Utility scale underground liquid hydrogen storage
Methods of hydrogen storage for subsequent use span many approaches including high pressures, cryogenics, and chemical compounds that reversibly release H2 upon heating. Underground hydrogen storage is useful to provide grid energy storage for intermittent energy sources, like wind power, as well as providing fuel for transportation, particularly for ships and airplanes.
Most research into hydrogen storage is focused on storing hydrogen as a lightweight, compact energy carrier for mobile applications.
Liquid hydrogen or slush hydrogen may be used, as in the Space Shuttle. However liquid hydrogen requires cryogenic storage and boils around 20.268 K (−252.882 °C or −423.188 °F). Hence, its liquefaction
imposes a large energy loss (as energy is needed to cool it down to
that temperature). The tanks must also be well insulated to prevent boil off but adding insulation increases cost. Liquid hydrogen has less energy density by volume than hydrocarbon fuels such as gasoline
by approximately a factor of four. This highlights the density problem
for pure hydrogen: there is actually about 64% more hydrogen in a liter
of gasoline (116 grams hydrogen) than there is in a liter of pure liquid
hydrogen (71 grams hydrogen). The carbon in the gasoline also
contributes to the energy of combustion.
Compressed hydrogen, by comparison, is stored quite differently. Hydrogen gas has good energy density by weight, but poor energy density by volume versus hydrocarbons, hence it requires a larger tank to store. A large hydrogen tank
will be heavier than the small hydrocarbon tank used to store the same
amount of energy, all other factors remaining equal. Increasing gas
pressure would improve the energy density by volume, making for smaller,
but not lighter container tanks. Compressed hydrogen is estimated to cost about 2.1% of the energy content
to power the compressor for a large scale underground facility such as
an underground cavern or aquifer from 1 to 200 bar. Higher compression
without energy recovery will mean more energy lost to the compression step. Compressed hydrogen storage can exhibit very low permeation.
Established technologies
Net storage density of hydrogen
Compressed hydrogen
Compressed
hydrogen is a storage form where hydrogen gas is kept under pressures
to increase the storage density. Compressed hydrogen in hydrogen tanks
at 350 bar (5,000 psi) and 700 bar (10,000 psi) is used for hydrogen
tank systems in vehicles, based on type IV carbon-composite technology. Car manufacturers have been developing this solution, such as Honda or Nissan.
Liquid hydrogen
BMW has been working on liquid hydrogen tanks for cars, producing for example the BMW Hydrogen 7.
Japan have liquid hydrogen (LH2) storage at a tanker port in Kobe, and
are anticipated to receive the first shipment of liquid hydrogen via LH2
carrier in 2020.
Hydrogen is liquified by reducing its temperature to -253°C, similar to
liquified natural gas (LNG) which is stored at -162°C. A potential
efficiency loss of 12.79% can be achieved, or 4.26kWh/kg out of
33.3kWh/kg.
Proposals and research
Hydrogen
storage technologies can be divided into physical storage, where
hydrogen molecules are stored (including pure hydrogen storage via
compression and liquefaction), and chemical storage, where hydrides are
stored.
Chemical storage
Chemical
storage could offer high storage performance due to the strong binding
of hydrogen and the high storage densities. However, the regeneration of
storage material is still an issue. A large number of chemical storage
systems are under investigation, which involve hydrolysis reactions, hydrogenation/dehydrogenation reactions, ammonia borane and other boron hydrides, ammonia, and alane etc.
Storage in hydrocarbons may also be successful in overcoming the issue
with low density. For example, supercritical hydrogen at 30 °C and 500
bar only has a density of 15.0 mol/L while methanol has a density of 49.5 mol H2/L methanol and saturated dimethyl ether at 30 °C and 7 bar has a density of 42.1 mol H2/L dimethyl ether. These liquids would use much smaller, cheaper, safer storage tanks.
The most promising chemical approach is electrochemical hydrogen
storage, as the release of hydrogen can be controlled by the applied
electricity. Most of the materials listed below can be directly used for electrochemical hydrogen storage.
Metal hydrides
Metal hydride hydrogen storage
Metal hydrides, such as MgH2, NaAlH4, LiAlH4, LiH, LaNi5H6, TiFeH2 and palladium hydride, with varying degrees of efficiency, can be used as a storage medium for hydrogen, often reversibly.
Some are easy-to-fuel liquids at ambient temperature and pressure,
whereas others are solids which could be turned into pellets. These
materials have good energy density, although their specific energy is often worse than the leading hydrocarbon fuels.
