From Wikipedia, the free encyclopedia
Strictly speaking, both
actinium and
lawrencium have been labeled as
group 3 elements,
but both elements are often included in any general discussion of the
chemistry of the actinide elements. Actinium is the more often omitted
of the two, because its placement as a group 3 element is somewhat more
common in texts and for semantic reasons: since "actinide" means "like
actinium", it has been argued that actinium cannot logically be an
actinide, even though IUPAC acknowledges its inclusion based on common
usage.
The actinide series derives its name from the first element in the series, actinium. The informal chemical symbol
An is used in general discussions of actinide chemistry to refer to any actinide. All but one of the actinides are
f-block elements, with the exception being either actinium or lawrencium. The series mostly corresponds to the filling of the 5f
electron shell,
although actinium and thorium lack any f-electrons, and curium and
lawrencium have the same number as the preceding element. In comparison
with the
lanthanides, also mostly
f-block elements, the actinides show much more variable
valence. They all have very large
atomic and
ionic radii
and exhibit an unusually large range of physical properties. While
actinium and the late actinides (from americium onwards) behave
similarly to the lanthanides, the elements thorium, protactinium, and
uranium are much more similar to
transition metals in their chemistry, with neptunium and plutonium occupying an intermediate position.
In presentations of the
periodic table, the lanthanides and the actinides are customarily shown as two additional rows below the main body of the table, with placeholders or else a selected single element of each series (either
lanthanum or
lutetium, and either
actinium or
lawrencium, respectively) shown in a single cell of the main table, between
barium and
hafnium, and
radium and
rutherfordium, respectively. This convention is entirely a matter of
aesthetics and formatting practicality; a rarely used wide-formatted periodic
table inserts the lanthanide and actinide series in their proper places,
as parts of the table's sixth and seventh rows (periods).
Discovery, isolation and synthesis
Like the
lanthanides, the actinides form a family of elements with similar properties. Within the actinides, there are two overlapping groups:
transuranium elements, which follow uranium in the
periodic table—and
transplutonium elements, which follow plutonium. Compared to the lanthanides, which (except for
promethium)
are found in nature in appreciable quantities, most actinides are rare.
The majority of them do not even occur in nature, and of those that do,
only thorium and uranium do so in more than trace quantities. The most
abundant or easily synthesized actinides are uranium and thorium,
followed by plutonium, americium, actinium, protactinium, neptunium, and
curium.
\
The existence of transuranium elements was suggested by
Enrico Fermi based on his experiments in 1934.
However, even though four actinides were known by that time, it was not
yet understood that they formed a family similar to lanthanides. The
prevailing view that dominated early research into transuranics was that
they were regular elements in the 7th period, with thorium,
protactinium and uranium corresponding to 6th-period
hafnium,
tantalum and
tungsten,
respectively. Synthesis of transuranics gradually undermined this point
of view. By 1944 an observation that curium failed to exhibit oxidation
states above 4 (whereas its supposed 6th period homolog,
platinum, can reach oxidation state of 6) prompted
Glenn Seaborg
to formulate a so-called "actinide hypothesis". Studies of known
actinides and discoveries of further transuranic elements provided more
data in support of this point of view, but the phrase "actinide
hypothesis" (the implication being that a "hypothesis" is something that
has not been decisively proven) remained in active use by scientists
through the late 1950s.
At present, there are two major methods of producing
isotopes of
transplutonium elements: (1) irradiation of the lighter elements with either
neutrons
or (2) accelerated charged particles. The first method is most
important for applications, as only neutron irradiation using nuclear
reactors allows the production of sizeable amounts of synthetic
actinides; however, it is limited to relatively light elements. The
advantage of the second method is that elements heavier than plutonium,
as well as neutron-deficient isotopes, can be obtained, which are not
formed during neutron irradiation.
In 1962–1966, there were attempts in the United States to produce transplutonium isotopes using a series of six
underground nuclear explosions.
Small samples of rock were extracted from the blast area immediately
after the test to study the explosion products, but no isotopes with
mass number greater than 257 could be detected, despite predictions that such isotopes would have relatively long
half-lives of
α-decay. This non-observation was attributed to
spontaneous fission owing to the large speed of the products and to other decay channels, such as neutron emission and
nuclear fission.
From actinium to uranium
Enrico Fermi suggested the existence of transuranium elements in 1934.
Uranium and thorium were the first actinides
discovered. Uranium was identified in 1789 by the German chemist
Martin Heinrich Klaproth in
pitchblende ore. He named it after the planet
Uranus, which had been discovered only eight years earlier. Klaproth was able to precipitate a yellow compound (likely
sodium diuranate) by dissolving
pitchblende in
nitric acid and neutralizing the solution with
sodium hydroxide. He then reduced the obtained yellow powder with charcoal, and extracted a black substance that he mistook for metal. Only 60 years later, the French scientist
Eugène-Melchior Péligot identified it as uranium oxide. He also isolated the first sample of uranium metal by heating
uranium tetrachloride with metallic
potassium. The
atomic mass of uranium was then calculated as 120, but
Dmitri Mendeleev in 1872 corrected it to 240 using his periodicity laws. This value was confirmed experimentally in 1882 by K. Zimmerman.
Thorium oxide was discovered by
Friedrich Wöhler in the mineral
Thorianite, which was found in Norway (1827).
Jöns Jacob Berzelius
characterized this material in more detail by in 1828. By reduction of
thorium tetrachloride with potassium, he isolated the metal and named it
thorium after the
Norse god of thunder and lightning
Thor. The same isolation method was later used by Péligot for uranium.
Actinium was discovered in 1899 by
André-Louis Debierne, an assistant of
Marie Curie, in the pitchblende waste left after removal of radium and polonium. He described the substance (in 1899) as similar to
titanium and (in 1900) as similar to thorium. The discovery of actinium by Debierne was however questioned in 1971 and 2000,
arguing that Debierne's publications in 1904 contradicted his earlier
work of 1899–1900. This view instead credits the 1902 work of
Friedrich Oskar Giesel, who discovered a radioactive element named
emanium that behaved similarly to lanthanum. The name actinium comes from the Greek
aktis, aktinos
(ακτίς, ακτίνος), meaning beam or ray. This metal was discovered not by
its own radiation but by the radiation of the daughter products.
