From Wikipedia, the free encyclopedia
The two parts of a redox reaction
Rust, a slow redox reaction
A
bonfire; combustion is a fast redox reaction
Demonstration of the reaction between a strong oxidising and a reducing agent. When few drops of
glycerol (reducing agent) are added to powdered
potassium permanganate (strong oxidising agent), a vigorous reaction accompanied by self-ignition starts.
Redox (short for
reduction–
oxidation reaction) is a
chemical reaction in which the
oxidation states
of atoms are changed. Any such reaction involves both a reduction
process and a complementary oxidation process, two key concepts involved
with
electron transfer processes.
[1]
Redox reactions include all chemical reactions in which atoms have
their oxidation state changed; in general, redox reactions involve the
transfer of
electrons between
chemical species.
The chemical species from which the electron is stripped is said to
have been oxidized, while the chemical species to which the electron is
added is said to have been reduced. It can be explained in simple terms:
- Oxidation is the loss of electrons or an increase in oxidation state by a molecule, atom, or ion.
- Reduction is the gain of electrons or a decrease in oxidation state by a molecule, atom, or ion.
As an example, during the
combustion of wood, oxygen from the air is reduced, gaining electrons from the carbon.
[2]
Although oxidation reactions are commonly associated with the formation
of oxides from oxygen molecules, oxygen is not necessarily included in
such reactions, as other chemical species can serve the same function.
[2]
The reaction can occur relatively slowly, as in the case of
rust, or more quickly, as in the case of
fire. There are simple redox processes, such as the oxidation of
carbon to yield
carbon dioxide (CO
2) or the reduction of carbon by
hydrogen to yield
methane (CH
4), and more complex processes such as the oxidation of
glucose (C
6H
12O
6) in the
human body.
Etymology
"Redox" is a portmanteau of "reduction" and "oxidation".
The word
oxidation originally implied reaction with oxygen to form an oxide, since
dioxygen (O
2
(g)) was historically the first recognized oxidizing agent. Later, the
term was expanded to encompass oxygen-like substances that accomplished
parallel chemical reactions. Ultimately, the meaning was generalized to
include all processes involving loss of electrons.
The word
reduction originally referred to the loss in weight upon heating a metallic
ore such as a
metal oxide to extract the metal. In other words, ore was "reduced" to metal.
Antoine Lavoisier
(1743–1794) showed that this loss of weight was due to the loss of
oxygen as a gas. Later, scientists realized that the metal atom gains
electrons in this process. The meaning of
reduction then became
generalized to include all processes involving gain of electrons. Even
though "reduction" seems counter-intuitive when speaking of the
gain
of electrons, it might help to think of reduction as the loss of
oxygen, which was its historical meaning. Since electrons are negatively
charged, it is also helpful to think of this as reduction in electrical
charge.
The electrochemist
John Bockris has used the words
electronation and
deelectronation to describe reduction and oxidation processes respectively when they occur at
electrodes.
[3] These words are analogous to
protonation and
deprotonation, but they have not been widely adopted by chemists.
The term "hydrogenation" could be used instead of reduction, since
hydrogen is the reducing agent in a large number of reactions,
especially in organic chemistry and biochemistry. But, unlike oxidation,
which has been generalized beyond its root element, hydrogenation has
maintained its specific connection to reactions that
add hydrogen to another substance (e.g., the hydrogenation of unsaturated fats into saturated fats, R−CH=CH−R + H
2 → R−CH
2−CH
2−R). The word "redox" was first used in 1928.
[4]
Definitions
The processes of oxidation and reduction occur simultaneously and cannot happen independently of one another, similar to the
acid–base reaction.
[2] The oxidation alone and the reduction alone are each called a
half-reaction,
because two half-reactions always occur together to form a whole
reaction. When writing half-reactions, the gained or lost electrons are
typically included explicitly in order that the
half-reaction be balanced with respect to electric charge.
