| |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /mɛtˈfɔːrmɪn/, met-FOR-min |
| Trade names | Glucophage, other |
| Synonyms | N,N-dimethylbiguanide |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a696005 |
| License data | |
| Pregnancy category | |
| Routes of administration | by mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 50–60% |
| Protein binding | Minimal |
| Metabolism | Not by liver |
| Elimination half-life | 4–8.7 hours |
| Excretion | Urine (90%) |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard |
100.010.472 |
| Chemical and physical data | |
| Formula | C4H11N5 |
| Molar mass | 129.16364 g/mol |
| 3D model (JSmol) | |
| Density | 1.3±0.1[4] g/cm3 |
Metformin, marketed under the trade name Glucophage among others, is the first-line medication for the treatment of type 2 diabetes, particularly in people who are overweight. It is also used in the treatment of polycystic ovary syndrome (PCOS). Limited evidence suggests metformin may prevent the cardiovascular disease and cancer complications of diabetes. It is not associated with weight gain. It is taken by mouth.
Metformin is generally well tolerated. Common side effects include diarrhea, nausea and abdominal pain. It has a low risk of causing low blood sugar. High blood lactic acid level is a concern if the medication is prescribed inappropriately and in overly large doses. It should not be used in those with significant liver disease or kidney problems. While no clear harm comes from use during pregnancy, insulin is generally preferred for gestational diabetes. Metformin is in the biguanide class. It works by decreasing glucose production by the liver and increasing the insulin sensitivity of body tissues.
Metformin was discovered in 1922. French physician Jean Sterne began study in humans in the 1950s. It was introduced as a medication in France in 1957 and the United States in 1995. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. Metformin is believed to be the most widely used medication for diabetes which is taken by mouth. It is available as a generic medication. The wholesale price in the developed world was between US$0.21 and US$5.55 per month as of 2014. In the United States, it costs US$5 to US$25 per month.
Medical uses
Type 2 diabetes
The American Diabetes Association and the American College of Physicians each recommend metformin as a first-line agent to treat type 2 diabetes.Efficacy
The UK Prospective Diabetes Study, a large clinical trial performed in 1980-90s, provided evidence that metformin reduced the rate of adverse cardiovascular outcomes in overweight patients with type 2 diabetes relative to other antihyperglycemic agents. However, accumulated evidence from other and more recent trials reduced confidence in the efficacy of metformin for cardiovascular disease prevention.Treatment guidelines for major professional associations including the European Association for the Study of Diabetes, the European Society for Cardiology and the American Diabetes Association, now describe evidence for the cardiovascular benefits of metformin as equivocal.
In 2017, the American College of Physicians's guidelines were updated to recognize metformin as the first-line treatment for type-2 diabetes. These guidelines supersede earlier reviews. For example, a 2014 review found tentative evidence that people treated with sulfonylureas had a higher risk of severe low blood sugar events (RR 5.64), though their risk of non-fatal cardiovascular events was lower than the risk of those treated with metformin (RR 0.67). There was not enough data available at that time to determine the relative risk of death or of death from heart disease.
Metformin has little or no effect on body weight in type 2 diabetes compared with placebo, in contrast to sulfonylureas which are associated with weight gain. There is some evidence that metformin is associated with weight loss in obesity in the absence of diabetes. Metformin has a lower risk of hypoglycemia than the sulfonylureas, although hypoglycemia has uncommonly occurred during intense exercise, calorie deficit, or when used with other agents to lower blood glucose. Metformin modestly reduces LDL and triglyceride levels.
Prediabetes
Metformin treatment of people at a prediabetes stage of risk for type 2 diabetes may decrease their chances of developing the disease, although intensive physical exercise and dieting work significantly better for this purpose. In a large U.S. study known as the Diabetes Prevention Program, participants were divided into groups and given either placebo, metformin, or lifestyle intervention and followed for an average of three years.The intensive program of lifestyle modifications included a 16-lesson training on dieting and exercise followed by monthly individualized sessions with the goals of decreasing weight by 7% and engaging in physical activity for at least 150 minutes per week.