Owing to the close similarity of actinium and lanthanum and low
abundance, pure actinium could only be produced in 1950. The term
actinide was probably introduced by
Victor Goldschmidt in 1937.
Protactinium was possibly isolated in 1900 by
William Crookes. It was first identified in 1913, when
Kasimir Fajans and Oswald Helmuth Göhring encountered the short-lived isotope
234mPa (half-life 1.17 minutes) during their studies of the
238U decay. They named the new element
brevium (from Latin
brevis meaning brief); the name was changed to
protoactinium (from
Greek πρῶτος + ἀκτίς meaning "first beam element") in 1918 when two groups of scientists, led by the Austrian
Lise Meitner and
Otto Hahn of Germany and
Frederick Soddy and John Cranston of Great Britain, independently discovered the much longer-lived
231Pa. The name was shortened to
protactinium
in 1949. This element was little characterized until 1960, when A. G.
Maddock and his co-workers in the U.K. isolated 130 grams of
protactinium from 60 tonnes of waste left after extraction of uranium
from its ore.
Neptunium and above
Transuranium elements do not occur in sizeable quantities in nature and are commonly synthesized via
nuclear reactions conducted with nuclear reactors. For example, under irradiation with reactor neutrons,
uranium-238 partially converts to
plutonium-239:
![{\displaystyle {\ce {{^{238}_{92}U}+{}_{0}^{1}n->{}_{92}^{239}U->[\beta ^{-}][23.5\ {\ce {min}}]{}_{93}^{239}Np->[\beta ^{-}][2.3\ {\ce {days}}]{}_{94}^{239}Pu}}\left({\ce {->[\alpha ][2.4\cdot 10^{4}\ {\ce {years}}]}}\right){\ce {^{235}_{92}U}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e7a95dee843f1cf5e9156013e8ca01c30870d896)
This synthesis reaction was used by Fermi and his collaborators in their design of the reactors located at the
Hanford Site, which produced significant amounts of plutonium-239 for the nuclear weapons of the
Manhattan Project and the United States' post-war nuclear arsenal.
Actinides with the highest mass numbers are synthesized by bombarding uranium, plutonium, curium and californium with
ions of nitrogen, oxygen, carbon, neon or boron in a
particle accelerator. So,
nobelium was produced by bombarding uranium-238 with
neon-22 as
 .
.
The first isotopes of transplutonium elements,
americium-241 and
curium-242, were synthesized in 1944 by Glenn T. Seaborg, Ralph A. James and
Albert Ghiorso. Curium-242 was obtained by bombarding plutonium-239 with 32-MeV α-particles
 .
.
The americium-241 and curium-242 isotopes also were produced by
irradiating plutonium in a nuclear reactor. The latter element was named
after
Marie Curie and her husband
Pierre who are noted for discovering
radium and for their work in
radioactivity.
In 1945, B. B. Cunningham obtained the first bulk chemical compound of a transplutonium element, namely americium hydroxide.
Over the next three to four years, milligram quantities of americium
and microgram amounts of curium were accumulated that allowed production
of isotopes of berkelium (Thomson, 1949) and californium (Thomson, 1950). Sizeable amounts of these elements were produced only in 1958 (Burris B. Cunningham and Stanley G. Thomson), and the first californium compound (0.3 µg of CfOCl) was obtained only in 1960 by B. B. Cunningham and J. C. Wallmann.
Einsteinium and fermium were identified in 1952–1953 in the fallout from the "
Ivy Mike"
nuclear test (1 November 1952), the first successful test of a hydrogen
bomb. Instantaneous exposure of uranium-238 to a large neutron flux
resulting from the explosion produced heavy isotopes of uranium,
including uranium-253 and uranium-255, and their
β-decay yielded
einsteinium-253 and
fermium-255.
The discovery of the new elements and the new data on neutron capture
were initially kept secret on the orders of the U.S. military until 1955
due to
Cold War tensions.
Nevertheless, the Berkeley team were able to prepare einsteinium and
fermium by civilian means, through the neutron bombardment of
plutonium-239, and published this work in 1954 with the disclaimer that
it was not the first studies that had been carried out on the elements. The "Ivy Mike" studies were declassified and published in 1955.
The first significant (submicrograms) amounts of einsteinium were
produced in 1961 by Cunningham and colleagues, but this has not been
done for fermium yet.
The first isotope of mendelevium,
256Md
(half-life 87 min), was synthesized by Albert Ghiorso, Glenn T.
Seaborg, Gregory R. Choppin, Bernard G. Harvey and Stanley G. Thompson
when they bombarded an
253Es target with
alpha particles in the 60-inch
cyclotron of
Berkeley Radiation Laboratory; this was the first isotope of any element to be synthesized one atom at a time.
There were several attempts to obtain isotopes of nobelium by
Swedish (1957) and American (1958) groups, but the first reliable result
was the synthesis of
256No by the Russian group (
Georgy Flyorov et al.) in 1965, as acknowledged by the
IUPAC in 1992. In their experiments, Flyorov
et al. bombarded uranium-238 with neon-22.
In 1961, Ghiorso
et al. obtained the first isotope of lawrencium by irradiating californium (mostly
californium-252) with
boron-10 and
boron-11 ions. The
mass number of this isotope was not clearly established (possibly 258 or 259) at the time. In 1965,
256Lr was synthesized by Flyorov
et al. from
243Am and
18O. Thus IUPAC recognized the nuclear physics teams at Dubna and Berkeley as the co-discoverers of lawrencium.
Isotopes
Actinides have 89−103 protons and usually 117−159 neutrons.
Thirty-one
isotopes of actinium and eight excited
isomeric states of some of its
nuclides were identified by 2010. Three isotopes,
225Ac,
227Ac and
228Ac,
were found in nature and the others were produced in the laboratory;
only the three natural isotopes are used in applications. Actinium-225
is a member of the radioactive
neptunium series; it was first discovered in 1947 as a decay product of
uranium-233,
it is an α-emitter with a half-life of 10 days. Actinium-225 is less
available than actinium-228, but is more promising in radiotracer
applications.
Actinium-227 (half-life 21.77 years) occurs in all uranium ores, but in
small quantities. One gram of uranium (in radioactive equilibrium)
contains only 2
×10
−10 gram of
227Ac. Actinium-228 is a member of the
radioactive thorium series formed by the decay of
228Ra; it is a β
− emitter with a half-life of 6.15 hours. In one tonne of thorium there is 5
×10
−8 gram of
228Ac. It was discovered by
Otto Hahn in 1906.
