From Wikipedia, the free encyclopedia
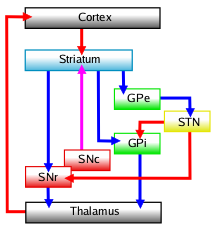
Diagram of the main components of the basal ganglia and their interconnections
The
basal ganglia
form a major brain system in all species of vertebrates, but in
primates (including humans) there are special features that justify a
separate consideration. As in other vertebrates, the
primate basal ganglia can be divided into
striatal,
pallidal,
nigral, and
subthalamic components. In primates, however, there are two pallidal subdivisions called the
external globus pallidus (GPe) and
internal globus pallidus (GPi). Also in primates, the dorsal striatum is divided by a large
tract called the
internal capsule into two masses named the
caudate nucleus and the
putamen—in
most other species no such division exists, and only the striatum as a
whole is recognized. Beyond this, there is a complex circuitry of
connections between the striatum and cortex that is specific to
primates. This complexity reflects the difference in functioning of
different cortical areas in the primate brain.
Functional imaging studies have been performed mainly using human subjects. Also, several major
degenerative diseases of the basal ganglia, including
Parkinson's disease and
Huntington's disease, are specific to humans, although "models" of them have been proposed for other species.
Corticostriatal connection
A
major output from the cortex, with axons from most of the cortical
regions connecting to the striatum, is called the corticostriatal
connection, part of the
cortico-basal ganglia-thalamo-cortical loop.
In the primate most of these axons are thin and unbranched. The
striatum does not receive axons from the primary olfactory, visual or
auditory cortices. The corticostriatal connection is an excitatory
glutamatergic pathway. One small cortical site can project many axon branches to several parts of the striatum.
Striatum
The
striatum is the largest structure of the basal ganglia.
Structure
Neuronal constitution
Medium spiny neurons
(MSN)s, account for up to 95 per cent of the striatal neurons. There
are two populations of these projection neurons, MSN1 and MSN2, both of
which are inhibitory
GABAergic. There are also various groups of GABAergic interneurons and a single
group of cholinergic interneurons. These few types are responsible for
the reception, processing, and relaying of all the cortical input.
Most of the
dendritic spines on the medium spiny neurons synapse with cortical afferents and their axons project numerous collaterals to other neurons. The
cholinergic interneurons of the primate, are very different from those of non-primates. These are said to be
tonically active.
The dorsal striatum and the ventral striatum have different
populations of the cholinergic interneurons showing a marked difference
in shape.
Physiology
Unless stimulated by cortical input the striatal neurons are usually inactive.
Levels of organisation
The
striatum is one mass of grey matter that has two different parts, a
ventral and a dorsal part. The dorsal striatum contains the caudate
nucleus and the putamen, and the ventral striatum contains the
nucleus accumbens and the
olfactory tubercle. The
internal capsule is seen as dividing the two parts of the dorsal striatum.
Sensorimotor input is mostly to the putamen. An
associative input goes to the caudate nucleus and possibly to the nucleus accumbens.
There are two different components of the striatum differentiated by
staining –
striosomes and a matrix. Striosomes are located in the matrix of the striatum and these contain
μ-opioid receptors and
dopamine receptor D1 binding sites.
The
striatopallidal fibers give a connection from the putamen to the
globus pallidus and substantia nigra.
Connectomics
Unlike
the inhibitory GABAergic neurons in the neocortex that only send local
connections, in the striatum these neurons send long axons to targets in
the
pallidum and substantia nigra. A study in
macaques showed that the medium spiny neurons have several targets.
Most striatal axons first target the GPe, some of these also target the
GPi and both parts of the substantia nigra. There are no single axon
projections to either the GPi, or to the SN, or to both of these areas;
only connecting as continuing targets via axon collaterals from the
striatum to the GPe.
The only difference between the axonal
connectomes
of the striosomes and the axons of those neurons in the matrix, is in
the numbers of their branching axons. Striosomal axons cross the extent
of the SN, and in macaques emit 4 to 6 vertical collaterals that form
vertical columns which enter deep into the SN pars compacta (SNpc); the
axons from those in the matrix are more sparsely branched. This pattern
of connectivity is problematic. The main mediator of the
striatopallidonigral system is
GABA and there are also
cotransmitters. The GPe stains for
met-enkephalin, the GPi stains for either
substance P or
dynorphin or both, and the SN stains for both.