The incidence of diabetes was 58% lower in the lifestyle group and 31% lower in individuals given metformin. Among younger people with a higher body mass index, lifestyle modification was no more effective than metformin, and for older individuals with a lower body mass index, metformin was no better than placebo in preventing diabetes. After ten years, the incidence of diabetes was 34% lower in the group of participants given diet and exercise and 18% lower in those given metformin. It is unclear whether metformin slowed down the progression of prediabetes to diabetes (true preventative effect), or the decrease of diabetes in the treated population was simply due to its glucose-lowering action (treatment effect).
Polycystic ovary syndrome
Antidiabetic therapy has been proposed as a treatment for polycystic ovary syndrome (PCOS), a condition frequently associated with insulin resistance, since the late 1980s.The use of metformin in PCOS was first reported in 1994, in a small study conducted at the University of the Andes, Venezuela. The United Kingdom's National Institute for Health and Clinical Excellence recommended in 2004 that women with PCOS and a body mass index above 25 be given metformin for anovulation and infertility when other therapies fail to produce results. However, two clinical studies completed in 2006–2007 returned mostly negative results, with metformin performing no better than placebo, and a metformin-clomifene combination no better than clomifene alone. Reflecting this, subsequent reviews large randomized controlled trials in general have not shown the promise suggested by the early studies.
UK and international clinical practice guidelines do not recommend metformin as a first-line treatment or do not recommend it at all, except for women with glucose intolerance. The guidelines suggest clomiphene as the first medication option and emphasize lifestyle modification independently from medical treatment. Metformin treatment decreases the risk of developing type 2 diabetes mellitus in women with PCOS who exhibited impaired glucose tolerance (IGT) at baseline.
Female infertility
Metformin or clomiphene are both first line treatments for infertility in women with PCOS. In a dissenting opinion, a systematic review of four head-to-head comparative trials of metformin and clomifene found them equally effective for infertility.Four positive studies of metformin were in women not responding to clomifene, while the population in the negative studies was drug-naive or uncontrolled for the previous treatment. Metformin should be used as a second-line medication if clomifene treatment fails. Another review recommended metformin unreservedly as a first-line treatment option because it has positive effects not only on anovulation, but also on insulin resistance, hirsutism and obesity often associated with PCOS. A Cochrane review found metformin improves ovulation and pregnancy rates, particularly when combined with clomifene, and tentative evidence that it may increase the number of live births.
The use of metformin during all parts of pregnancy is controversial. One review found that when taken during pregnancy it reduces the number of complications during pregnancy and does not appear to cause developmental delays. Another review found good short term safety for both the mother and baby but unclear long term safety.
Metformin use among women with PCOS before they are pregnant does not appear to reduce abortion risk.
Gestational diabetes
Several observational studies and randomized, controlled trials found metformin to be as effective and safe as insulin for the management of gestational diabetes.Nonetheless, several concerns were raised and evidence on the long-term safety of metformin for both mother and child is lacking.
Metformin is safe in pregnancy and women with gestational diabetes treated with metformin have less weight gain during pregnancy than those treated with insulin. Babies born to women treated with metformin have been found to develop less visceral fat, making them less prone to insulin resistance in later life.
Other
Metformin appears to be safe and effective in counteracting the weight gain caused by the antipsychotic medications olanzapine and clozapine. Although modest reversal of clozapine-associated weight gain is found with metformin, primary prevention of weight gain is more valuable.Metformin may reduce the insulin requirement in type 1 diabetes.
Contraindications
Metformin is contraindicated in people with any condition that could increase the risk of lactic acidosis, including kidney disorders (arbitrarily defined as creatinine levels over 150 μmol/l (1.7 mg/dl)), lung disease and liver disease. According to the prescribing information, heart failure (in particular, unstable or acute congestive heart failure) increases the risk of lactic acidosis with metformin. A 2007 systematic review of controlled trials, however, suggested metformin is the only antidiabetic medication not associated with any measurable harm in people with heart failure, and it may reduce mortality in comparison with other antidiabetic agents.Metformin is recommended to be temporarily discontinued before any radiographic study involving iodinated contrast agents, (such as a contrast-enhanced CT scan or angiogram), as the contrast dye may temporarily impair kidney function, indirectly leading to lactic acidosis by causing retention of metformin in the body. Metformin can be resumed after two days, assuming kidney function is normal.