28
isotopes of protactinium are known with mass numbers 212–239 as well as three excited
isomeric states. Only
231Pa and
234Pa
have been found in nature. All the isotopes have short lifetime, except
for protactinium-231 (half-life 32,760 years). The most important
isotopes are
231Pa and
233Pa,
which is an intermediate product in obtaining uranium-233 and is the
most affordable among artificial isotopes of protactinium.
233Pa has convenient half-life and energy of
γ-radiation, and thus was used in most studies of protactinium chemistry. Protactinium-233 is a
β-emitter with a half-life of 26.97 days.
Uranium has the highest number (25) of both natural and synthetic
isotopes. They have mass numbers of 215–242 (except 220 and 241), and three of them,
234U,
235U and
238U, are present in appreciable quantities in nature. Among others, the most important is
233U, which is a final product of transformations of
232Th irradiated by slow neutrons.
233U has a much higher fission efficiency by low-energy (thermal) neutrons, compared e.g. with
235U. Most uranium chemistry studies were carried out on uranium-238 owing to its long half-life of 4.4
×10
9 years.
There are 23
isotopes of neptunium with mass numbers of 219 and 223–244; they are all highly radioactive. The most popular among scientists are long-lived
237Np (t
1/2 = 2.20
×10
6 years) and short-lived
239Np,
238Np (t
1/2 ~ 2 days).
Eighteen
isotopes of americium are known with mass numbers from 229 to 247 (with the exception of 231). The most important are
241Am and
243Am,
which are alpha-emitters and also emit soft, but intense γ-rays; both
of them can be obtained in an isotopically pure form. Chemical
properties of americium were first studied with
241Am, but later shifted to
243Am, which is almost 20 times less radioactive. The disadvantage of
243Am is production of the short-lived daughter isotope
239Np, which has to be considered in the data analysis.
Among 19
isotopes of curium, the most accessible are
242Cm and
244Cm;
they are α-emitters, but with much shorter lifetime than the americium
isotopes. These isotopes emit almost no γ-radiation, but undergo
spontaneous fission with the associated emission of neutrons. More long-lived isotopes of curium (
245–248Cm,
all α-emitters) are formed as a mixture during neutron irradiation of
plutonium or americium. Upon short irradiation, this mixture is
dominated by
246Cm, and then
248Cm begins to accumulate. Both of these isotopes, especially
248Cm, have a longer half-life (3.48
×10
5 years) and are much more convenient for carrying out chemical research than
242Cm and
244Cm, but they also have a rather high rate of spontaneous fission.
247Cm has the longest lifetime among isotopes of curium (1.56
×10
7 years), but is not formed in large quantities because of the strong fission induced by thermal neutrons.
Eighteen
isotopes of berkelium were identified with mass numbers 233–234, 236, and 238–252. Only
249Bk is available in large quantities; it has a relatively short half-life of 330 days and emits mostly soft
β-particles, which are inconvenient for detection. Its
alpha radiation is rather weak (1.45
×10
−3% with respect to β-radiation), but is sometimes used to detect this isotope.
247Bk
is an alpha-emitter with a long half-life of 1,380 years, but it is
hard to obtain in appreciable quantities; it is not formed upon neutron
irradiation of plutonium because of the β-stability of isotopes of
curium isotopes with mass number below 248.
Isotopes of californium with mass numbers 237–256 are formed in nuclear reactors; californium-253 is a β-emitter and the rest are α-emitters. The isotopes with even mass numbers (
250Cf,
252Cf and
254Cf) have a high rate of spontaneous fission, especially
254Cf
of which 99.7% decays by spontaneous fission. Californium-249 has a
relatively long half-life (352 years), weak spontaneous fission and
strong γ-emission that facilitates its identification.
249Cf is not formed in large quantities in a nuclear reactor because of the slow β-decay of the parent isotope
249Bk
and a large cross section of interaction with neutrons, but it can be
accumulated in the isotopically pure form as the β-decay product of
(pre-selected)
249Bk. Californium produced by reactor-irradiation of plutonium mostly consists of
250Cf and
252Cf, the latter being predominant for large neutron fluences, and its study is hindered by the strong neutron radiation.
Properties of some transplutonium isotope pairs
Parent
isotope
|
t1/2
|
Daughter
isotope
|
t1/2
|
Time to establish
radioactive equilibrium
|
| 243Am |
7370 years |
239Np |
2.35 days |
47.3 days
|
| 245Cm |
8265 years |
241Pu |
14 years |
129 years
|
| 247Cm |
1.64×107 years |
243Pu |
4.95 hours |
7.2 days
|
| 254Es |
270 days |
250Bk |
3.2 hours |
35.2 hours
|
| 255Es |
39.8 days |
255Fm |
22 hours |
5 days
|
| 257Fm |
79 days |
253Cf |
17.6 days |
49 days
|
Among the 18 known
isotopes of einsteinium with mass numbers from 240 to 257, the most affordable is
253Es.
It is an α-emitter with a half-life of 20.47 days, a relatively weak
γ-emission and small spontaneous fission rate as compared with the
isotopes of californium. Prolonged neutron irradiation also produces a
long-lived isotope
254Es (t
1/2 = 275.5 days).
Twenty
isotopes of fermium are known with mass numbers of 241–260.
254Fm,
255Fm and
256Fm are
α-emitters with a short half-life (hours), which can be isolated in significant amounts.
257Fm (t
1/2
= 100 days) can accumulate upon prolonged and strong irradiation. All
these isotopes are characterized by high rates of spontaneous fission.
Among the 16 known
isotopes of mendelevium (mass numbers from 245 to 260), the most studied is
256Md,
which mainly decays through the electron capture (α-radiation is ≈10%)
with the half-life of 77 minutes. Another alpha emitter,
258Md, has a half-life of 53 days. Both these isotopes are produced from rare einsteinium (
253Es and
255Es respectively), that therefore limits their availability.