This probably means that a single axon is able to concentrate different
co-mediators in different subtrees, depending on the target.
Selectivity of striatal territories for targets
A
study of the percentage of striatal axons from the sensorimotor
(putamen) and associative striatum (caudate nucleus) to the globus
pallidus
found important differences. The GPe for instance receives a large
input of axons from the associative areas. The GPi is strongly
sensorimotor connected. The SN is at first associative. This is
confirmed by the effects of striatal stimulations.
All the projections from the primary somatosensory cortex to the
putamen, avoid the striosomes and innervate areas within the matrix.
Pallidonigral set and pacemaker
Constitution
The pallidonigral set comprises the direct targets of the striatal axons: the two nuclei of the pallidum, and the
pars compacta (SNpc) and
pars reticulata
(SNpr) of the substantia nigra. One character of this ensemble is given
by the very dense striato-pallidonigral bundle giving it its whitish
aspect (pallidus means pale). In no ways has the pallidum the shape of a
globe. After Foix and Nicolesco (1925) and some others, Cécile and
Oskar Vogt (1941)
suggested the term pallidum - also used by the Terminologia Anatomica
(1998). They also proposed the term nigrum for replacing nigra, which is
indeed not a substance; but this is generally not followed. The whole
pallidonigral set is made up the same neuronal components. The majority
is made up of very large neurons, poorly branched, strongly stained for
parvalbumin, having very large dendritic arborisations (much larger in
primates than in rodents) with straight and thick dendrites.
Only the shape and direction of the dendritic arborizations differ
between the pallidum and the SN neurons. The pallidal dendritic
arborisations are very large flat and disc-shaped.
Their principal plane is parallel to the others and also parallel to
the lateral border of the pallidum; thus perpendicular to the axis of
the afferences.
Since the pallidal discs are thin, they are crossed only for a short
distance by striatal axons. However, since they are wide, they are
crossed by many striatal axons from wide striatal parts. Since they are
loose, the chances of contact are not very high. Striatal arborisations,
emit perpendicular branches participating in flat bands parallel to the
lateral border, which increases the density of synapses in this
direction. This is true for not only for the striatal afferent but also
for the subthalamic (see below).
The synaptology of the set is uncommon and characteristic.
The dendrites of the pallidal or nigral axons are entirely covered by
synapses, without any apposition of glia. More than 90% of synapses are
of striatal origin. One noticeable property of this ensemble is that not one of its
elements receives cortical afferents.
Initial collaterals are present. However, in addition to the presence of
various appendages at the distal extremity of the pallidal neurons that could act as elements of local circuitry, there are weak or no functional interrelations between pallidal neurons.
External globus pallidus
The
external globus pallidus
(GPe) or lateral globus pallidus, is flat, curved and extended in depth
and width. The branching dendritic trees are disc-shaped, flat, run
parallel to each other and to the pallidum border, and are perpendicular
to those axons coming from the striatum.
The GPe also receives input from the subthalamic nucleus, and
dopaminergic input from the SNpc. The GPe does not give output to the
thalamus only intrasystemically connecting to the other basal ganglia
structures. It can be seen as a GABA inhibitory mediator regulating the
basal ganglia. Its firing activity is very fast and exhibits long
intervals of up to several seconds of silence.
In monkeys an initial inhibition was seen in response to striatal
input, followed by a regulated excitation. In the study this suggested
that the excitation was used temporarily to control the magnitude of the
incoming signal and to spatially focus this into a limited number of
pallidal neurons. GPe neurons are often multi-targeted and may respond to a number of
neuron types. In macaques, axons from the GPe to the striatum account
for about 15%; those to the GPi, SNpr and subthalamic nucleus are about
84%. The subthalamic nucleus was seen to be the preferred target which
also sends most of its axons to the GPe.
Internal globus pallidus
The
internal globus pallidus
(GPi) or medial globus pallidus is only found in the primate brain and
so is a younger portion of the globus pallidus. Like the GPe and the
substantia nigra the GPi is a fast-spiking pacemaker but its activity
does not show the long intervals of silence seen in the others.