Though sufficient for many purposes, these general descriptions are
not precisely correct. Although oxidation and reduction properly refer
to
a change in oxidation state
— the actual transfer of electrons may never occur. The oxidation state
of an atom is the fictitious charge that an atom would have if all
bonds between atoms of different elements were 100% ionic. Thus,
oxidation is best defined as an
increase in oxidation state, and reduction as a
decrease in oxidation state.
In practice, the transfer of electrons will always cause a change in
oxidation state, but there are many reactions that are classed as
"redox" even though no electron transfer occurs (such as those involving
covalent bonds).
Oxidizing and reducing agents
In redox processes, the reductant transfers electrons to the oxidant. Thus, in the reaction, the reductant or
reducing agent loses electrons and is oxidized, and the oxidant or
oxidizing agent
gains electrons and is reduced. The pair of an oxidizing and reducing
agent that are involved in a particular reaction is called a
redox pair. A
redox couple is a reducing species and its corresponding oxidizing form, e.g., Fe
2+/Fe
3+.
Oxidizers
Substances that have the ability to
oxidize other substances (cause them to lose electrons) are said to be
oxidative or
oxidizing and are known as
oxidizing agents,
oxidants, or oxidizers. That is, the oxidant (oxidizing agent) removes
electrons from another substance, and is thus itself reduced. And,
because it "accepts" electrons, the oxidizing agent is also called an
electron acceptor.
Oxygen is the quintessential oxidizer.
Oxidants are usually chemical substances with elements in high oxidation states (e.g.,
H
2O
2,
MnO−
4,
CrO
3,
Cr
2O2−
7,
OsO
4), or else highly
electronegative elements (
O2,
F2,
Cl2,
Br2) that can gain extra electrons by oxidizing another substance.
Reducers
Substances that have the ability to
reduce other substances (cause them to gain electrons) are said to be
reductive or
reducing and are known as
reducing agents,
reductants, or reducers. The reductant (reducing agent) transfers
electrons to another substance, and is thus itself oxidized. And,
because it "donates" electrons, the reducing agent is also called an
electron donor. Electron donors can also form
charge transfer complexes with electron acceptors.
Reductants in chemistry are very diverse.
Electropositive elemental
metals, such as
lithium,
sodium,
magnesium,
iron,
zinc, and
aluminium, are good reducing agents. These metals donate or
give away electrons readily.
Hydride transfer reagents, such as
NaBH4 and
LiAlH4, are widely used in
organic chemistry,
[5][6] primarily in the reduction of
carbonyl compounds to
alcohols. Another method of reduction involves the use of hydrogen gas (H
2) with a
palladium,
platinum, or
nickel catalyst. These
catalytic reductions are used primarily in the reduction of carbon-carbon double or triple bonds.
Standard electrode potentials (reduction potentials)
Each half-reaction has a
standard electrode potential (
E0
cell), which is equal to the potential difference or
voltage at equilibrium under
standard conditions of an
electrochemical cell in which the
cathode reaction is the
half-reaction considered, and the
anode is a
standard hydrogen electrode where hydrogen is oxidized:
- 1⁄2 H2 → H+ + e−.
The electrode potential of each half-reaction is also known as its
reduction potential E0
red, or potential when the half-reaction takes place at a
cathode. The reduction potential is a measure of the tendency of the
oxidizing agent to be reduced. Its value is zero for H
+ + e
− →
1⁄2 H
2 by definition, positive for oxidizing agents stronger than H
+ (e.g., +2.866 V for F
2) and negative for oxidizing agents that are weaker than H
+ (e.g., −0.763 V for Zn
2+).
[7]
For a redox reaction that takes place in a cell, the potential difference is:
- E0
cell = E0
cathode – E0
anode
However, the potential of the reaction at the anode was sometimes expressed as an
oxidation potential:
- E0
ox = –E0
red.