Long-lived
isotopes of nobelium and
isotopes of lawrencium
(and of heavier elements) have relatively short half-lives. For
nobelium, 11 isotopes are known with mass numbers 250–260 and 262. The
chemical properties of nobelium and lawrencium were studied with
255No (t
1/2 = 3 min) and
256Lr (t
1/2 = 35 s). The longest-lived nobelium isotope,
259No, has a half-life of approximately 1 hour.
Among all of these, the only isotopes that occur in sufficient
quantities in nature to be detected in anything more than traces and
have a measurable contribution to the atomic weights of the actinides
are the primordial 232Th, 235U, and 238U, and three long-lived decay products of natural uranium, 230Th, 231Pa, and 234U. Natural thorium consists of 0.02(2)% 230Th and 99.98(2)% 232Th; natural protactinium consists of 100% 231Pa; and natural uranium consists of 0.0054(5)% 234U, 0.7204(6)% 235U, and 99.2742(10)% 238U.
Distribution in nature
Thorium and uranium are the most abundant actinides in nature with the respective mass concentrations of 16 ppm and 4 ppm. Uranium mostly occurs in the Earth's crust as a mixture of its oxides in the minerals
uraninite, which is also called pitchblende because of its black color. There are several dozens of other
uranium minerals such as
carnotite (KUO
2VO
4·3H
2O) and
autunite (Ca(UO
2)
2(PO
4)
2·nH
2O). The isotopic composition of natural uranium is
238U (relative abundance 99.2742%),
235U (0.7204%) and
234U (0.0054%); of these
238U has the largest half-life of 4.51
×10
9 years. The worldwide production of uranium in 2009 amounted to 50,572
tonnes, of which 27.3% was mined in
Kazakhstan. Other important uranium mining countries are Canada (20.1%), Australia (15.7%),
Namibia (9.1%),
Russia (7.0%), and
Niger (6.4%).
Content of plutonium in uranium and thorium ores
| Ore
|
Location
|
Uranium
content, %
|
Mass ratio 239Pu/ore
|
Ratio 239Pu/U (×1012)
|
| Uraninite |
Canada |
13.5 |
9.1×10−12 |
7.1
|
| Uraninite |
Congo |
38 |
4.8×10−12 |
12
|
| Uraninite |
Colorado, US |
50 |
3.8×10−12 |
7.7
|
| Monazite |
Brazil |
0.24 |
2.1×10−14 |
8.3
|
| Monazite |
North Carolina, US |
1.64 |
5.9×10−14 |
3.6
|
| Fergusonite |
- |
0.25 |
<1 span="" style="margin: 0 .15em 0 .25em;">×10−14 |
<4 span=""> |
| Carnotite |
- |
10 |
<4 span="" style="margin: 0 .15em 0 .25em;">× |
10
−14
<0 .4="" span=""> |
The most abundant
thorium minerals are
thorianite (ThO
2),
thorite (ThSiO
4) and
monazite, ((Th,Ca,Ce)PO
4).
Most thorium minerals contain uranium and vice versa; and they all have
significant fraction of lanthanides. Rich deposits of thorium minerals
are located in the United States (440,000 tonnes), Australia and India
(~300,000 tonnes each) and Canada (~100,000 tonnes).
The abundance of actinium in the Earth's crust is only about 5×10−15%.
Actinium is mostly present in uranium-containing, but also in other
minerals, though in much smaller quantities. The content of actinium in
most natural objects corresponds to the isotopic equilibrium of parent
isotope 235U, and it is not affected by the weak Ac migration. Protactinium is more abundant (10−12%) in the Earth's crust than actinium. It was discovered in the uranium ore in 1913 by Fajans and Göhring. As actinium, the distribution of protactinium follows that of 235U.
The half-life of the longest-lived isotope of neptunium,
237Np,
is negligible compared to the age of the Earth. Thus neptunium is
present in nature in negligible amounts produced as intermediate decay
products of other isotopes. Traces of plutonium in uranium minerals were first found in 1942, and the more systematic results on
239Pu
are summarized in the table (no other plutonium isotopes could be
detected in those samples). The upper limit of abundance of the
longest-living isotope of plutonium,
244Pu, is 3
×10
−20%.
Plutonium could not be detected in samples of lunar soil. Owing to its
scarcity in nature, most plutonium is produced synthetically.
Owing to the low abundance of actinides, their extraction is a complex, multistep process.
Fluorides of actinides are usually used because they are insoluble in water and can be easily separated with
redox reactions. Fluorides are reduced with
calcium,
magnesium or
barium:
![{\displaystyle {\begin{array}{l}{}\\{\ce {2AmF3{}+3Ba->[{\ce {1150-1350^{\circ }C}}]3BaF2{}+2Am}}\\{\ce {PuF4{}+2Ba->[{\ce {1200^{\circ }C}}]2BaF2{}+Pu}}\\{\ce {UF4{}+2Mg->[{\ce {>500^{\circ }C}}]U{}+2MgF2}}\\{}\end{array}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/057c8209f7427fca3331e574fa79f36c0e2a81db)
Among the actinides, thorium and uranium are the easiest to isolate. Thorium is extracted mostly from
monazite: thorium
pyrophosphate (ThP
2O
7) is reacted with
nitric acid, and the produced thorium nitrate treated with
tributyl phosphate.
Rare-earth impurities are separated by increasing the
pH in sulfate solution.
In another extraction method, monazite is decomposed with a 45% aqueous solution of
sodium hydroxide at 140 °C. Mixed metal hydroxides are extracted first, filtered at 80 °C, washed with water and dissolved with concentrated
hydrochloric acid.
Next, the acidic solution is neutralized with hydroxides to pH = 5.8
that results in precipitation of thorium hydroxide (Th(OH)
4)
contaminated with ~3% of rare-earth hydroxides; the rest of rare-earth
hydroxides remains in solution. Thorium hydroxide is dissolved in an
inorganic acid and then purified from the
rare earth elements. An efficient method is the dissolution of thorium hydroxide in nitric acid, because the resulting solution can be purified by
extraction with organic solvents:
Separation of uranium and plutonium from nuclear fuel
- Th(OH)4 + 4 HNO3 → Th(NO3)4 + 4 H2O
Metallic thorium is separated from the anhydrous oxide, chloride or fluoride by reacting it with calcium in an inert atmosphere:
- ThO2 + 2 Ca → 2 CaO + Th
Sometimes thorium is extracted by
electrolysis of a fluoride in a mixture of sodium and potassium chloride at 700–800 °C in a
graphite crucible. Highly pure thorium can be extracted from its iodide with the
crystal bar process.