Adverse effects
The most common adverse effect of metformin is gastrointestinal irritation, including diarrhea, cramps, nausea, vomiting, and increased flatulence; metformin is more commonly associated with gastrointestinal side effects than most other antidiabetic medications. The most serious potential side effect of metformin use is lactic acidosis; this complication is very rare, and the vast majority of these cases seem to be related to comorbid conditions, such as impaired liver or kidney function, rather than to the metformin itself.Metformin has also been reported to decrease the blood levels of thyroid-stimulating hormone in people with hypothyroidism. The significance of this is still unknown.
Gastrointestinal
In a clinical trial of 286 subjects, 53.2% of the 141 given immediate-release metformin (as opposed to placebo) reported diarrhea, versus 11.7% for placebo, and 25.5% reported nausea/vomiting, versus 8.3% for those on placebo.Gastrointestinal upset can cause severe discomfort; it is most common when metformin is first administered, or when the dose is increased. The discomfort can often be avoided by beginning at a low dose (1.0 to 1.7 grams per day) and increasing the dose gradually but even with low doses 5% of people may be unable to tolerate metformin. Use of slow- or extended-release preparations may improve tolerability.
Long-term use of metformin has been associated with increased homocysteine levels and malabsorption of vitamin B12. Higher doses and prolonged use are associated with increased incidence of vitamin B12 deficiency, and some researchers recommend screening or prevention strategies.
Lactic acidosis
The most serious potential adverse effect of biguanide use is metformin-associated lactic acidosis (MALA). Though the incidence for MALA is about nine per 100,000 person-years, this is similar to the background incidence of lactic acidosis in the general population. A systematic review concluded no data exists to definitively link metformin to lactic acidosis. Lactic acidosis can be fatal.Phenformin, another biguanide, was withdrawn from the market because of an increased risk of lactic acidosis (rate of 40-64 per 100,000 patient-years). However, metformin is safer than phenformin, and the risk of developing lactic acidosis is not increased except for known high-risk groups.
Lactate uptake by the liver is diminished with metformin administration because lactate is a substrate for hepatic gluconeogenesis, a process that metformin inhibits. In healthy individuals, this slight excess is cleared by other mechanisms (including uptake by unimpaired kidneys), and no significant elevation in blood levels of lactate occurs. Given impaired kidney function, clearance of metformin and lactate is reduced, increasing levels of both, and possibly causing lactic acid buildup. Because metformin decreases liver uptake of lactate, any condition that may precipitate lactic acidosis is a contraindication. Common causes include alcoholism (due to depletion of NAD+ stores), heart failure and respiratory disease (due to inadequate tissue oxygenation); the most common cause is kidney disease.
Metformin has been suggested as increasing production of lactate in the large intestine, which could potentially contribute to lactic acidosis in those with risk factors. However, the clinical significance of this is unknown, and the risk of metformin-associated lactic acidosis is most commonly attributed to decreased hepatic uptake rather than increased intestinal production.
Lactic acidosis is initially treated with sodium bicarbonate, although high doses are not recommended, as this may increase intracellular acidosis. Acidosis that does not respond to administration of sodium bicarbonate may require further management with standard hemodialysis or continuous venovenous hemofiltration.
Overdose
A review of metformin overdoses reported to poison control centers over a five-year period found serious adverse events were rare, though the elderly appeared to be at greater risk. A similar study in which cases were reported to Texas poison control centers between 2000 and 2006 found ingested doses of more than 5,000 mg were more likely to involve serious medical outcomes in adults. Survival following intentional overdoses with up to 63,000 mg (63 g) of metformin have been reported. Fatalities following overdose are rare. In healthy children, unintentional doses of less than 1,700 mg are unlikely to cause significant toxic effects.The most common symptoms following overdose include vomiting, diarrhea, abdominal pain, tachycardia, drowsiness, and, rarely, hypoglycemia or hyperglycemia. Treatment of metformin overdose is generally supportive, as no specific antidote is known. Extracorporeal treatments are recommended in severe overdoses. Due to metformin's low molecular weight and lack of plasma protein binding, these techniques have the benefit of removing metformin from blood plasma, preventing further lactate overproduction.
Metformin may be quantified in blood, plasma, or serum to monitor therapy, confirm a diagnosis of poisoning, or assist in a forensic death investigation. Blood or plasma metformin concentrations are usually in a range of 1–4 mg/l in persons receiving therapeutic doses, 40–120 mg/l in victims of acute overdosage, and 80–200 mg/l in fatalities. Chromatographic techniques are commonly employed.