Most metal hydrides bind with hydrogen very strongly. As a
result, high temperatures around 120 °C (248 °F) – 200 °C (392 °F) are
required to release their hydrogen content. This energy cost can be
reduced by using alloys which consists of a strong hydride former and a
weak one such as in LiNH2, LiBH4 and NaBH4.
These are able to form weaker bonds, thereby requiring less input to
release stored hydrogen. However, if the interaction is too weak, the
pressure needed for rehydriding is high, thereby eliminating any energy
savings. The target for onboard hydrogen fuel systems is roughly
<100 and="" bar="" for="" h="" kj="" mol="" nbsp="" recharge="" release="" sub="">2
).
An alternative method for reducing dissociation temperatures is doping with activators. This has been successfully used for aluminium hydride but its complex synthesis makes it undesirable for most applications as it is not easily recharged with hydrogen.
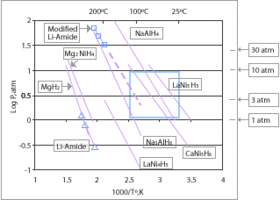
Currently the only hydrides which are capable of achieving the 9
wt% gravimetric goal for 2015 (see chart above) are limited to lithium,
boron and aluminium based compounds; at least one of the second-row
elements or Al must be added. Research is being done to determine new
compounds which can be used to meet these requirements.
Proposed hydrides for use in a hydrogen economy include simple hydrides of magnesium or transition metals and complex metal hydrides, typically containing sodium, lithium, or calcium and aluminium or boron.
Hydrides chosen for storage applications provide low reactivity (high
safety) and high hydrogen storage densities. Leading candidates are lithium hydride, sodium borohydride, lithium aluminium hydride and ammonia borane.
A French company McPhy Energy is developing the first industrial
product, based on magnesium hydride, already sold to some major clients
such as Iwatani and ENEL.
New Scientist reported that Arizona State University is investigating using a borohydride solution to store hydrogen, which is released when the solution flows over a catalyst made of ruthenium. Researchers at University of Pittsburgh and Georgia Tech
performed extensive benchmarking simulations on mixtures of several
light metal hydrides to predict possible reaction thermodynamics for
hydrogen storage.
Nanostructured metal hydrides
Metal hydrides have proven to be a good alternative for hydrogen
storage systems because of their favorable properties such as high
volumetric and gravimetric density. However, more research is necessary
to satisfy the United States Department of Energy’s
requirements for storage capacity, kinetics, cyclability, cost, and
release temperature.
Nano-metal hydrides possess a number of properties that make them even
better candidates for future hydrogen storage systems compared to their
bulk equivalents. At the nanoscale, structural and chemical properties
(such as particle size and sorption site density) show a significant
improvement in properties such as sorption kinetics, hydrogen diffusion
or hydrogen release temperature. However, the downsides of nanoscale
materials include poor heat transfer and total sorption capacity.
The Key Laboratory of Advanced Materials Technology of Liaoning, China,
studied the difference in the reaction kinetics between bulk MgH2 and nanoparticles of this compound.
They observed that the amount of hydrogen released by the 30 nm
particle was 5wt%, 10 times greater than the bulk material (0.5wt%) over
the same period of time (1 hour) and at the same temperature (300ºC).
However, a 5 wt% capacity is still not sufficient for large scale
applications. Palladium
(Pd) proved to be an effective alternative with favorable kinetics, but
its high cost suggests favorability in using more affordable metals
such as vanadium (V). A MgH2-5 wt% V showed fast adsorption kinetics in much larger quantities than Pd. A mixture of titanium and iron was also successfully used, despite these two metals being ineffective if used separately. The addition of LiBH4 has also been studied for the enhancement of sorption kinetics.
Thermodynamics of nanometal hydrides
As shown before, nanomaterials
have proven to be superior for hydrogen storage systems. Nanomaterials
offer an alternative that overcomes the two major barriers of bulk
materials, rate of sorption and release temperature.
The rate of hydrogen sorption improves at the nanoscale due to the short diffusion distance in comparison to bulk materials. This, combined with the notably greater surface-area-to-volume ratio of nanoparticles, accounts for the significant advantage over bulk metals used for hydrogen storage.