The oxidation potential is a measure of the tendency of the reducing
agent to be oxidized, but does not represent the physical potential at
an electrode. With this notation, the cell voltage equation is written
with a plus sign
- E0
cell = E0
red(cathode) + E0
ox(anode)
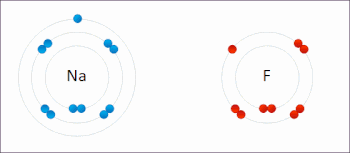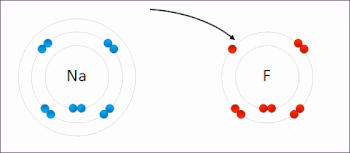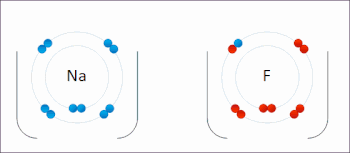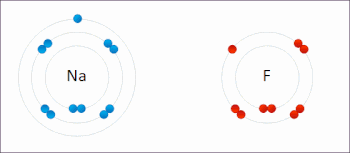
Examples of redox reactions
Illustration of a redox reaction
A good example is the reaction between
hydrogen and
fluorine in which hydrogen is being oxidized and fluorine is being reduced:
- H
2 + F
2 → 2 HF
We can write this overall reaction as two
half-reactions:
the oxidation reaction:
- H
2 → 2 H+ + 2 e−
and the reduction reaction:
- F
2 + 2 e− → 2 F−
Analyzing each half-reaction in isolation can often make the overall
chemical process clearer. Because there is no net change in charge
during a redox reaction, the number of electrons in excess in the
oxidation reaction must equal to the number consumed by the reduction
reaction (as shown above).
Elements, even in molecular form, always have an oxidation state of
zero. In the first half-reaction, hydrogen is oxidized from an oxidation
state of zero to an oxidation state of +1. In the second half-reaction,
fluorine is reduced from an oxidation state of zero to an oxidation
state of −1.
When adding the reactions together the electrons are canceled:
-
H
2 |
→ |
2 H+ + 2 e− |
F
2 + 2 e− |
→ |
2 F− |
|
| H2 + F2 |
→ |
2 H+ + 2 F− |
And the ions combine to form
hydrogen fluoride:
- 2 H+ + 2 F− → 2 HF
The overall reaction is:
- H
2 + F
2 → 2 HF
Metal displacement
A redox reaction is the force behind an
electrochemical cell like the
Galvanic cell pictured. The battery is made out of a zinc electrode in a ZnSO
4 solution connected with a wire and a porous disk to a copper electrode in a CuSO
4 solution.
In this type of reaction, a metal atom in a compound (or in a solution) is replaced by an atom of another metal. For example,
copper is deposited when
zinc metal is placed in a
copper(II) sulfate solution:
Zn(s)+ CuSO
4(aq) → ZnSO
4(aq) + Cu(s)
In the above reaction, zinc metal displaces the copper(II) ion from
copper sulfate solution and thus liberates free copper metal.
The ionic equation for this reaction is:
- Zn + Cu2+ → Zn2+ + Cu
As two
half-reactions, it is seen that the zinc is oxidized:
- Zn → Zn2+ + 2 e−
And the copper is reduced:
- Cu2+ + 2 e− → Cu
Other examples
- The reduction of nitrate to nitrogen in the presence of an acid (denitrification):
- 2 NO−
3 + 10 e− + 12 H+ → N2 + 6 H2O
- The combustion of hydrocarbons, such as in an internal combustion engine, which produces water, carbon dioxide, some partially oxidized forms such as carbon monoxide, and heat energy. Complete oxidation of materials containing carbon produces carbon dioxide.
- In organic chemistry, the stepwise oxidation of a hydrocarbon by oxygen produces water and, successively, an alcohol, an aldehyde or a ketone, a carboxylic acid, and then a peroxide.
Corrosion and rusting
- The term corrosion refers to the electrochemical oxidation of metals in reaction with an oxidant such as oxygen. Rusting, the formation of iron oxides, is a well-known example of electrochemical corrosion; it forms as a result of the oxidation of iron metal. Common rust often refers to iron(III) oxide, formed in the following chemical reaction:
- 4 Fe + 3 O2 → 2 Fe2O3
- The oxidation of iron(II) to iron(III) by hydrogen peroxide in the presence of an acid:
- Fe2+ → Fe3+ + e−
- H2O2 + 2 e− → 2 OH−
- Overall equation:
- 2 Fe2+ + H2O2 + 2 H+ → 2 Fe3+ + 2 H2O
Redox reactions in industry
Cathodic protection
is a technique used to control the corrosion of a metal surface by
making it the cathode of an electrochemical cell. A simple method of
protection connects protected metal to a more easily corroded "
sacrificial anode"
to act as the anode. The sacrificial metal instead of the protected
metal, then, corrodes. A common application of cathodic protection is in
galvanized steel, in which a sacrificial coating of zinc on steel parts protects them from rust.
