster and potentially increase the solar day of a planet in
all other respects like earth to over 120 hours within a few billion
years. This long solar day would make effective heat dissipation for
organisms in the tropics and subtropics extremely difficult in a similar
manner to tidal locking to a red dwarf star. Short days (high rotation
speed) causes high wind speeds at ground level. Long days (slow rotation
speed) cause the day and night temperatures to be too extreme.
[120]
Many Rare Earth proponents argue that the Earth's plate tectonics
would probably not exist if not for the tidal forces of the Moon.
[121][122]
The hypothesis that the Moon's tidal influence initiated or sustained
Earth's plate tectonics remains unproven, though at least one study
implies a temporal correlation to the formation of the Moon.
[123] Evidence for the past existence of plate tectonics on planets like Mars
[124]
which may never have had a large moon would counter this argument.
Kasting argues that a large moon is not required to initiate plate
tectonics.
[78]
Complex life may arise in alternative habitats
Complex life may exist in environments similar to
black smokers on Earth.
Rare Earth proponents argue that simple life may be common, though
complex life requires specific environmental conditions to arise.
Critics consider life could arise on a
moon
of a gas giant, though this is less likely if life requires
volcanicity. The moon must have stresses to induce tidal heating, but
not so dramatic as seen on Jupiter's Io. However, the moon is within the
gas giant's intense radiation belts, sterilizing any biodiversity
before it can get established.
Dirk Schulze-Makuch disputes this, hypothesizing alternative biochemistries for alien life.
[125]
While Rare Earth proponents argue that only microbial extremophiles
could exist in subsurface habitats beyond Earth, some argue that complex
life can also arise in these environments. Examples of extremophile
animals such as the
Hesiocaeca methanicola, an animal that inhabits ocean floor
methane clathrates substances more commonly found in the outer Solar System, the
tardigrades which can survive in the vacuum of space
[126] or
Halicephalobus mephisto
which exists in crushing pressure, scorching temperatures and extremely
low oxygen levels 3.6 kilometres deep in the Earth's crust,
[127] are sometimes cited by critics as complex life capable of thriving in "alien" environments.
Jill Tarter
counters the classic counterargument that these species adapted to
these environments rather than arose in them, by suggesting that we
cannot assume conditions for life to emerge which are not actually
known.
[128] There are suggestions that complex life could arise in sub-surface
conditions which may be similar to those where life may have arisen on
Earth, such as the
tidally heated subsurfaces of Europa or Enceladus.
[129][130] Ancient circumvental ecosystems such as these support complex life on Earth such as
Riftia pachyptila that exist completely independent of the surface biosphere.
[131]



![{\displaystyle {\ce {{NO2}->[h \nu] {NO}+ {O}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/34fed23155c119954fa08461965c09c3d04f2921)

![{\displaystyle {\ce {{NO2}+ {O2}->[h \nu] {NO}+ {O3}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/1e1d3ed7bb48f69dfa453b9727ecbe7863804988)

![{\displaystyle {\ce {{CFCS}->[h \nu] {Cl.}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/088ca5853b063101511fb5a21520e648c6d87466)

![{\displaystyle {\ce {{O3}->[h \nu] {O}+ {O2}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/f2db8df4adfc00e58fc18167823562cdbaa169d8)

![{\displaystyle {\ce {{2O3}->[h \nu] 3O2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/29b154561607496cd0feb37e02045d192ecbb4e8)