The release temperature of a material is defined as the temperature at which the desorption
process begins. It is paramount to achieve the lowest release
temperature possible to reduce the cost of the heating process required
to liberate the gas. To describe the improvement over bulk materials
regarding this parameter, researchers base their studies on a modified van ’t Hoff equation,
shown below, that relates temperature and partial pressure of hydrogen
during the desorption process. The modifications to the standard
equation are related to size effects at the nanoscale.
Where pH2 is the partial pressure of hydrogen, ΔH is the enthalpy of the sorption process (exothermic), ΔS is the change in entropy, R is the ideal gas constant, T is the temperature in Kelvin, Vm is the molar volume of the metal, r is the radius of the nanoparticle and γ is the surface free energy of the particle.
From the above relation we see that the enthalpy and entropy
change of desorption processes depend on the radius of the nanoparticle.
Moreover, a new term is included that takes into account the specific
surface area of the particle and it can be mathematically proven that a
decrease in particle radius leads to a decrease in the release
temperature for a given partial pressure.
Non-metal hydrides
The Italian catalyst manufacturer Acta has proposed using hydrazine as an alternative to hydrogen in fuel cells.
As the hydrazine fuel is liquid at room temperature, it can be handled
and stored more easily than hydrogen. By storing it in a tank full of a
double-bonded carbon-oxygen carbonyl, it reacts and forms a safe solid called hydrazone.
By then flushing the tank with warm water, the liquid hydrazine hydrate
is released. Hydrazine breaks down in the cell to form nitrogen and hydrogen which bonds with oxygen, releasing water.
Silicon hydrides and germanium hydrides are also candidates of hydrogen
storage materials, as they can subject to energetically favored
reaction to form covalently bonded dimers with loss of a hydrogen
molecule.
Carbohydrates
Carbohydrates (polymeric C6H10O5) releases H2
in a bioreformer mediated by the enzyme cocktail—cell-free synthetic
pathway biotransformation. Carbohydrate provides high hydrogen storage
densities as a liquid with mild pressurization and cryogenic
constraints: It can also be stored as a solid powder. Carbohydrate is
the most abundant renewable bioresource in the world.
In May 2007 biochemical engineers from the Virginia Polytechnic Institute and State University
and biologists and chemists from the Oak Ridge National Laboratory
announced a method of producing high-yield pure hydrogen from starch and
water. In 2009, they demonstrated to produce nearly 12 moles of hydrogen per glucose unit from cellulosic materials and water. Thanks to complete conversion and modest reaction conditions, they propose to use carbohydrate as a high energy density hydrogen carrier with a density of 14.8 wt%.
Synthesized hydrocarbons
An alternative to hydrides is to use regular hydrocarbon fuels as the hydrogen carrier. Then a small hydrogen reformer would extract the hydrogen as needed by the fuel cell. However, these reformers are slow to react to changes in demand and add a large incremental cost to the vehicle powertrain.
Direct methanol fuel cells
do not require a reformer, but provide a lower energy density compared
to conventional fuel cells, although this could be counterbalanced with
the much better energy densities of ethanol and methanol over hydrogen. Alcohol fuel is a renewable resource.
Solid-oxide fuel cells can operate on light hydrocarbons such as propane and methane
without a reformer, or can run on higher hydrocarbons with only partial
reforming, but the high temperature and slow startup time of these fuel
cells are problematic for automotive applications.
Aluminum
Aluminum has been proposed as an energy storage method by a number of researchers. Hydrogen can be extracted from aluminum by reacting it with water. To react with water, however, aluminum must be stripped of its natural oxide layer, a process which requires pulverization, chemical reactions with caustic substances, or alloys. The byproduct of the reaction to create hydrogen is aluminum oxide, which can be recycled back into aluminum with the Hall–Héroult process, making the reaction theoretically renewable.
Liquid organic hydrogen carriers (LOHC)
Reversible hydrogenation of N-ethylcarbazole
Unsaturated organic compounds can store huge amounts of hydrogen. These Liquid Organic Hydrogen Carriers
(LOHC) are hydrogenated for storage and dehydrogenated again when the
energy/hydrogen is needed. Research on LOHC was concentrated on
cycloalkanes at an early stage, with its relatively high hydrogen
capacity (6-8 wt %) and production of COx-free hydrogen. Heterocyclic aromatic compounds (or N-Heterocycles) are also appropriate for this task. A compound featuring in LOHC research is N-Ethylcarbazole (NEC) but many others do exist. Dibenzyltoluene,
which is already industrially used as a heat transfer fluid in
industry, was identified as potential LOHC. With a wide liquid range
between -39 °C (melting point) and 390 °C (boiling point) and a hydrogen
storage density of 6.2 wt% dibenzyltoluene is ideally suited as LOHC
material. Formic acid has been suggested as a promising hydrogen storage material with a 4.4wt% hydrogen capacity.