Interactions
The H2-receptor antagonist cimetidine causes an increase in the plasma concentration of metformin by reducing clearance of metformin by the kidneys; both metformin and cimetidine are cleared from the body by tubular secretion, and both, particularly the cationic (positively charged) form of cimetidine, may compete for the same transport mechanism. A small double-blind, randomized study found the antibiotic cephalexin to also increase metformin concentrations by a similar mechanism; theoretically, other cationic medications may produce the same effect.Metformin also interacts with anticholinergic medications, due to their effect on gastric motility. Anticholinergic drugs reduce gastric motility, prolonging the time drugs spend in the gastrointestinal tract. This impairment may lead to more metformin being absorbed than without the presence of an anticholinergic drug, thereby increasing the concentration of metformin in the plasma and increasing the risk for adverse effects.
Mechanism of action
Metformin's main effect is to decrease liver glucose production. It also has an insulin-sensitizing effect with multiple actions on tissues including the liver, skeletal muscle, endothelium, adipose tissue, and the ovary.Metformin decreases high blood sugar, primarily by suppressing liver glucose production (hepatic gluconeogenesis). The average patient with type 2 diabetes has three times the normal rate of gluconeogenesis; metformin treatment reduces this by over one-third. The molecular mechanism of metformin is incompletely understood. Multiple potential mechanisms of action have been proposed, including; inhibition of the mitochondrial respiratory chain (complex I), activation of AMP-activated protein kinase (AMPK), inhibition of glucagon-induced elevation of cyclic adenosine monophosphate (cAMP) with reduced activation of protein kinase A (PKA), inhibition of mitochondrial glycerophosphate dehydrogenase, and an effect on gut microbiota.
Activation of AMPK was required for metformin's inhibitory effect on liver glucose production. AMPK is an enzyme that plays an important role in insulin signaling, whole body energy balance and the metabolism of glucose and fats. AMPK Activation was required for an increase in the expression of small heterodimer partner, which in turn inhibited the expression of the hepatic gluconeogenic genes phosphoenolpyruvate carboxykinase and glucose 6-phosphatase. Metformin is frequently used in research along with AICA ribonucleotide as an AMPK agonist. Mouse models in which the genes for AMPKα1 and α2 catalytic subunits (Prkaa1/2) or LKB1, an upstream kinase of AMPK, had been knocked out in hepatocytes, have raised doubts over the role of AMPK, since the effect of metformin was not abolished by loss of AMPK function. The mechanism by which biguanides increase the activity of AMPK remains uncertain; however, metformin increases the concentration of cytosolic adenosine monophosphate (AMP) (as opposed to a change in total AMP or total AMP/adenosine triphosphate). Increased cellular AMP has been proposed to explain the inhibition of glucagon-induced increase in cAMP and activation of PKA. Metformin and other biguanides may antagonize the action of glucagon, thus reducing fasting glucose levels. Metformin also induces a profound shift in the faecal microbial community profile in diabetic mice and this may contribute to its mode of action possibly through an effect on glucagon-like peptide-1 secretion.
In addition to suppressing hepatic glucose production, metformin increases insulin sensitivity, enhances peripheral glucose uptake (by inducing the phosphorylation of GLUT4 enhancer factor), decreases insulin-induced suppression of fatty acid oxidation, and decreases absorption of glucose from the gastrointestinal tract. Increased peripheral use of glucose may be due to improved insulin binding to insulin receptors. The increase in insulin binding after metformin treatment has also been demonstrated in patients with NIDDM.
AMPK probably also plays a role in increased peripheral insulin sensitivity, as metformin administration increases AMPK activity in skeletal muscle. AMPK is known to cause GLUT4 deployment to the plasma membrane, resulting in insulin-independent glucose uptake. Some metabolic actions of metformin do appear to occur by AMPK-independent mechanisms; the metabolic actions of metformin in the heart muscle can occur independent of changes in AMPK activity and may be mediated by p38 MAPK- and PKC-dependent mechanisms.
