From Wikipedia, the free encyclopedia
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, symbol | iodine, I | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pronunciation | /ˈaɪ.ədaɪn/, /ˈaɪ.ədɨn/, or /ˈaɪ.ədiːn/ EYE-ə-dyn, EYE-ə-dən, or EYE-ə-deen |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | lustrous metallic gray, violet as a gas | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Iodine in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number | 53 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | 126.90447(3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | diatomic nonmetal | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, block | group 17 (halogens), p-block | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Kr] 4d10 5s2 5p5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| per shell | 2, 8, 18, 18, 7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 386.85 K (113.7 °C, 236.66 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 457.4 K (184.3 °C, 363.7 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density near r.t. | 4.933 g·cm−3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Triple point | 386.65 K, 12.1 kPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Critical point | 819 K, 11.7 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | (I2) 15.52 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | (I2) 41.57 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | (I2) 54.44 J·mol−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
vapor pressure (rhombic)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 7, 5, 3, 1, −1 (a strongly acidic oxide) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 2.66 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies | 1st: 1008.4 kJ·mol−1 2nd: 1845.9 kJ·mol−1 3rd: 3180 kJ·mol−1 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 140 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 139±3 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 198 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellanea | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | orthorhombic
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 0.449 W·m−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 1.3×107 Ω·m (at 0 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic[1] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 7.7 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 7553-56-2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery and first isolation | Bernard Courtois (1811) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Most stable isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Decay modes in parentheses are predicted, but have not yet been observed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Iodine is a chemical element with symbol I and atomic number 53. The name is from Greek ἰοειδής ioeidēs, meaning violet or purple, due to the color of elemental iodine vapor.[2]
Iodine and its compounds are primarily used in nutrition, and industrially in the production of acetic acid and certain polymers. Iodine's relatively high atomic number, low toxicity, and ease of attachment to organic compounds have made it a part of many X-ray contrast materials in modern medicine. Iodine has only one stable isotope. A number of iodine radioisotopes are also used in medical applications.
Iodine is found on Earth mainly as the highly water-soluble iodide ion I−, which concentrates it in oceans and brine pools. Like the other halogens, free iodine occurs mainly as a diatomic molecule I2, and then only momentarily after being oxidized from iodide by an oxidant like free oxygen. In the universe and on Earth, iodine's high atomic number makes it a relatively rare element. However, its presence in ocean water has given it a role in biology. It is the heaviest essential element utilized widely by life in biological functions (only tungsten, employed in enzymes by a few species of bacteria, is heavier). Iodine's rarity in many soils, due to initial low abundance as a crust-element, and also leaching of soluble iodide by rainwater, has led to many deficiency problems in land animals and inland human populations. Iodine deficiency affects about two billion people and is the leading preventable cause of intellectual disabilities.[3]
Iodine is required by higher animals for synthesizing thyroid hormones, which contain the element. Because of this function, radioisotopes of iodine are concentrated in the thyroid gland along with nonradioactive iodine. If inhaled, the radioisotope iodine-131, which has a high fission product yield, concentrates in the thyroid, but is easily remedied with non-radioactive potassium iodide treatment.
Characteristics
Under standard conditions, iodine is a bluish-black solid appearing to sublimate into a noxious violet-pink gas, the colour due to absorption of visible light by electronic transitions between the highest occupied and lowest unoccupied molecular orbitals. Melting at 113.7 °C (236.7 °F), it forms compounds with many elements but is less reactive than the other members of its group, the halogens, and has some metallic light reflectance.Elemental iodine is slightly soluble in water, with one gram dissolving in 3450 ml at 20 °C and 1280 ml at 50 °C; potassium iodide may be added to increase solubility via formation of triiodide ions.[citation needed] Nonpolar solvents such as hexane and carbon tetrachloride provide a higher solubility.[4] Polar solutions are brown, reflecting the role of these solvents as Lewis bases, while nonpolar solutions are violet, the color of iodine vapor.[5] Charge-transfer complexes form when iodine is dissolved in polar solvents, modifying the energy distribution of iodine's molecular orbitals, hence changing the colour. A metal ion may replace the solvent, in which case the two species exchange electrons, the ion undergoing π backbonding.[6]

I2•PPh3 charge-transfer complexes in CH2Cl2. From left to right: (1) I2 dissolved in dichloromethane. (2) A few seconds after excess PPh3 was added. (3) One minute later after excess PPh3 was added, which contains [Ph3PI]+I−. (4) Immediately after excess I2 was added, which contains [Ph3PI]+[I3]−.[6]
Structure and bonding
Iodine normally exists as a diatomic molecule with an I-I bond length of 270 pm,[7] one of the longest single bonds known. The I2 molecules tend to interact via the weak van der Waals forces called the London dispersion forces, and this interaction is responsible for the higher melting point compared to more compact halogens, which are also diatomic. Since the atomic size of iodine is larger, its melting point is higher. The solid crystallizes as orthorhombic crystals. The crystal motif in the Hermann–Mauguin notation is Cmca (No 64), Pearson symbol oS8. The I-I bond is relatively weak, with a bond dissociation energy of 36 kcal/mol, and most bonds to iodine are weaker than for the lighter halides. One consequence of this weak bonding is the relatively high tendency of I2 molecules to dissociate into atomic iodine.