Uranium is extracted from its ores in various ways. In one
method, the ore is burned and then reacted with nitric acid to convert
uranium into a dissolved state. Treating the solution with a solution of
tributyl phosphate (TBP) in kerosene transforms uranium into an organic
form UO
2(NO
3)
2(TBP)
2. The insoluble impurities are filtered and the uranium is extracted by reaction with hydroxides as (NH
4)
2U
2O
7 or with
hydrogen peroxide as UO
4·2H
2O.
When the uranium ore is rich in such minerals as
dolomite,
magnesite,
etc., those minerals consume much acid. In this case, the carbonate
method is used for uranium extraction. Its main component is an aqueous
solution of
sodium carbonate, which converts uranium into a complex [UO
2(CO
3)
3]
4−,
which is stable in aqueous solutions at low concentrations of hydroxide
ions. The advantages of the sodium carbonate method are that the
chemicals have low
corrosivity
(compared to nitrates) and that most non-uranium metals precipitate
from the solution. The disadvantage is that tetravalent uranium
compounds precipitate as well. Therefore, the uranium ore is treated
with sodium carbonate at elevated temperature and under oxygen pressure:
- 2 UO2 + O2 + 6 CO2−
3 → 2 [UO2(CO3)3]4−
This equation suggests that the best solvent for the uranium
carbonate processing is a mixture of carbonate with bicarbonate. At high
pH, this results in precipitation of diuranate, which is treated with
hydrogen in the presence of nickel yielding an insoluble uranium tetracarbonate.
Another separation method uses polymeric resins as a
polyelectrolyte. Ion exchange processes in the resins result in separation of uranium. Uranium from resins is washed with a solution of
ammonium nitrate or nitric acid that yields
uranyl nitrate, UO
2(NO
3)
2·6H
2O. When heated, it turns into UO
3, which is converted to UO
2 with hydrogen:
- UO3 + H2 → UO2 + H2O
- 4 HF + UO2 → UF4 + 2 H2O
To extract plutonium, neutron-irradiated uranium is dissolved in nitric acid, and a reducing agent (
FeSO4, or
H2O2)
is added to the resulting solution. This addition changes the oxidation
state of plutonium from +6 to +4, while uranium remains in the form of
uranyl nitrate (UO
2(NO
3)
2). The solution is treated with a reducing agent and neutralized with
ammonium carbonate to pH = 8 that results in precipitation of Pu
4+ compounds.
In another method, Pu
4+ and
UO2+
2 are first extracted with tributyl phosphate, then reacted with
hydrazine washing out the recovered plutonium.
The major difficulty in separation of actinium is the similarity
of its properties with those of lanthanum. Thus actinium is either
synthesized in nuclear reactions from isotopes of radium or separated
using ion-exchange procedures.
Properties
Actinides have similar properties to lanthanides. The 6
d and 7
s electronic shells are filled in actinium and thorium, and the 5
f shell is being filled with further increase in atomic number; the 4
f shell is filled in the lanthanides. The first experimental evidence for the filling of the 5
f shell in actinides was obtained by McMillan and Abelson in 1940. As in lanthanides (see
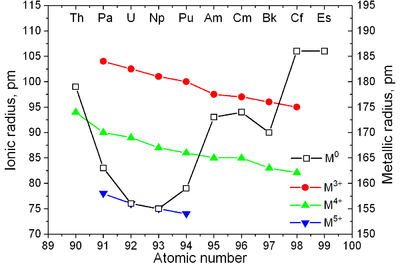
lanthanide contraction), the
ionic radius of actinides monotonically decreases with atomic number.
Physical properties
A pellet of
238PuO
2 to be used in a
radioisotope thermoelectric generator for either the
Cassini or
Galileo
mission. The pellet produces 62 watts of heat and glows because of the
heat generated by the radioactive decay (primarily α). Photo is taken
after insulating the pellet under a
graphite blanket for minutes and removing the blanket.
Actinides are typical metals. All of them are soft and have a silvery color (but tarnish in air), relatively high
density and plasticity. Some of them can be cut with a knife. Their
electrical resistivity varies between 15 and 150 µOhm·cm.
The hardness of thorium is similar to that of soft steel, so heated
pure thorium can be rolled in sheets and pulled into wire. Thorium is
nearly half as dense as uranium and plutonium, but is harder than either
of them. All actinides are radioactive,
paramagnetic,
and, with the exception of actinium, have several crystalline phases:
plutonium has seven, and uranium, neptunium and californium three. The
crystal structures
of protactinium, uranium, neptunium and plutonium do not have clear
analogs among the lanthanides and are more similar to those of the 3
d-
transition metals.
All actinides are
pyrophoric, especially when finely divided, that is, they spontaneously ignite upon reaction with air. The
melting point of actinides does not have a clear dependence on the number of
f-electrons. The unusually low melting point of neptunium and plutonium (~640 °C) is explained by
hybridization of 5
f and 6
d orbitals and the formation of directional bonds in these metals.
Chemical properties
Like the lanthanides, all actinides are highly reactive with
halogens and
chalcogens; however, the actinides react more easily. Actinides, especially those with a small number of 5
f-electrons, are prone to
hybridization. This is explained by the similarity of the electron energies at the 5
f, 7
s and 6
d
shells. Most actinides exhibit a larger variety of valence states, and
the most stable are +6 for uranium, +5 for protactinium and neptunium,
+4 for thorium and plutonium and +3 for actinium and other actinides.
Chemically, actinium is similar to lanthanum, which is explained
by their similar ionic radii and electronic structure. Like lanthanum,
actinium almost always has an oxidation state of +3 in compounds, but it
is less reactive and has more pronounced
basic properties. Among other trivalent actinides Ac
3+ is least acidic, i.e. has the weakest tendency to hydrolyze in aqueous solutions.
Thorium is rather active chemically. Owing to lack of
electrons on 6
d and 5
f
orbitals, the tetravalent thorium compounds are colorless. At pH <
3, the solutions of thorium salts are dominated by the cations [Th(H
2O)
8]
4+. The Th
4+ ion is relatively large, and depending on the
coordination number
can have a radius between 0.95 and 1.14 Å. As a result, thorium salts
have a weak tendency to hydrolyse. The distinctive ability of thorium
salts is their high solubility, not only in water, but also in polar
organic solvents.