Using LOHCs relatively high gravimetric storage densities can be
reached (about 6 wt-%) and the overall energy efficiency is higher than
for other chemical storage options such as producing methane from the hydrogen.
Cycloalkanes
Cycloalkanes
reported as LOHC include cyclohexane, methyl-cyclohexane and decalin.
The dehydrogenation of cycloalkanes is highly endothermic (63-69 kJ/mol H2), which means this process requires high temperature.
Dehydrogenation of decalin is the most thermodynamically favored among
the three cycloalkanes, and methyl-cyclohexane is second because of the
presence of the methyl group.
Research on catalyst development for dehydrogenation of cycloalkanes
has been carried out for decades. Nickel (Ni), Molybdenum (Mo) and
Platinum (Pt) based catalysts are highly investigated for
dehydrogenation. However, coking is still a big challenge for catalyst's
long-term stability.
N-Heterocycles
Both hydrogenation and dehydrogenation of LOHCs requires catalysts.
It was demonstrated that replacing hydrocarbons by hetero-atoms, like
N, O etc. improves reversible de/hydrogenation properties. The
temperature required for hydrogenation and dehydrogenation drops
significantly with increasing numbers of heteroatoms.
Among all the N-heterocycles, the saturated-unsaturated pair of
dodecahydro-N-ethylcarbazole (12H-NEC) and NEC has been considered as a
promising candidate for hydrogen storage with a fairly large hydrogen
content (5.8wt%).
The figure on the top right shows dehydrogenation and hydrogenation of
the 12H-NEC and NEC pair. The standard catalyst for NEC to 12H-NEC is Ru
and Rh based. The selectivity of hydrogenation can reach 97% at 7 MPa
and 130 °C-150 °C.
Although N-Heterocyles can optimize the unfavorable thermodynamic
properties of cycloalkanes, a lot of issues remain unsolved, such as
high cost, high toxicity and kinetic barriers etc.
Formic acid
In 2006, Swiss researchers at EPFL reported the use of formic acid as a hydrogen storage material.
Carbon monoxide free hydrogen has been generated in a very wide
pressure range (1–600 bar). A homogeneous catalytic system based on
water-soluble ruthenium catalysts selectively decompose HCOOH into H2 and CO2 in aqueous solution.
This catalytic system overcomes the limitations of other catalysts
(e.g. poor stability, limited catalytic lifetimes, formation of CO) for
the decomposition of formic acid making it a viable hydrogen storage
material.
And the co-product of this decomposition, carbon dioxide, can be used
as hydrogen vector by hydrogenating it back to formic acid in a second
step. The catalytic hydrogenation of CO2 has long been studied and efficient procedures have been developed. Formic acid contains 53 g L−1
hydrogen at room temperature and atmospheric pressure. By weight, pure
formic acid stores 4.3 wt% hydrogen. Pure formic acid is a liquid with a
flash point 69 °C (cf. gasoline −40 °C, ethanol 13 °C). 85% formic acid
is not flammable.
Ammonia
Ammonia (NH3) releases H2
in an appropriate catalytic reformer. Ammonia provides high hydrogen
storage densities as a liquid with mild pressurization and cryogenic
constraints: It can also be stored as a liquid at room temperature and
pressure when mixed with water. Ammonia is the second most commonly
produced chemical in the world and a large infrastructure for making,
transporting, and distributing ammonia exists. Ammonia can be reformed
to produce hydrogen with no harmful waste, or can mix with existing
fuels and under the right conditions burn efficiently. Since there is no
carbon in ammonia, no carbon by-products are produced; thereby making
this possibility a "carbon neutral" option for the future. Pure ammonia
burns poorly at the atmospheric pressures found in natural gas fired
water heaters and stoves. Under compression in an automobile engine it
is a suitable fuel for slightly modified gasoline engines. Ammonia is
the suitable alternative fuel because it has 18.6 MJ/kg energy density
at NTP and carbon-free combustion byproducts. However, ammonia is a toxic gas at normal temperature and pressure and has a potent odor.
In 2018, researchers at CSIRO in Australia powered a Toyota Mirai and Hyundai Nexo with hydrogen separated from ammonia using a membrane technology.