Chemistry
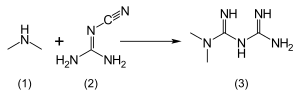
The usual synthesis of metformin, originally described in 1922, involves the one-pot reaction of dimethylamine hydrochloride and 2-cyanoguanidine over heat.According to the procedure described in the 1975 Aron patent, and the Pharmaceutical Manufacturing Encyclopedia, equimolar amounts of dimethylamine and 2-cyanoguanidine are dissolved in toluene with cooling to make a concentrated solution, and an equimolar amount of hydrogen chloride is slowly added. The mixture begins to boil on its own, and after cooling, metformin hydrochloride precipitates with a 96% yield.
Pharmacokinetics
Metformin has an oral bioavailability of 50–60% under fasting conditions, and is absorbed slowly. Peak plasma concentrations (Cmax) are reached within one to three hours of taking immediate-release metformin and four to eight hours with extended-release formulations. The plasma protein binding of metformin is negligible, as reflected by its very high apparent volume of distribution (300–1000 l after a single dose). Steady state is usually reached in one or two days.Metformin has acid dissociation constant values (pKa) of 2.8 and 11.5, so exists very largely as the hydrophilic cationic species at physiological pH values. The metformin pKa values make metformin a stronger base than most other basic medications with less than 0.01% nonionized in blood. Furthermore, the lipid solubility of the nonionized species is slight as shown by its low logP value (log(10) of the distribution coefficient of the nonionized form between octanol and water) of -1.43. These chemical parameters indicate low lipophilicity and, consequently, rapid passive diffusion of metformin through cell membranes is unlikely. As a result of its low lipid solubility it requires the transporter SLC22A1 in order for it to enter cells. The logP of metformin is less than that of phenformin (-0.84) because two methyl substituents on metformin impart lesser lipophilicity than the larger phenylethyl side chain in phenformin. More lipophilic derivatives of metformin are presently under investigation with the aim of producing prodrugs with superior oral absorption than metformin.
Metformin is not metabolized. It is cleared from the body by tubular secretion and excreted unchanged in the urine; metformin is undetectable in blood plasma within 24 hours of a single oral dose. The average elimination half-life in plasma is 6.2 hours. Metformin is distributed to (and appears to accumulate in) red blood cells, with a much longer elimination half-life: 17.6 hours (reported as ranging from 18.5 to 31.5 hours in a single-dose study of nondiabetics).
History
The biguanide class of antidiabetic medications, which also includes the withdrawn agents phenformin and buformin, originates from the French lilac or goat's rue (Galega officinalis), a plant used in folk medicine for several centuries.
Galega officinalis, a natural source of galegine
Metformin was first described in the scientific literature in 1922, by Emil Werner and James Bell, as a product in the synthesis of N,N-dimethylguanidine. In 1929, Slotta and Tschesche discovered its sugar-lowering action in rabbits, finding it the most potent biguanide analog they studied. This result was completely forgotten, as other guanidine analogs, such as the synthalins, took over and were themselves soon overshadowed by insulin.
Interest in metformin resumed at the end of the 1940s. In 1950, metformin, unlike some other similar compounds, was found not to decrease blood pressure and heart rate in animals. That year, Filipino physician Eusebio Y. Garcia used metformin (he named it Fluamine) to treat influenza; he noted the medication "lowered the blood sugar to minimum physiological limit" and was not toxic. Garcia believed metformin to have bacteriostatic, antiviral, antimalarial, antipyretic and analgesic actions. In a series of articles in 1954, Polish pharmacologist Janusz Supniewski was unable to confirm most of these effects, including lowered blood sugar. Instead he observed antiviral effects in humans.
French diabetologist Jean Sterne studied the antihyperglycemic properties of galegine, an alkaloid isolated from Galega officinalis, which is related in structure to metformin and had seen brief use as an antidiabetic before the synthalins were developed. Later, working at Laboratoires Aron in Paris, he was prompted by Garcia's report to reinvestigate the blood sugar-lowering activity of metformin and several biguanide analogs. Sterne was the first to try metformin on humans for the treatment of diabetes; he coined the name "Glucophage" (glucose eater) for the medication and published his results in 1957.
Metformin became available in the British National Formulary in 1958. It was sold in the UK by a small Aron subsidiary called Rona.