Protactinium exhibits two valence states; the +5 is stable, and
the +4 state easily oxidizes to protactinium(V). Thus tetravalent
protactinium in solutions is obtained by the action of strong reducing
agents in a hydrogen atmosphere. Tetravalent protactinium is chemically
similar to uranium(IV) and thorium(IV). Fluorides, phosphates,
hypophosphate, iodate and phenylarsonates of protactinium(IV) are
insoluble in water and dilute acids. Protactinium forms soluble
carbonates. The hydrolytic properties of pentavalent protactinium are
close to those of
tantalum(V) and
niobium(V). The complex chemical behavior of protactinium is a consequence of the start of the filling of the 5
f shell in this element.
Uranium has a valence from 3 to 6, the last being most stable. In the hexavalent state, uranium is very similar to the
group 6 elements. Many compounds of uranium(IV) and uranium(VI) are
non-stoichiometric, i.e. have variable composition. For example, the actual chemical formula of uranium dioxide is UO
2+x, where
x varies between −0.4 and 0.32. Uranium(VI) compounds are weak
oxidants. Most of them contain the linear "
uranyl" group,
UO2+
2.
Between 4 and 6 ligands can be accommodated in an equatorial plane
perpendicular to the uranyl group. The uranyl group acts as a
hard acid and forms stronger complexes with oxygen-donor ligands than with nitrogen-donor ligands.
NpO2+
2 and
PuO2+
2
are also the common form of Np and Pu in the +6 oxidation state.
Uranium(IV) compounds exhibit reducing properties, e.g., they are easily
oxidized by atmospheric oxygen. Uranium(III) is a very strong reducing
agent. Owing to the presence of d-shell, uranium (as well as many other
actinides) forms
organometallic compounds, such as U
III(C
5H
5)
3 and U
IV(C
5H
5)
4.
Neptunium has valence states from 3 to 7, which can be
simultaneously observed in solutions. The most stable state in solution
is +5, but the valence +4 is preferred in solid neptunium compounds.
Neptunium metal is very reactive. Ions of neptunium are prone to
hydrolysis and formation of
coordination compounds.
Plutonium also exhibits valence states between 3 and 7 inclusive,
and thus is chemically similar to neptunium and uranium. It is highly
reactive, and quickly forms an oxide film in air. Plutonium reacts with
hydrogen even at temperatures as low as 25–50 °C; it also easily forms
halides and
intermetallic compounds. Hydrolysis reactions of plutonium ions of different oxidation states are quite diverse. Plutonium(V) can enter
polymerization reactions.
The largest chemical diversity among actinides is observed in
americium, which can have valence between 2 and 6. Divalent americium is
obtained only in dry compounds and non-aqueous solutions (
acetonitrile).
Oxidation states +3, +5 and +6 are typical for aqueous solutions, but
also in the solid state. Tetravalent americium forms stable solid
compounds (dioxide, fluoride and hydroxide) as well as complexes in
aqueous solutions. It was reported that in alkaline solution americium
can be oxidized to the heptavalent state, but these data proved
erroneous. The most stable valence of americium is 3 in the aqueous
solutions and 3 or 4 in solid compounds.
Valence 3 is dominant in all subsequent elements up to lawrencium
(with the exception of nobelium). Curium can be tetravalent in solids
(fluoride, dioxide). Berkelium, along with a valence of +3, also shows
the valence of +4, more stable than that of curium; the valence 4 is
observed in solid fluoride and dioxide. The stability of Bk
4+ in aqueous solution is close to that of
Ce4+.
[92]
Only valence 3 was observed for californium, einsteinium and fermium.
The divalent state is proven for mendelevium and nobelium, and in
nobelium it is more stable than the trivalent state. Lawrencium shows
valence 3 both in solutions and solids.
The redox potential

increases from −0.32 V in uranium, through 0.34 V (Np) and 1.04 V (Pu)
to 1.34 V in americium revealing the increasing reduction ability of the
An
4+ ion from americium to uranium. All actinides form AnH
3 hydrides of black color with salt-like properties. Actinides also produce
carbides with the general formula of AnC or AnC
2 (U
2C
3 for uranium) as well as sulfides An
2S
3 and AnS
2.
Uranyl nitrate (UO2(NO3)2)
Aqueous solutions of uranium III, IV, V, VI salts
Aqueous solutions of neptunium III, IV, V, VI, VII salts
Aqueous solutions of plutonium III, IV, V, VI, VII salts
-
-
-
Compounds
Oxides and hydroxides
Some actinides can exist in several oxide forms such as An
2O
3, AnO
2, An
2O
5 and AnO
3. For all actinides, oxides AnO
3 are
amphoteric and An
2O
3, AnO
2 and An
2O
5 are basic, they easily react with water, forming bases:
- An2O3 + 3 H2O → 2 An(OH)3.
These bases are poorly soluble in water and by their activity are close to the
hydroxides of rare-earth metals.
Np(OH)
3 has not yet been synthesized, Pu(OH)
3 has a blue color while Am(OH)
3 is pink and
curium hydroxide Cm(OH)
3 is colorless. Bk(OH)
3 and Cf(OH)
3 are also known, as are tetravalent hydroxides for Np, Pu and Am and pentavalent for Np and Am.
Thorium reacting with oxygen exclusively forms the dioxide:
![{\displaystyle {\ce {Th{}+O2->[{\ce {1000^{\circ }C}}]\overbrace {ThO2} ^{Thorium~dioxide}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/13e4a27b9ed3d03fc5420ed4a04020bf77562406)
Thorium dioxide is a refractory material with the highest melting point among any known oxide (3390 °C). Adding 0.8–1% ThO
2 to tungsten stabilizes its structure, so the doped filaments have better mechanical stability to vibrations. To dissolve ThO
2 in acids, it is heated to 500–600 °C; heating above 600 °C produces a very resistant to acids and other reagents form of ThO
2. Small addition of fluoride ions
catalyses dissolution of thorium dioxide in acids.
Two protactinium oxides have been obtained: PaO
2 (black) and Pa
2O
5 (white); the former is
isomorphic with ThO
2 and the latter is easier to obtain. Both oxides are basic, and Pa(OH)
5 is a weak, poorly soluble base.