Isotopes
Of the 37 known (characterized) isotopes of iodine, only one, 127I, is stable.The longest-lived radioisotope, 129I, has a half-life of 15.7 million years. This is long enough to make it a permanent fixture of the environment on human time scales, but far too short for it to exist as a primordial isotope today. Instead, iodine-129 is an extinct radionuclide, and its presence in the early Solar System is inferred from the observation of an excess of its daughter xenon-129. This nuclide is also newly made by cosmic rays and as a byproduct of artificial nuclear fission, which it is used to monitor as a very long-lived environmental contaminant.
The next-longest-lived radioisotope, iodine-125, has a half-life of 59 days. It is used as a convenient gamma-emitting tag for proteins in biological assays, and a few nuclear medicine imaging tests where a longer half-life is required. It is also commonly used in brachytherapy implanted capsules, which kill tumors by local short-range gamma radiation (but where the isotope is never released into the body).
Iodine-123 (half-life 13 hours) is the isotope of choice for nuclear medicine imaging of the thyroid gland, which naturally accumulates all iodine isotopes.
Iodine-131 (half-life 8 days) is a beta-emitting isotope, which is a common nuclear fission product. It is preferably administered to humans only in very high doses that destroy all tissues that accumulate it (usually the thyroid), which in turn prevents these tissues from developing cancer from a lower dose (paradoxically, a high dose of this isotope appears safer for the thyroid than a low dose). Like other radioiodines, I-131 accumulates in the thyroid gland, but unlike the others, in small amounts it is highly carcinogenic there, it seems, owing to the high local cell mutation due to damage from beta decay. Because of this tendency of 131I to cause high damage to cells that accumulate it and other cells near them (0.6 to 2 mm away, the range of the beta rays), it is the only iodine radioisotope used as direct therapy, to kill tissues such as cancers that take up artificially iodinated molecules (example, the compound iobenguane, also known as MIBG). For the same reason, only the iodine isotope I-131 is used to treat Grave's disease and those types of thyroid cancers (sometimes in metastatic form) where the tissue that requires destruction, still functions to naturally accumulate iodide.
Nonradioactive ordinary potassium iodide (iodine-127), in a number of convenient forms (tablets or solution) may be used to saturate the thyroid gland's ability to take up further iodine, and thus protect against accidental contamination from iodine-131 generated by nuclear fission accidents, such as the Chernobyl disaster and more recently the Fukushima I nuclear accidents, as well as from contamination from this isotope in nuclear fallout from nuclear weapons.