In September 2005 chemists from the Technical University of Denmark announced a method of storing hydrogen in the form of ammonia saturated into a salt tablet. They claim it will be an inexpensive and safe storage method.
Amine borane complexes
Prior to 1980, several compounds were investigated for hydrogen
storage including complex borohydrides, or aluminohydrides, and ammonium
salts. These hydrides have an upper theoretical hydrogen yield limited
to about 8.5% by weight. Amongst the compounds that contain only B, N,
and H (both positive and negative ions), representative examples
include: amine boranes, boron hydride ammoniates, hydrazine-borane
complexes, and ammonium octahydrotriborates or tetrahydroborates. Of
these, amine boranes (and especially ammonia borane)
have been extensively investigated as hydrogen carriers. During the
1970s and 1980s, the U.S. Army and Navy funded efforts aimed at
developing hydrogen/deuterium gas-generating compounds for use in the
HF/DF and HCl chemical lasers,
and gas dynamic lasers. Earlier hydrogen gas-generating formulations
used amine boranes and their derivatives. Ignition of the amine
borane(s) forms boron nitride (BN) and hydrogen gas. In addition to ammonia borane
(H3BNH3), other gas-generators include diborane diammoniate, H2B(NH3)2BH4.
Nano borohydrides and nanocatalyst doping
Enhancement of sorption kinetics and storage capacity can be improved through nanomaterial-based catalyst doping, as shown in the work of the Clean Energy Research Center in the University of South Florida. This research group studied LiBH4 doped with nickel
nanoparticles and analyzed the weight loss and release temperature of
the different species. They observed that an increasing amount of
nanocatalyst lowers the release temperature by approximately 20ºC and
increases the weight loss of the material by 2-3%. The optimum amount of
Ni particles was found to be 3 mol%, for which the temperature was
within the limits established (around 100ºC) and the weight loss was
notably greater than the undoped species.
Imidazolium ionic liquids
In 2007 Dupont
and others reported hydrogen-storage materials based on imidazolium
ionic liquids. Simple alkyl(aryl)-3-methylimidazolium
N-bis(trifluoromethanesulfonyl)imidate salts that possess very low
vapour pressure, high density, and thermal stability and are not
inflammable can add reversibly 6–12 hydrogen atoms in the presence of
classical Pd/C or Ir0 nanoparticle catalysts and can be used as
alternative materials for on-board hydrogen-storage devices. These salts
can hold up to 30 g L−1 of hydrogen at atmospheric pressure.
Phosphonium borate
In 2006 researchers of University of Windsor reported on reversible hydrogen storage in a non-metal phosphonium borate frustrated Lewis pair:
The phosphino-borane on the left accepts one equivalent of hydrogen
at one atmosphere and 25 °C and expels it again by heating to 100 °C.
The storage capacity is 0.25 wt% still rather below the 6 to 9 wt%
required for practical use.
Carbonite substances
Research has proven that graphene can store hydrogen efficiently. After taking up hydrogen, the substance becomes graphane. After tests, conducted by dr André Geim at the University of Manchester,
it was shown that not only can graphene store hydrogen easily, it can
also release the hydrogen again, after heating to 450 °C.
Metal-organic frameworks
Metal-organic frameworks
represent another class of synthetic porous materials that store
hydrogen and energy at the molecular level. MOFs are highly crystalline
inorganic-organic hybrid structures that contain metal clusters or ions
(secondary building units) as nodes and organic ligands as linkers. When
guest molecules (solvent) occupying the pores are removed during
solvent exchange and heating under vacuum, porous structure of MOFs can
be achieved without destabilizing the frame and hydrogen molecules will
be adsorbed onto the surface of the pores by physisorption. Compared to
traditional zeolites and porous carbon materials, MOFs have very high
number of pores and surface area which allow higher hydrogen uptake in a
given volume. Thus, research interests on hydrogen storage in MOFs have
been growing since 2003 when the first MOF-based hydrogen storage was
introduced. Since there are infinite geometric and chemical variations
of MOFs based on different combinations of SBUs and linkers, many
researches explore what combination will provide the maximum hydrogen
uptake by varying materials of metal ions and linkers.
In 2006, chemists at UCLA and the University of Michigan have achieved hydrogen storage concentrations of up to 7.5 wt% in MOF-74 at a low temperature of 77 K. In 2009, researchers at University of Nottingham reached 10 wt% at 77 bar (1,117 psi) and 77 K with MOF NOTT-112.