Decomposition of certain salts of uranium, for example UO
2(NO
3)·6H
2O in air at 400 °C, yields orange or yellow UO
3. This oxide is amphoteric and forms several hydroxides, the most stable being
uranyl hydroxide UO
2(OH)
2. Reaction of uranium(VI) oxide with hydrogen results in uranium dioxide, which is similar in its properties with ThO
2. This oxide is also basic and corresponds to the uranium hydroxide (U(OH)
4).
Plutonium, neptunium and americium form two basic oxides: An2O3 and AnO2. Neptunium trioxide is unstable; thus, only Np3O8 could be obtained so far. However, the oxides of plutonium and neptunium with the chemical formula AnO2 and An2O3 are well characterized.
Salts
Actinides easily react with halogens forming salts with the formulas MX
3 and MX
4 (X =
halogen). So the first berkelium compound, BkCl
3, was synthesized in 1962 with an amount of 3 nanograms. Like the halogens of rare earth elements, actinide
chlorides,
bromides, and
iodides are water-soluble, and
fluorides are insoluble. Uranium easily yields a colorless hexafluoride, which
sublimates at a temperature of 56.5 °C; because of its volatility, it is used in the separation of uranium isotopes with
gas centrifuge or
gaseous diffusion. Actinide hexafluorides have properties close to
anhydrides. They are very sensitive to moisture and hydrolyze forming AnO
2F
2. The pentachloride and black hexachloride of uranium were synthesized, but they are both unstable.
Action of acids on actinides yields salts, and if the acids are
non-oxidizing then the actinide in the salt is in low-valence state:
- U + 2H2SO4 → U(SO4)2 + 2H2
- 2Pu + 6HCl → 2PuCl3 + 3H2
However, in these reactions the regenerating hydrogen can react with
the metal, forming the corresponding hydride. Uranium reacts with acids
and water much more easily than thorium.
Actinide salts can also be obtained by dissolving the
corresponding hydroxides in acids. Nitrates, chlorides, sulfates and
perchlorates of actinides are water-soluble. When crystallizing from
aqueous solutions, these salts forming a hydrates, such as Th(NO
3)
4·6H
2O, Th(SO
4)
2·9H
2O and Pu
2(SO
4)
3·7H
2O.
Salts of high-valence actinides easily hydrolyze. So, colorless
sulfate, chloride, perchlorate and nitrate of thorium transform into
basic salts with formulas Th(OH)
2SO
4 and Th(OH)
3NO
3. The solubility and insolubility of trivalent and tetravalent actinides is like that of lanthanide salts. So
phosphates,
fluorides,
oxalates,
iodates and
carbonates of actinides are weakly soluble in water; they precipitate as hydrates, such as ThF
4·3H
2O and Th(CrO
4)
2·3H
2O.
Actinides with oxidation state +6, except for the AnO
22+-type cations, form [AnO
4]
2−, [An
2O
7]
2− and other complex anions. For example, uranium, neptunium and plutonium form salts of the Na
2UO
4 (uranate) and (NH
4)
2U
2O
7 (diuranate) types. In comparison with lanthanides, actinides more easily form
coordination compounds,
and this ability increases with the actinide valence. Trivalent
actinides do not form fluoride coordination compounds, whereas
tetravalent thorium forms K
2ThF
6, KThF
5, and even K
5ThF
9 complexes. Thorium also forms the corresponding
sulfates (for example Na
2SO
4·Th(SO
4)
2·5H
2O),
nitrates and thiocyanates. Salts with the general formula An
2Th(NO
3)
6·
nH
2O are of coordination nature, with the
coordination number
of thorium equal to 12. Even easier is to produce complex salts of
pentavalent and hexavalent actinides. The most stable coordination
compounds of actinides – tetravalent thorium and uranium – are obtained
in reactions with diketones, e.g.
acetylacetone.
Applications
While actinides have some established daily-life applications, such as in smoke detectors (americium) and
gas mantles (thorium), they are mostly used in
nuclear weapons and use as a
fuel in nuclear reactors.
The last two areas exploit the property of actinides to release
enormous energy in nuclear reactions, which under certain conditions may
become self-sustaining
chain reaction.
The most important isotope for
nuclear power applications is
uranium-235. It is used in the
thermal reactor, and its concentration in natural uranium does not exceed 0.72%. This isotope strongly absorbs
thermal neutrons releasing much energy. One fission act of 1 gram of
235U converts into about 1 MW·day. Of importance, is that
235
92U
emits more neutrons than it absorbs; upon reaching the
critical mass,
235
92U
enters into a self-sustaining chain reaction. Typically, uranium nucleus is divided into two fragments with the release of 2–3 neutrons, for example:
- 235
92U
+ 1
0n
⟶ 115
45Rh
+ 118
47Ag
+ 31
0n
Emission of neutrons during the fission of uranium is important not
only for maintaining the nuclear chain reaction, but also for the
synthesis of the heavier actinides.
Uranium-239 converts via
β-decay
into plutonium-239, which, like uranium-235, is capable of spontaneous
fission. The world's first nuclear reactors were built not for energy,
but for producing plutonium-239 for nuclear weapons.
About half of the produced thorium is used as the light-emitting material of gas mantles. Thorium is also added into multicomponent
alloys of
magnesium and
zinc.
So the Mg-Th alloys are light and strong, but also have high melting
point and ductility and thus are widely used in the aviation industry
and in the production of
missiles. Thorium also has good
electron emission properties, with long lifetime and low potential barrier for the emission.
The relative content of thorium and uranium isotopes is widely used to
estimate the age of various objects, including stars (see
radiometric dating).
The major application of plutonium has been in
nuclear weapons,
where the isotope plutonium-239 was a key component due to its ease of
fission and availability. Plutonium-based designs allow reducing the
critical mass to about a third of that for uranium-235. The "
Fat Man"-type plutonium bombs produced during the
Manhattan Project
used explosive compression of plutonium to obtain significantly higher
densities than normal, combined with a central neutron source to begin
the reaction and increase efficiency. Thus only 6.2 kg of plutonium was
needed for an
explosive yield equivalent to 20 kilotons of
TNT. Hypothetically, as little as 4 kg of plutonium—and maybe even
less—could be used to make a single atomic bomb using very sophisticated
assembly designs.