Occurrence
Iodine is rare in the Solar System and Earth's crust (47–60th in abundance); however, iodide salts are often very soluble in water. Iodine occurs in slightly greater concentrations in seawater than in rocks, 0.05 vs. 0.04 ppm. Minerals containing iodine include caliche, found in Chile. The brown algae Laminaria and Fucus found in temperate zones of the Northern Hemisphere contain 0.028–0.454 dry weight percent of iodine. Aside from tungsten, iodine is the heaviest element to be essential in living organisms. About 19,000 tonnes are produced annually from natural sources.[8]
Organoiodine compounds are produced by marine life forms, the most notable being iodomethane (commonly called methyl iodide). About 214 kilotonnes/year of iodomethane is produced by the marine environment, by microbial activity in rice paddies and by the burning of biological material.[9] The volatile iodomethane is broken up in the atmosphere as part of a global iodine cycle.[9][10]
Chemistry
Iodine adopts a variety of oxidation states, commonly ranging from (formally) I(VII) to I(-I), and including the intermediate states of I(V), I(III) and I(I). Practically, only the −1 oxidation state is of significance, being the form found in iodide salts and organoiodine compounds. Iodine is a Lewis acid. With electron donors such as triphenylphosphine and pyridine it forms a charge-transfer complex. With the iodide anion it forms the triiodide ion.[11] Iodine and the iodide ion form a redox couple. I2 is easily reduced and I− is easily oxidized.Redox reactions
In everyday life, iodides are slowly oxidized by atmospheric oxygen to give free iodine. Evidence for this conversion is the yellow tint of certain aged samples of iodide salts and some organoiodine compounds.[8] The oxidation of iodide to iodine in air is also responsible for the slow loss of iodide content in iodized salt if exposed to air.[12] Some salts use iodate (IO−3) to prevent the loss of iodine.
Iodine is easily reduced. Most common is the interconversion of I− and I2. Molecular iodine can be prepared by oxidizing iodides with chlorine:
- 2 I− + Cl2 → I2 + 2 Cl−
- 2 I− + 4 H+ + MnO2 → I2 + 2 H2O + Mn2+
- 8 I2 + 8 H2S → 16 HI + S8
- 2 I2 + N2H4 → 4 HI + N2
2 cation, the result of iodine being oxidized by SO
3:[15]
- 2 I
2 + 2 SO
3 + H
2SO
4 → 2 I+
2 + SO
2 + 2 HSO−
4
2 cation is also formed in the oxidation of iodine by SbF
5 or TaF
5. The resulting I+
2Sb
2F−
11 or I+
2Ta
2F−
11 can be isolated as deep blue crystals. The solutions of these salts turn red when cooled below −60 °C, owing to the formation of the I2+
4 cation:[15]
- 2 I+
2 I2+
I2+
4
4 disproportionates into I+
3 and an iodine(III) compound. Excess iodine can then react with I+
3 to form I+
5 (green) and I3+
15 (black).[15]
Oxides
The best-known oxides are the anions, IO−3 and IO−
4, but several other oxides are known, such as the strong oxidant iodine pentoxide.
By contrast with chlorine, the formation of the hypohalite ion (IO−) in neutral aqueous solutions of iodine is negligible.
- I2 + H2O
 H+ + I− + HIO (K = 2.0×10−13)[13] In basic solutions (such as aqueous sodium hydroxide), iodine converts in a two stage reaction to iodide and iodate:[13]
H+ + I− + HIO (K = 2.0×10−13)[13] In basic solutions (such as aqueous sodium hydroxide), iodine converts in a two stage reaction to iodide and iodate:[13]
I2 + 2 OH− → I− + IO− + H2O (K = 30) 3 IO− → 2 I− + IO−
3(K = 1020)
Iodic acid (HIO3), periodic acid (HIO4) and their salts are strong oxidizers and are of some use in organic synthesis. Iodine is oxidized to iodate by nitric acid as well as by chlorates:[16]
- I2 + 10 HNO3 → 2 HIO3 + 10 NO2 + 4 H2O
- I2 + 2 ClO−
3 → 2 IO−
3 + Cl2
Other inorganic compounds
Iodine forms compounds with all the elements except for the noble gases. From the perspective of commercial applications, an important compound is hydroiodic acid, used as a co-catalyst in the Cativa process for the production of acetic acid. Titanium and aluminium iodides are used in the production of butadiene, a precursor to rubber tires.[8]Alkali metal salts are common colourless solids that are highly soluble in water. Potassium iodide is a convenient source of the iodide anion; it is easier to handle than sodium iodide because it is not hygroscopic. Both salts are mainly used in the production of iodized salt. Sodium iodide is especially useful in the Finkelstein reaction, because it is soluble in acetone, whereas potassium iodide is less so. In this reaction, an alkyl chloride is converted to an alkyl iodide. This relies on the insolubility of sodium chloride in acetone to drive the reaction:
- R-Cl (acetone) + NaI (acetone) → R-I (acetone) + NaCl (s)
- 3 I2 + 2 Al → 2 AlI3
Interhalogen compounds are well known; examples include iodine monochloride and trichloride; iodine pentafluoride and heptafluoride.