Most articles about hydrogen storage in MOFs report hydrogen uptake
capacity at a temperature of 77K and a pressure of 1 bar because these
conditions are commonly available and the binding energy between
hydrogen and the MOF at this temperature is large compared to the
thermal vibration energy. Varying several factors such as surface area,
pore size, catenation, ligand structure, and sample purity can result in
different amounts of hydrogen uptake in MOFs.
Encapsulation
Cella Energy
technology is based around the encapsulation of hydrogen gas and
nano-structuring of chemical hydrides in small plastic balls, at room
temperature and pressure.
Physical storage
In
this case hydrogen remains in physical forms, i.e., as gas,
supercritical fluid, adsorbate, or molecular inclusions. Theoretical
limitations and experimental results are considered
concerning the volumetric and gravimetric capacity of glass
microvessels, microporous, and nanoporous media, as well as safety and
refilling-time demands.
Activated carbons
Activated carbons are highly porous amorphous carbon materials with high apparent surface area. Hydrogen physisorption can be increased in these materials by increasing the apparent surface area and optimizing pore diameter to around 7 Å.
These materials are of particular interest due to the fact that they
can be made from waste materials, such as cigarette butts which have
shown great potential as precursor materials for high-capacity hydrogen
storage materials.
Cryo-compressed
Cryo-compressed
storage of hydrogen is the only technology that meets 2015 DOE targets
for volumetric and gravimetric efficiency (see "CcH2" on slide 6 in ).
Furthermore, another study has shown that cryo-compressed
exhibits interesting cost advantages: ownership cost (price per mile)
and storage system cost (price per vehicle) are actually the lowest when
compared to any other technology.
For example, a cryo-compressed hydrogen system would cost $0.12 per
mile (including cost of fuel and every associated other cost), while
conventional gasoline vehicles cost between $0.05 and $0.07 per mile.
Like liquid storage, cryo-compressed uses cold hydrogen (20.3 K
and slightly above) in order to reach a high energy density. However,
the main difference is that, when the hydrogen would warm-up due to heat
transfer with the environment ("boil off"), the tank is allowed to go
to pressures much higher (up to 350 bars versus a couple of bars for
liquid storage). As a consequence, it takes more time before the
hydrogen has to vent, and in most driving situations, enough hydrogen is
used by the car to keep the pressure well below the venting limit.
Consequently, it has been demonstrated that a high driving range
could be achieved with a cryo-compressed tank : more than 650 miles
(1,050 km) were driven with a full tank mounted on an hydrogen-fueled
engine of Toyota Prius. Research is still on its way in order to study and demonstrate the full potential of the technology.
As of 2010, the BMW Group has started a thorough component and
system level validation of cryo-compressed vehicle storage on its way to
a commercial product.
Carbon nanotubes
Carbon nanotubes
Hydrogen carriers based on nanostructured carbon (such as carbon buckyballs and nanotubes)
have been proposed. However, since Hydrogen usually amounts up to
~3.0-7.0 wt% at 77K which is far from the value set by US department of
Energy (6 wt% at nearly ambient conditions), it makes carbon materials
poor candidates for hydrogen storage.
To realize carbon materials as effective hydrogen storage
technologies, the Clean Energy Research Center has doped carbon
nanotubes (CNTs) with MgH2. The metal hydride has proven to have a theoretical storage capacity (7.6 wt%) that fulfills the United States Department of Energy
requirement of 6 wt%, but has limited practical applications due to its
high release temperature. The proposed mechanism involves the creation
of fast diffusion channels by CNTs within the MgH2 lattice. Fullerene
is other carbonaceous nanomaterials that has been tested for hydrogen
storage in this center. Fullerene molecules are composed of a C60 close-caged structure, that allows for hydrogenation of the double bonded carbons leading to a theoretical C60H60 isomer with a hydrogen content of 7.7 wt%. However, the release temperature in these systems is too high (600oC) for practical applications.
Clathrate hydrates
H2 caged in a clathrate hydrate was first reported in 2002, but requires very high pressures to be stable. In 2004, researchers from Delft University of Technology and Colorado School of Mines showed solid H2-containing hydrates could be formed at ambient temperature and 10s of bar by adding small amounts of promoting substances such as THF. These clathrates have a theoretical maximum hydrogen densities of around 5 wt% and 40 kg/m3.