Broad interest in metformin was not rekindled until the withdrawal of the other biguanides in the 1970s. Metformin was approved in Canada in 1972, but did not receive approval by the U.S. Food and Drug Administration (FDA) for type 2 diabetes until 1994. Produced under license by Bristol-Myers Squibb, Glucophage was the first branded formulation of metformin to be marketed in the U.S., beginning on March 3, 1995. Generic formulations are now available in several countries, and metformin is believed to have become the world's most widely prescribed antidiabetic medication.
Formulations
Generic metformin 500-mg tablets, as sold in the United Kingdom
The name "Metformin" is the BAN, USAN and INN for the medication. It is sold under several trade names, including Glucophage XR, Carbophage SR, Riomet, Fortamet, Glumetza, Obimet, Gluformin, Dianben, Diabex, Diaformin, Siofor, Metfogamma and Glifor.
Liquid metformin is sold under the name Riomet in India. Each 5 ml of Riomet is equivalent to the 500-mg tablet form.
Metformin IR (immediate release) is available in 500, 850, and 1000-mg tablets. All of these are available as generic medications in the U.S.
Metformin SR (slow release) or XR (extended release) was introduced in 2004. It is available in 500, 750, and 1000-mg strengths, mainly to counteract common gastrointestinal side effects, as well as to increase compliance by reducing pill burden. No difference in effectiveness exists between the two preparations.
Combination with other medications
When used for type 2 diabetes, metformin is often prescribed in combination with other medications.Several are available as fixed-dose combinations, to reduce pill burden and simplify administration.
Thiazolidinediones (glitazones)
Rosiglitazone
A combination of metformin and rosiglitazone was released in 2002 and sold as Avandamet by GlaxoSmithKline.By 2009 it had become the most popular metformin combination.
In 2005, the stock of Avandamet was removed from the market, after inspections showed the factory where it was produced was violating good manufacturing practices. The medication pair continued to be prescribed separately and Avandamet was again available by the end of that year. A generic formulation of metformin/rosiglitazone from Teva received tentative approval from the FDA and reached the market in early 2012.
However, following a meta-analysis in 2007 that linked the medication's use to an increased risk of heart attack, concerns were raised over the safety of medicines containing rosiglitazone. In September 2010 the European Medicines Agency (EMA) recommended that the medication be suspended from the European market because the benefits of rosiglitazone no longer outweighed the risks.
It was withdrawn from the market in the UK and India in 2010, and in New Zealand and South Africa in 2011. From November 2011 until November 2013 the FDA did not allow rosiglitazone or metformin/rosiglitazone to be sold without a prescription; moreover, makers were required to notify patients of the risks associated with its use, and the drug had to be purchased by mail order through specified pharmacies.
In November 2013, the FDA lifted its earlier restrictions on rosiglitazone after reviewing the results of the 2009 RECORD clinical trial (a six-year, open label randomized control trial), which failed to show elevated risk of heart attack or death associated with the medication.
Pioglitazone
The combination of metformin and pioglitazone (Actoplus Met, Piomet, Politor) remains available in U.S. and Europe.DPP-4 inhibitors
Dipeptidyl peptidase-4 inhibitors inhibit dipeptidyl peptidase-4 and thus reduce glucagon and blood glucose levels.DPP-4 inhibitors combined with metformin include a sitagliptin/metformin combination and a saxagliptin combination (Komboglyze), and with alogliptin as Kazano among others.
In Europe, Canada, and elsewhere metformin combined with linagliptin is marketed under the trade name Jentadueto.
Sulfonylureas
Sulfonylureas act by increasing insulin release from the beta cells in the pancreas. Metformin is available combined with the sulfonylureas glipizide (Metaglip) and glibenclamide (US: glyburide) (Glucovance).Generic formulations of metformin/glipizide and metformin/glibenclamide are available (the latter is more popular).
Meglitinide
Meglitinides are similar to sulfonylureas.A repaglinide/metformin combination is sold as Prandimet.
Triple combination
The combination of metformin with pioglitazone and glibenclamide is available in India as Triformin.Research
Metformin has been studied for its effects on multiple other conditions, including:- Non-alcoholic fatty liver disease.
- Premature puberty.
- Cancer.
- Cardiovascular disease in people with diabetes.
- Aging (C. elegans and crickets). A 2017 review found that people with diabetes who were taking metformin had lower all-cause mortality. They also had reduced cancer and cardiovascular disease compared with those on other therapies. As of 2016 it is being studied to see what effect it may have on aging.