Organic compounds
Organoiodine compounds can be made in many ways. For example, methyl iodide can be prepared from methanol, red phosphorus, and iodine.[17] The iodinating reagent is phosphorus triiodide that is formed in situ:- 3 CH3OH + PI3 → 3 CH3I + H3PO3
Some drawbacks to the use of organoiodine compounds in chemical synthesis are:
- iodine compounds are more expensive than the corresponding bromides and chlorides, in that order
- iodides are much stronger alkylating agents, and so are more toxic (e.g., methyl iodide is very toxic (T+)).[18]
- low-molecular-weight iodides tend to have a much higher equivalent weight, compared to other alkylating agents (e.g., methyl iodide versus dimethyl carbonate), owing to the atomic mass of iodine.
Production
Of the several places in which iodine occurs in nature, only two sources are useful commercially: the caliche, found in Chile, and the iodine-containing brines of gas and oil fields, especially in Japan and the United States. The caliche contains sodium nitrate, which is the main product of the mining activities, and small amounts of sodium iodate and sodium iodide. In the extraction of sodium nitrate, the sodium iodate and sodium iodide are also extracted.[19] The high concentration of iodine in the caliche and the extensive mining made Chile the largest producer of iodine in 2007.Most other producers use naturally occurring brine for the production of iodine. The Japanese Minami Kanto gas field east of Tokyo and the American Anadarko Basin gas field in northwest Oklahoma are the two largest sources for iodine from brine. The brine has a temperature of over 60 °C owing to the depth of the source. The brine is first purified and acidified using sulfuric acid, then the iodide present is oxidized to iodine with chlorine. An iodine solution is produced, but is dilute and must be concentrated. Air is blown into the solution, causing the iodine to evaporate, then it is passed into an absorbing tower containing acid where sulfur dioxide is added to reduce the iodine. The hydrogen iodide (HI) is reacted with chlorine to precipitate the iodine. After filtering and purification the iodine is packed.[19][20]
- 2 HI + Cl2 → I2↑ + 2 HCl
- I2 + 2 H2O + SO2 → 2 HI + H2SO4
- 2 HI + Cl2 → I2↓ + 2 HCl
Commercial samples often contain high concentrations of impurities, which can be removed by sublimation. The element may also be prepared in an ultra-pure form through the reaction of potassium iodide with copper(II) sulfate, which gives copper(II) iodide initially, which then decomposes spontaneously to copper(I) iodide and iodine:
- Cu2+ + 2 I− → CuI2
- 2 CuI2 → 2 CuI + I2
History
Iodine was discovered by French chemist Bernard Courtois in 1811.[22][23] He was born to a manufacturer of saltpeter (a vital part of gunpowder). At the time of the Napoleonic Wars, France was at war and saltpeter was in great demand. Saltpeter produced from French niter beds required sodium carbonate, which could be isolated from seaweed collected on the coasts of Normandy and Brittany.To isolate the sodium carbonate, seaweed was burned and the ash washed with water. The remaining waste was destroyed by adding sulfuric acid. Courtois once added excessive sulfuric acid and a cloud of purple vapor rose. He noted that the vapor crystallized on cold surfaces, making dark crystals. Courtois suspected that this was a new element but lacked funding to pursue it further.
Courtois gave samples to his friends, Charles Bernard Desormes (1777–1862) and Nicolas Clément (1779–1841), to continue research. He also gave some of the substance to chemist Joseph Louis Gay-Lussac (1778–1850), and to physicist André-Marie Ampère (1775–1836). On 29 November 1813, Desormes and Clément made public Courtois's discovery. They described the substance to a meeting of the Imperial Institute of France.[24] On 6 December, Gay-Lussac announced that the new substance was either an element or a compound of oxygen.[25][26][27] It was Gay-Lussac who suggested the name "iode", from the Greek word ιώδες (iodes) for violet (because of the color of iodine vapor).[22][25] Ampère had given some of his sample to English chemist Humphry Davy (1778–1829). Davy did some experiments on the substance and noted its similarity to chlorine.[28] Davy sent a letter dated 10 December to the Royal Society of London stating that he had identified a new element.[29] Arguments erupted between Davy and Gay-Lussac over who identified iodine first, but both scientists acknowledged Courtois as the first to isolate the element.