Glass capillary arrays
A
team of Russian, Israeli and German scientists have collaboratively
developed an innovative technology based on glass capillary arrays for
the safe infusion, storage and controlled release of hydrogen in mobile
applications. The C.En technology has achieved the United States Department of Energy (DOE) 2010 targets for on-board hydrogen storage systems.
DOE 2015 targets can be achieved using flexible glass capillaries and cryo-compressed method of hydrogen storage.
Glass microspheres
Hollow glass microspheres (HGM) can be utilized for controlled storage and release of hydrogen.
Stationary hydrogen storage
Unlike
mobile applications, hydrogen density is not a huge problem for
stationary applications. As for mobile applications, stationary
applications can use established technology:
- Compressed hydrogen (CGH2) in a hydrogen tank
- Liquid hydrogen in a (LH2) cryogenic hydrogen tank
- Slush hydrogen in a cryogenic hydrogen tank
Underground hydrogen storage
'Available storage technologies, their capacity and discharge time.'
Underground hydrogen storage is the practice of hydrogen storage in underground caverns, salt domes and depleted oil and gas fields. Large quantities of gaseous hydrogen have been stored in underground caverns by ICI for many years without any difficulties. The storage of large quantities of liquid hydrogen underground can function as grid energy storage. The round-trip efficiency is approximately 40% (vs. 75-80% for pumped-hydro (PHES)), and the cost is slightly higher than pumped hydro, if only a limited number of hours of storage is required.
Another study referenced by a European staff working paper found that
for large scale storage, the cheapest option is hydrogen at €140/MWh for
2,000 hours of storage using an electrolyser, salt cavern storage and
combined-cycle power plant. The European project Hyunder
indicated in 2013 that for the storage of wind and solar energy an
additional 85 caverns are required as it cannot be covered by PHES and CAES systems.
A German case study on storage of hydrogen in salt caverns found that
if the German power surplus (7% of total variable renewable generation
by 2025 and 20% by 2050) would be converted to hydrogen and stored
underground, these quantities would require some 15 caverns of 500,000
cubic metres each by 2025 and some 60 caverns by 2050 – corresponding to
approximately one third of the number of underground gas caverns
currently operated in Germany.
In the US, Sandia Labs are conducting research into the storage of
hydrogen in depleted oil and gas fields, which could easily absorb large
amounts of renewably produced hydrogen as there are some 2.7 million
depleted wells in existence.
Power to gas
Power to gas is a technology which converts electrical power to a gas fuel. There are two methods: the first is to use the electricity for water splitting and inject the resulting hydrogen into the natural gas grid; the second, less efficient method is used to convert carbon dioxide and hydrogen to methane, (see natural gas) using electrolysis and the Sabatier reaction.
A third option is to combine the hydrogen via electrolysis with a
source of carbon (either carbon dioxide or carbon monoxide from biogas, from industrial processes or via direct air-captured carbon dioxide) via biomethanation, where biomethanogens (archaea) consume carbon dioxide and hydrogen and produce methane within an anaerobic
environment. This process is highly efficient, as the archaea are
self-replicating and only require low-grade (60°C) heat to perform the
reaction.
Another process has also been achieved by SoCalGas
to convert the carbon dioxide in raw biogas to methane in a single
electrochemical step, representing a simpler method of converting excess
renewable electricity into storable natural gas.
The UK has completed surveys and is preparing to start injecting
hydrogen into the gas grid as the grid previously carried 'town gas'
which is a 50% hydrogen-methane gas formed from coal. Auditors KPMG
found that converting the UK to hydrogen gas could be £150bn to £200bn
cheaper than rewiring British homes to use electric heating powered by
lower-carbon sources.
Excess power or off peak power generated by wind generators or
solar arrays can then be used for load balancing in the energy grid.
Using the existing natural gas system for hydrogen, Fuel cell maker Hydrogenics and natural gas distributor Enbridge have teamed up to develop such a power to gas system in Canada.
Pipeline storage of hydrogen where a natural gas network is used for the storage of hydrogen. Before switching to natural gas, the German gas networks were operated using towngas,
which for the most part (60-65%) consisted of hydrogen. The storage
capacity of the German natural gas network is more than 200,000 GW·h
which is enough for several months of energy requirement. By comparison,
the capacity of all German pumped storage power plants amounts to only
about 40 GW·h. The transport of energy through a gas network is done
with much less loss (<0 .1="" a="" existing="" href="https://en.wikipedia.org/wiki/List_of_natural_gas_pipelines" in="" network="" of="" power="" than="" the="" title="List of natural gas pipelines" use="">natural gas pipelines
for hydrogen was studied by NaturalHy
Automotive Onboard hydrogen storage
Targets for on-board hydrogen storage assuming storage of 5 kg of hydrogen.