The primary process of reducing ore at high temperature to produce
metals is known as
smelting.
Oxidation is used in a wide variety of industries such as in the production of
cleaning products and oxidizing
ammonia to produce
nitric acid, which is used in most
fertilizers.
Redox reactions are the foundation of
electrochemical cells, which can generate electrical energy or support
electrosynthesis.
The process of
electroplating uses redox reactions to coat objects with a thin layer of a material, as in
chrome-plated automotive parts,
silver plating cutlery, and
gold-plated jewelry.
The production of
compact discs depends on a redox reaction, which coats the disc with a thin layer of metal film.
[clarification needed]
Redox reactions in biology
Many important
biological processes involve redox reactions.
Cellular respiration, for instance, is the oxidation of
glucose (C
6H
12O
6) to
CO2 and the reduction of
oxygen to
water. The summary equation for cell respiration is:
- C6H12O6 + 6 O2 → 6 CO2 + 6 H2O
The process of cell respiration also depends heavily on the reduction of
NAD+ to NADH and the reverse reaction (the oxidation of NADH to NAD
+).
Photosynthesis and cellular respiration are complementary, but photosynthesis is not the reverse of the redox reaction in cell respiration:
- 6 CO2 + 6 H2O + light energy → C6H12O6 + 6 O2
Biological energy is frequently stored and released by means of redox reactions. Photosynthesis involves the reduction of
carbon dioxide into
sugars and the oxidation of
water
into molecular oxygen. The reverse reaction, respiration, oxidizes
sugars to produce carbon dioxide and water. As intermediate steps, the
reduced carbon compounds are used to reduce
nicotinamide adenine dinucleotide (NAD
+), which then contributes to the creation of a
proton gradient, which drives the synthesis of
adenosine triphosphate (ATP) and is maintained by the reduction of oxygen. In animal cells,
mitochondria perform similar functions. See the
Membrane potential article.
Free radical reactions are redox reactions that occur as a part of
homeostasis
and killing microorganisms, where an electron detaches from a molecule
and then reattaches almost instantaneously. Free radicals are a part of
redox molecules and can become harmful to the human body if they do not
reattach to the redox molecule or an
antioxidant. Unsatisfied free radicals can spur the mutation of cells they encounter and are, thus, causes of cancer.
The term
redox state is often used to describe the balance of
GSH/GSSG, NAD
+/NADH and
NADP+/NADPH
in a biological system such as a cell or organ. The redox state is
reflected in the balance of several sets of metabolites (e.g.,
lactate and
pyruvate,
beta-hydroxybutyrate, and
acetoacetate),
whose interconversion is dependent on these ratios. An abnormal redox
state can develop in a variety of deleterious situations, such as
hypoxia,
shock, and
sepsis.
Redox mechanism also control some cellular processes. Redox proteins
and their genes must be co-located for redox regulation according to the
CoRR hypothesis for the function of DNA in mitochondria and chloroplasts.
Redox cycling
A wide variety of
aromatic compounds are
enzymatically reduced to form
free radicals
that contain one more electron than their parent compounds. In general,
the electron donor is any of a wide variety of flavoenzymes and their
coenzymes. Once formed, these anion free radicals reduce molecular oxygen to
superoxide,
and regenerate the unchanged parent compound. The net reaction is the
oxidation of the flavoenzyme's coenzymes and the reduction of molecular
oxygen to form superoxide. This catalytic behavior has been described as
futile cycle or redox cycling.
Examples of redox cycling-inducing molecules are the
herbicide paraquat and other
viologens and
quinones such as
menadione.