Applications
The production of ethylenediamine dihydroiodide, provided as a nutritional supplement for livestock, consumes a large fraction of available iodine. Another significant use is as a co-catalyst for the production of acetic acid by the Monsanto and Cativa processes. In these technologies, which support the world's demand for acetic acid, hydroiodic acid converts the methanol feedstock into methyl iodide, which undergoes carbonylation. Hydrolysis of the resulting acetyl iodide regenerates hydroiodic acid and gives acetic acid.[8]Disinfectants
Elemental iodine is used as a disinfectant in various forms. The iodine exists as the element, or as the water-soluble triiodide anion I3− generated in situ by adding iodide to poorly water-soluble elemental iodine (the reverse chemical reaction makes some free elemental iodine available for antisepsis). In alternative fashion, iodine may come from iodophors, which contain iodine complexed with a solubilizing agent (iodide ion may be thought of loosely as the iodophor in triiodide water solutions). Examples of such preparations include:[30]- Tincture of iodine: iodine in ethanol, or iodine and sodium iodide in a mixture of ethanol and water.
- Lugol's iodine: iodine and iodide in water alone, forming mostly triiodide. Unlike tincture of iodine, Lugol's has a minimized amount of the free iodine (I2) component.
- Povidone iodine (an iodophor).
Analysis
Iodine is useful in analytical chemistry because of its reactions with alkenes, starch and oxidizing and reducing agents. The highly colored species involved in these reactions make it easy to detect the endpoints in many analytical determinations. Iodine is a common general stain used in thin-layer chromatography. Iodine forms an intense blue complex with the glucose polymers starch and glycogen. Several analytical methods rely on this property:
- Iodometry. The concentration of an oxidant can be determined by adding it to an excess of iodide, to destroy elemental iodine/triiodide as a result of oxidation by the oxidant. A starch indicator is then used as the indicator close to the end-point, in order to increase the visual contrast (dark blue becomes colorless, instead of the yellow of dilute triiodide becoming colorless).
- An Iodine test may be used to test a sample substance for the presence of starch. The Iodine clock reaction is an extension of the techniques in iodometry.
- Iodine solutions are used in counterfeit banknote detection pens; the premise being that counterfeit banknotes are made using commercially available paper containing starch.
- Starch-iodide paper are used to test for the presence of oxidants such as peroxides. The oxidants convert iodide to iodine, which shows up as blue. A solution of starch and iodide can perform the same function.[31]
- During colposcopy, Lugol's iodine is applied to the vagina and cervix. Normal vaginal tissue stains brown owing to its high glycogen content (a color-reaction similar to that with starch), while abnormal tissue suspicious for cancer does not stain, and thus appears pale compared to the surrounding tissue. Biopsy of suspicious tissue can then be performed. This is called a Schiller's Test.