Targets were set by the FreedomCAR Partnership in January 2002 between the United States Council for Automotive Research (USCAR) and U.S. DOE (Targets assume a 5-kg H2 storage system). The 2005 targets were not reached in 2005. The targets were revised in 2009 to reflect new data on system efficiencies obtained from fleets of test cars. The ultimate goal for volumetric storage is still above the theoretical density of liquid hydrogen.
It is important to note that these targets are for the hydrogen
storage system, not the hydrogen storage material. System densities are
often around half those of the working material, thus while a material
may store 6 wt% H2,
a working system using that material may only achieve 3 wt% when the
weight of tanks, temperature and pressure control equipment, etc., is
considered.
In 2010, only two storage technologies were identified as having
the potential to meet DOE targets: MOF-177 exceeds 2010 target for
volumetric capacity, while cryo-compressed H2 exceeds more restrictive 2015 targets for both gravimetric and volumetric capacity.
The existing options for hydrogen storage require large storage
volumes which makes them impractical for stationary and portable
applications. Portability is one of the biggest challenges in the automotive industry, where high density storage systems are problematic due to safety concerns.
Fuel cell powered vehicles
are required to provide a driving range over 300 miles—this cannot be
achieved with traditional storage methods. A long term goal set by the
Fuel Cell Technology Office involves the usage of nanomaterials to
improve maximum range.
U.S. Department of Energy's requirements
The
Department of Energy has set the targets for onboard hydrogen storage
for light vehicles. The list of requirements include parameters related
to gravimetric and volumetric capacity, operability, durability and
cost. These targets have been set as the goal for a multiyear research
plan expected to offer an alternative to fossil fuels.
Fuel cells and storage
Due
to its clean-burning characteristics, hydrogen is one of the most
promising fuel alternatives in the automotive industry. Hydrogen based
fuel could significantly reduce the emissions of greenhouse gases such as CO2, SO2 and NOx. The three limiting factor for the use of hydrogen fuel cells
(HFC) include efficiency, size, and safe onboard storage of the gas.
Other major disadvantages of this emerging technology involve cost,
operability and durability issues, that are still to be improved from
the existing systems. To address these challenges, the use of
nanomaterials has been proposed as an alternative option to the
traditional hydrogen storage systems. The use of nanomaterials could
provide a higher density system that is expected to increase the driving
range limit set by the DOE at 300 miles. Carbonaceous materials such as CNTs
and metal hydrides are the main focus of researchers. Carbonaceous
materials are currently being considered for onboard storage systems due
to their versatility, multifunctionality, mechanical properties and low
cost with respect to other alternatives.
Other advantages of nanomaterials in fuel cells
The
introduction of nanomaterials in onboard hydrogen storage systems can
be a major turning point in the automotive industry. However, storing is
not the only practical aspect of the fuel cell to which nanomaterials
may contribute. Different studies have shown that the transport and catalytic properties of Nafion membranes used in HFCs can be enhanced with TiO2/SnO2 nanoparticles. The increased performance is caused by an improvement in hydrogen splitting kinetics due to catalytic activity of the nanoparticles. Furthermore, this system exhibits faster transport of protons across the cell which makes HFCs with nanoparticle composite membranes a promising alternative.
Another application of nanomaterials in water splitting has been introduced by a research group at Manchester Metropolitan University in the UK using screen-printed electrodes consisting of a graphene-like material. Similar systems have been developed using photoelectrochemical techniques.


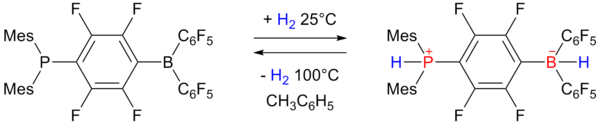












![Hydrogenation of nitrogen {\displaystyle {\ce {{\underset {nitrogen}{N{\equiv }N}}+{\underset {hydrogen \atop (200atm)}{3H2}}->[{\ce {Fe\ catalyst}}][350-550^{\circ }{\ce {C}}]{\underset {ammonia}{2NH3}}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/2dd5645111a3ad991987a7b9e10599029c287e98)