[8]
Redox reactions in geology
In
geology, redox is important to both the formation of minerals and the mobilization of minerals, and is also important in some
depositional environments.
In general, the redox state of most rocks can be seen in the color of
the rock. The rock forms in oxidizing conditions, giving it a red color.
It is then "bleached" to a green—or sometimes white—form when a
reducing fluid passes through the rock. The reduced fluid can also carry
uranium-bearing
minerals. Famous examples of redox conditions affecting geological processes include
uranium deposits and
Moqui marbles.
Balancing redox reactions
Describing the overall electrochemical reaction for a redox process requires a
balancing of the component
half-reactions for oxidation and reduction. In general, for reactions in aqueous solution, this involves adding
H+,
OH−,
H2O, and electrons to compensate for the oxidation changes.
Acidic media
In acidic media, H
+ ions and water are added to half-reactions to balance the overall reaction.
For instance, when
manganese(II) reacts with
sodium bismuthate:
-
| Unbalanced reaction: |
Mn2+(aq) + NaBiO3(s) → Bi3+(aq) + MnO−
4 (aq) |
| Oxidation: |
4 H2O(l) + Mn2+(aq) → MnO−
4(aq) + 8 H+(aq) + 5 e− |
| Reduction: |
2 e− + 6 H+ + BiO−
3(s) → Bi3+(aq) + 3 H2O(l) |
The reaction is balanced by scaling the two half-cell reactions to
involve the same number of electrons (multiplying the oxidation reaction
by the number of electrons in the reduction step and vice versa):
- 8 H2O(l) + 2 Mn2+(aq) → 2 MnO−
4(aq) + 16 H+(aq) + 10 e− - 10 e− + 30 H+ + 5 BiO−
3(s) → 5 Bi3+(aq) + 15 H2O(l)
Adding these two reactions eliminates the electrons terms and yields the balanced reaction:
- 14 H+(aq) + 2 Mn2+(aq) + 5 NaBiO3(s) → 7 H2O(l) + 2 MnO−
4(aq) + 5 Bi3+(aq) + 5 Na+(aq)
Basic media
In basic media,
OH− ions and water are added to half reactions to balance the overall reaction.
For example, in the reaction between
potassium permanganate and
sodium sulfite:
-
| Unbalanced reaction: |
KMnO4 + Na2SO3 + H2O → MnO2 + Na2SO4 + KOH |
| Reduction: |
3 e− + 2 H2O + MnO−
4 → MnO2 + 4 OH− |
| Oxidation: |
2 OH− + SO2−
3 → SO2−
4 + H2O + 2 e− |
Balancing the number of electrons in the two half-cell reactions gives:
- 6 e− + 4 H2O + 2 MnO−
4 → 2 MnO2 + 8 OH− - 6 OH− + 3 SO2−
3 → 3 SO2−
4 + 3 H2O + 6 e−
Adding these two half-cell reactions together gives the balanced equation:
- 2 KMnO4 + 3 Na2SO3 + H2O → 2 MnO2 + 3 Na2SO4 + 2 KOH
Memory aids
The key terms involved in redox are often confusing to students.
[9][10]
For example, an element that is oxidized loses electrons; however, that
element is referred to as the reducing agent. Likewise, an element that
is reduced gains electrons and is referred to as the oxidizing agent.
[11] Acronyms or mnemonics are commonly used
[12] to help remember the terminology:
- "OIL RIG" — oxidation is loss of electrons, reduction is gain of electrons.[9][10][11][12]
- "LEO the lion says GER" — loss of electrons is oxidation, gain of electrons is reduction.[9][10][11][12]
- "LEORA says GEROA" — loss of electrons is oxidation (reducing agent), gain of electrons is reduction (oxidizing agent).[11]
- "RED CAT" and "AN OX", or "AnOx RedCat" ("an ox-red cat") — reduction occurs at the cathode and the anode is for oxidation.
- "RED CAT gains what AN OX loses" – reduction at the cathode gains (electrons) what anode oxidation loses (electrons).