Plutonium-238
is potentially more efficient isotope for nuclear reactors, since it
has smaller critical mass than uranium-235, but it continues to release
much thermal energy (0.56 W/g)
by decay even when the fission chain reaction is stopped by control
rods. Its application is limited by the high price (about US$1000/g).
This isotope has been used in
thermopiles and water
distillation systems of some space satellites and stations. So
Galileo and
Apollo spacecraft (e.g.
Apollo 14)
had heaters powered by kilogram quantities of plutonium-238 oxide; this
heat is also transformed into electricity with thermopiles. The decay
of plutonium-238 produces relatively harmless alpha particles and is not
accompanied by gamma-irradiation. Therefore, this isotope (~160 mg) is
used as the energy source in heart pacemakers where it lasts about 5
times longer than conventional batteries.
Actinium-227
is used as a neutron source. Its high specific energy (14.5 W/g) and
the possibility of obtaining significant quantities of thermally stable
compounds are attractive for use in long-lasting thermoelectric
generators for remote use.
228Ac is used as an indicator of
radioactivity in chemical research, as it emits high-energy electrons (2.18 MeV) that can be easily detected.
228Ac-
228Ra mixtures are widely used as an intense gamma-source in industry and medicine.
Development of self-glowing actinide-doped materials with durable
crystalline matrices is a new area of actinide utilization as the
addition of alpha-emitting radionuclides to some glasses and crystals
may confer luminescence.
Toxicity
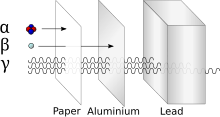
Schematic illustration of penetration of radiation through sheets of paper, aluminium and lead brick
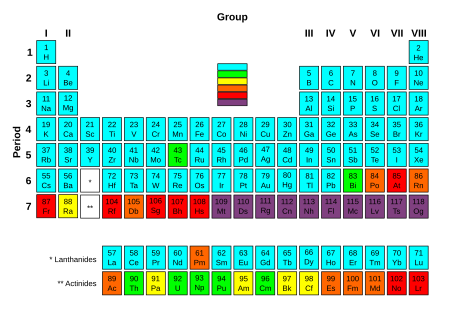
Periodic table with elements colored according to the half-life of their most stable isotope.
Elements which contain at least one stable isotope.
Slightly radioactive elements: the most stable isotope is very long-lived, with a half-life of over two million years.
Significantly radioactive elements: the most stable isotope has half-life between 800 and 34,000 years.
Radioactive elements: the most stable isotope has half-life between one day and 130 years.
Highly radioactive elements: the most stable isotope has half-life between several minutes and one day.
Extremely radioactive elements: the most stable isotope has half-life less than several minutes.
Radioactive substances can harm human health via (i) local skin
contamination, (ii) internal exposure due to ingestion of radioactive
isotopes, and (iii) external overexposure by
β-activity and
γ-radiation. Together with radium and transuranium elements, actinium is one of the most dangerous radioactive poisons with high specific
α-activity. The most important feature of actinium is its ability to accumulate and remain in the surface layer of
skeletons. At the initial stage of poisoning, actinium accumulates in the
liver. Another danger of actinium is that it undergoes radioactive decay faster than being excreted.
Adsorption from the digestive tract is much smaller (~0.05%) for actinium than radium.
Protactinium in the body tends to accumulate in the kidneys and
bones. The maximum safe dose of protactinium in the human body is 0.03
µCi that corresponds to 0.5 micrograms of
231Pa. This isotope, which might be present in the air as
aerosol, is 2.5
×10
8 times more toxic than
hydrocyanic acid.
Plutonium, when entering the body through air, food or blood
(e.g. a wound), mostly settles in the lungs, liver and bones with only
about 10% going to other organs, and remains there for decades. The long
residence time of plutonium in the body is partly explained by its poor
solubility in water. Some isotopes of plutonium emit ionizing
α-radiation, which damages the surrounding cells. The
median lethal dose (LD
50)
for 30 days in dogs after intravenous injection of plutonium is 0.32
milligram per kg of body mass, and thus the lethal dose for humans is
approximately 22 mg for a person weighing 70 kg; the amount for
respiratory exposure should be approximately four times greater. Another
estimate assumes that plutonium is 50 times less toxic than
radium,
and thus permissible content of plutonium in the body should be 5 µg or
0.3 µCi. Such amount is nearly invisible in under microscope. After
trials on animals, this maximum permissible dose was reduced to 0.65 µg
or 0.04 µCi. Studies on animals also revealed that the most dangerous
plutonium exposure route is through inhalation, after which 5–25% of
inhaled substances is retained in the body. Depending on the particle
size and solubility of the plutonium compounds, plutonium is localized
either in the lungs or in the
lymphatic system,
or is absorbed in the blood and then transported to the liver and
bones. Contamination via food is the least likely way. In this case,
only about 0.05% of soluble 0.01% insoluble compounds of plutonium
absorbs into blood, and the rest is excreted. Exposure of damaged skin
to plutonium would retain nearly 100% of it.
Using actinides in nuclear fuel, sealed radioactive sources or
advanced materials such as self-glowing crystals has many potential
benefits. However, a serious concern is the extremely high radiotoxicity
of actinides and their migration in the environment.
Use of chemically unstable forms of actinides in MOX and sealed
radioactive sources is not appropriate by modern safety standards. There
is a challenge to develop stable and durable actinide-bearing
materials, which provide safe storage, use and final disposal. A key
need is application of actinide solid solutions in durable crystalline
host phases.















![{\displaystyle {\begin{array}{l}{}\\{\ce {2AmF3{}+3Ba->[{\ce {1150-1350^{\circ }C}}]3BaF2{}+2Am}}\\{\ce {PuF4{}+2Ba->[{\ce {1200^{\circ }C}}]2BaF2{}+Pu}}\\{\ce {UF4{}+2Mg->[{\ce {>500^{\circ }C}}]U{}+2MgF2}}\\{}\end{array}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/057c8209f7427fca3331e574fa79f36c0e2a81db)











![{\displaystyle {\ce {Th{}+O2->[{\ce {1000^{\circ }C}}]\overbrace {ThO2} ^{Thorium~dioxide}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/13e4a27b9ed3d03fc5420ed4a04020bf77562406)