Medical applications
Potassium iodide has been used as an expectorant, although this use is increasingly uncommon. In medicine, potassium iodide is usually used to treat acute thyrotoxicosis, usually as a saturated solution of potassium iodide called SSKI. It is also used to block uptake of iodine-131 in the thyroid gland (see isotopes section above), when this isotope is used as part of radiopharmaceuticals (such as iobenguane) that are not targeted to the thyroid or thyroid-type tissues.Iodine-131 (usually in the chemical form of iodide) is a component of nuclear fallout, and is particularly dangerous owing to the thyroid gland's propensity to concentrate ingested iodine, where it is kept for periods longer than this isotope's radiological half-life of eight days. For this reason, if people are expected to be exposed to a significant amount of environmental radioactive iodine (iodine-131 in fallout), they may be instructed to take non-radioactive potassium iodide tablets. The typical adult dose is one 130 mg tablet per 24 hours, supplying 100 mg (100,000 micrograms) iodine, as iodide ion. (Typical daily dose of iodine to maintain normal health is of order 100 micrograms; see "Dietary Intake" below.) By ingesting this large amount of non-radioactive iodine, radioactive iodine uptake by the thyroid gland is minimized. See the article on potassium iodide for more on this topic.[32]
Iodine, as an element with high electron density and atomic number, absorbs X-rays well. Therefore, it may be used as a radiocontrast agent by filtering out imaging X-rays weaker than 33.3 keV, where iodine's innermost electrons begin absorbing X-rays strongly due to the photoelectric effect.[33] Organic compounds of a certain type (typically iodine-substituted benzene derivatives) are thus used in medicine as X-ray radiocontrast agents for intravenous injection. This is often in conjunction with advanced X-ray techniques such as angiography and CT scanning. At present, all water-soluble radiocontrast agents rely on iodine. It is on the World Health Organization's List of Essential Medicines, a list of the most important medication needed in a basic health system.[34]
Iodine and cancer risk
- Breast cancer. The breast strongly and actively concentrates iodine into breast-milk for the benefit of the developing infant, and may develop a goiter-like hyperplasia, sometimes manifesting as fibrocystic breast disease, when iodine level is low. Studies indicate that iodine deficiency, either dietary or pharmacologic, can lead to breast atypia and increased incidence of malignancy in animal models, while iodine treatment can reverse dysplasia,[35][36][37] with elemental iodine (I2) having been found to be more effective in reducing ductal hyperplasias and perilobular fibrosis in iodine-deficient rats than iodide (I−).[36] A protective effect of iodine against breast cancer has been suggested on the basis of the observation that Japanese women who consume iodine-rich seaweed have a relatively low rate of breast cancer.[38][39] Iodine is known to induce apoptosis in breast cancer cells.[40] Laboratory evidence has demonstrated an effect of iodine on breast cancer that is in part independent of thyroid function, with iodine inhibiting cancer promotion through modulation of the estrogen pathway. Gene array profiling of estrogen responsive breast cancer cell line shows that the combination of iodine and iodide alters gene expression and inhibits the estrogen response through up-regulating proteins involved in estrogen metabolism. Whether iodine/iodide will be useful as an adjuvant therapy in the pharmacologic manipulation of the estrogen pathway in women with breast cancer has not been determined clinically.[35]
- Iodine and stomach cancer. Some researchers have found an epidemiologic correlation between iodine deficiency, iodine-deficient goitre and gastric cancer;[41][42][43] a decrease of the incidence of death rate from stomach cancer after implementation of the effective iodine-prophylaxis has been reported also.[44] The proposed mechanism of action is that iodide ion can function in gastric mucosa as an antioxidant reducing species that can detoxify poisonous reactive oxygen species, such as hydrogen peroxide.
Historical medical applications
In the early 1900s, the Encyclopaedia Britannica described iodine as being "of definite value" for treatment of multiple conditions including "metallic poisonings, as by lead and mercury, asthma, aneurism, arteriosclerosis, angina pectoris, gout, goitre, syphilis, haemophilia, Bright's disease (nephritis) and bronchitis" with "usual doses" of iodide salts ranging from "five to thirty grains or more" (324,000mcg to 1,944,000mcg, though this is hundred of times higher than what is considered generally safe per today's tolerable UL).[45] For treatment of syphilis, it states "in its tertiary stages and also earlier this disease yields in the most rapid and unmistakable fashion to iodides; so much so that the administration of these salts is at present the best means of determining whether, for instance, a cranial tumour be syphilitic or not" (modern treatment for syphilis involves the use of antibiotics to kill syphilis bacteria - see Syphilis). For the treatment of chronic lead poisoning, it states "the essential part of the medicinal treatment of this condition is the administration of iodides, which are able to decompose the insoluble albuminates of lead which have become locked up in the tissues, rapidly causing their degeneration, and to cause the excretion of the poisonous metal by means of the intestine and the kidneys" (modern treatment for lead poisoning involves the use of a variety of substances - see Lead poisoning).[46]Other uses
Inorganic iodides find specialized uses. Hafnium, zirconium, titanium are purified by the van Arkel Process, which involves the reversible formation of the tetraiodides of these elements. Silver iodide is a major ingredient to traditional photographic film. Thousands of kilograms of silver iodide are consumed annually for cloud seeding.[8]The organoiodine compound erythrosine is an important food coloring agent. Perfluoroalkyl iodides are precursors to important surfactants, such as perfluorooctanesulfonic acid.[8]
Iodine was also used by the police, especially forensics, Iodine fuming was an effective way of revealing latent fingerprints on paper and other similar surfaces. It is still used mildly today.
In the United States, the Drug Enforcement Administration (DEA) regards iodine and compounds containing iodine (ionic iodides, iodoform, ethyl iodide, and so on) as reagents useful for the clandestine manufacture of methamphetamine.[47][48]
Iodine can be used to stabilize the wavelength of a helium–neon laser.[49]
Biological role
Iodine is an essential trace element for life, the heaviest element commonly needed by living organisms. Only tungsten, a component of a few bacterial enzymes, has a higher atomic number and atomic weight.Iodine's main role in animal biology is as a constituent of the thyroid hormones thyroxine (T4) and triiodothyronine (T3). These are made from addition condensation products of the amino acid tyrosine, and are stored prior to release in an iodine-containing protein called thyroglobulin. T4 and T3 contain four and three atoms of iodine per molecule, respectively. The thyroid gland actively absorbs iodide from the blood to make and release these hormones into the blood, actions that are regulated by a second hormone TSH from the pituitary. Thyroid hormones are phylogenetically very old molecules that are synthesized by most multicellular organisms, and that even have some effect on unicellular organisms.
Thyroid hormones play a basic role in biology, acting on gene transcription to regulate the basal metabolic rate.[citation needed] Total deficiency of thyroid hormones can reduce basal metabolic rate up to 50%. Excessive production of thyroid hormones can increase the basal metabolic rate by 100%.[citation needed] T4 acts largely as a precursor to T3, which is (with minor exceptions) the biologically active hormone. In amphibian metamorphosis iodine and thyroid hormones exert a well-studied experimental model of apoptosis on the cells of gills, tail, and fins of tadpoles.[50]
Iodine has a nutritional relationship with selenium. A family of selenium-dependent enzymes called deiodinases converts T4 to T3 (the active hormone) by removing an iodine atom from the outer tyrosine ring. These enzymes also convert T4 to reverse T3 (rT3) by removing an inner ring iodine atom, and convert T3 to 3,3'-diiodothyronine (T2) also by removing an inner ring atom. Both of the latter are inactivated hormones that are ready for disposal and have, in essence, no biological effects. A family of non-selenium-dependent enzymes then further deiodinates the products of these reactions.
Iodine accounts for 65% of the molecular weight of T4 and 59% of the T3. Fifteen to 20 mg of iodine is concentrated in thyroid tissue and hormones, but 70% of the body's iodine is distributed in other tissues, including mammary glands, eyes, gastric mucosa, fetal thymus, cerebro-spinal fluid and coroid plexus, arterial walls, the cervix, and salivary glands. In the cells of these tissues, iodide enters directly by sodium-iodide symporter (NIS). Its role in mammary tissue is related to fetal and neonatal development, but its role in the other tissues is partially unknown.[51]

Sequence of 123-iodide total-body scintiscans of a woman after intravenous injection of 123-iodide (half-life: 13 hours); (from left) respectively at 30 minutes, and at 6, 20 and 48 hours. High and rapid concentration of radio-iodide (in white) in extra-thyroidal organs is evident in gastric mucosa of the stomach, epidermis, salivary glands, periencephalic and cerebro-spinal fluid, choroid plexus and oral mucosa. In gastric mucosa 131-iodide (half-life: 8 days) persists in scintiscans for more than 72 hours. In the thyroid, iodide-concentration is more progressive, as in a reservoir [from 1% (after 30 minutes) to 5.8% (after 48 hours) of the total injected dose]. High iodide-concentration by the mammary gland is evident only in pregnancy and lactation. High excretion of radio-iodide is observed in the urine. (Venturi, 2011)
Dietary intake
The daily Dietary Reference Intake recommended by the United States Institute of Medicine is between 110 and 130 µg for infants up to 12 months, 90 µg for children up to eight years, 130 µg for children up to 13 years, 150 µg for adults, 220 µg for pregnant women and 290 µg for lactating mothers.[52] The Tolerable Upper Intake Level (UL) for adults is 1,100 μg/day (1.1 mg/day).[53] The tolerable upper limit was assessed by analyzing the effect of supplementation on thyroid-stimulating hormone.[51]The thyroid gland needs no more than 70 μg/day to synthesize the requisite daily amounts of T4 and T3. The higher recommended daily allowance levels of iodine seem necessary for optimal function of a number of body systems, including lactating breast, gastric mucosa, salivary glands, oral mucosa, and arterial walls.[54][55]
Natural sources of dietary iodine include seafood, such as fish, kelp and shellfish, dairy products such as milk, cheese and eggs and plants grown on iodine-rich soil.[56][57] Iodized salt is fortified with iodine.[57][58]
As of 2000, the median intake of iodine from food in the United States was 240 to 300 μg/day for men and 190 to 210 μg/day for women.[53] In Japan, consumption was considered much higher, ranging between 5,280 μg/day to 13,800 μg/day, this owing to the frequent consumption of seaweed or kombu kelp.[51] However, new studies suggest that Japan's consumption is closer to 1,000–3,000 μg/day.[59]
After iodine fortification programs (e.g., iodized salt) have been implemented, some cases of iodine-induced hyperthyroidism have been observed (so-called Jod-Basedow phenomenon). The condition seems to occur mainly in people over forty, and the risk appears higher when iodine deficiency is severe and the initial rise in iodine intake is high.[60]
Information processing, fine motor skills, and visual problem solving are improved by iodine repletion in moderately iodine-deficient children.[61]
Deficiency
In an estimated two-thirds of households on Earth, table salt is iodized.[3] However, this still leaves an estimated two billion people iodine-deficient. Iodine is required for the essential thyroxin hormones produced by and concentrated in the thyroid gland.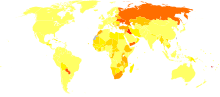
Disability-adjusted life year for iodine deficiency per 100,000 inhabitants in 2002.[62]
no data
less than 50
50–100
100–150
150–200
200–250
250–300
300–350
350–400
400–450
450–500
500–800
more than 800
In areas where there is little iodine in the diet,[10] typically remote inland areas and semi-arid equatorial climates where no marine foods are eaten, iodine deficiency gives rise to hypothyroidism, symptoms of which are extreme fatigue, goitre, mental slowing, depression, weight gain, and low basal body temperatures.[63] Iodine deficiency is the leading cause of preventable intellectual disability, a result that occurs primarily when babies or small children are rendered hypothyroidic by a lack of the element. The addition of iodine to table salt has largely eliminated this problem in the wealthier nations, but, as of March 2006, iodine deficiency remained a serious public health problem in the developing world.[64] Iodine deficiency is also a problem in certain areas of Europe.
Other possible health effects being investigated as being related to deficiency include:[35]
- Breast cancer. The breast strongly and actively concentrates iodine into breast-milk for the benefit of the developing infant, and may develop a goiter-like hyperplasia, sometimes manifesting as fibrocystic breast disease, when iodine levels are low.
- Stomach cancer. Some researchers have found an epidemiologic correlation between iodine deficiency, iodine-deficient goitre and gastric cancer.[65][66][67] A decrease of the incidence of death rate from stomach cancer after implementation of the effective iodine-prophylaxis has been reported also.[68]
- Autism. Although there is not a definitive answer for the cause of autism in children, research has shown that mothers with low levels of iodine are more likely to have an autistic child.[69]
Toxicity
Elemental iodine (I2) is toxic if taken orally. The lethal dose for an adult human is 30 mg/kg, which is about 2.1–2.4 grams (even if experiments on rats demonstrated that these animals could survive after eating a 14000 mg/kg dose). Excess iodine can be more cytotoxic in the presence of selenium deficiency.[70] Iodine supplementation in selenium-deficient populations is, in theory, problematic, partly for this reason.[51] Its toxicity derives from its oxidizing properties, which make it able to denaturate proteins (including enzymes).Elemental iodine is also a skin irritant, and direct contact with skin can cause damage, so iodine crystals should be handled with care. Solutions with high elemental iodine concentration such as tincture of iodine and Lugol's solution are capable of causing tissue damage if their use for cleaning and antisepsis is prolonged; similarly, cases have been reported where liquid Povidone-iodine (Betadine) trapped against the skin resulted in chemical burns.[71]