In addition to the striatal input there is also dopaminergic input from
the SNpc. Unlike the GPe the GPi does have a thalamic output and a
smaller output towards the
habenula. It also gives output to other areas including the
pedunculopontine nucleus and to the area behind the
red nucleus. The evolutionary increase of the internal pallidus also brought an associated increase in the
pallidothalamic tracts, and the appearance of the
ventral lateral nucleus in the thalamus. The mediator is GABA.
Substantia nigra
The substantia nigra is made up of two parts, the
pars compacta (SNpc) and the
pars reticulata
(SNpr), sometimes there is a reference to the pars lateralis but that
is usually included as part of the pars reticulata. The ‘’black
substance’’ that the term translates as, refers to the
neuromelanin
found in the dopaminergic neurons. These are found in a darker region
of the SNpc. The SNpr is a lighter coloured region. There are similar
cells in the substantia nigra and the globus pallidus. Both parts
receive input from the
striatopallidal fibres.
Pars compacta
The pars compacta is the most lateral part of the substantia nigra and sends axons to the
superior colliculus. The neurons have high firing rates which make them a fast-spiking pacemaker and they are involved in ocular
saccades.
Pars reticulata
The
border between the SNpc and SNpr is highly convoluted with deep
fringes. Its neuronal genus is the same as that of the pallidum, with
the same thick and long dendritic trees. It receives its synapses from
the striatum in the same way as the pallidum. Striatonigral axons from
the striosomes may form columns vertically oriented entering deeply in
the SNpr. The ventral dendrites of the SNpc from the reverse direction go also deeply in it. The SN also send axons to the
pedunculopontine nucleus and to the parafascicular part of the central complex. The SNpr is another "fast-spiking pacemaker". Stimulations provoke no movements. Confirming anatomical data, few
neurons respond to passive and active movements (there is no
sensorimotor map) "but a large proportion shows responses that may be
related to memory, attention or movement preparation"
that would correspond to a more elaborate level than that of the medial
pallidum. In addition to the massive striatopallidal connection, the
SNpr receives a dopamine innervation from the SNpc and glutamatergic
axons from the pars parafascicularis of the central complex. It sends
nigro-thalamic axons. There is no conspicuous nigro-thalamic bundle.
Axons arrive medially to the pallidal afferences at the anterior and
most medial part of the lateral region of the thalamus: the
ventral anterior nucleus (VA) differentiated from the
ventral lateral nucleus (VL) receiving pallidal afferences. The mediator is GABA.
Striatopallidonigral connection
The
striatopallidonigral connection is a very particular one. It engages
the totality of spiny striatal axons. Estimated numbers are 110 million
in man, 40 in chimpanzees and 12 in macaques.
The striato-pallido-nigral bundle is made up of thin, poorly myelinated
axons from the striatal spiny neurons grouped into pencils "converging
like the spokes of a wheel" (Papez, 1941). It gives its "pale" aspect to
the receiving areas. The bundle strongly stains for iron using
Perls' Prussian blue (in addition to iron it contains many heavy metals including
cobalt,
copper,
magnesium and
lead).
Convergence and focusing
After
the huge reduction in number of neurons between the cortex and the
striatum (see corticostriate connection), the striatopallido-nigral
connection is a further reduction in the number of transmitting compared
to receiving neurons. Numbers indicate that, for 31 million striatal
spiny neurons in macaques, there are only 166000 lateral pallidal
neurons, 63000 medial pallidal, 18000 lateral nigral and 35000 in the
pars reticulata.
If the number of striatal neurons is divided by their total number, as
an average, each target neuron may receive information from 117 striatal
neurons. (Numbers in man lead to about the same ratio). A different
approach starts from the mean surface of the pallidonigral target
neurons and the number of synapses that they may receive. Each
pallidonigral neuron may receive 70000 synapses. Each striatal neuron
may contribute 680 synapses. This leads again to an approximation of 100
striatal neurons for one target neuron. This represents a huge,
infrequent, reduction in neuronal connections. The consecutive
compression of maps cannot preserve finely distributed maps (as in the
case for instance of sensory systems). The fact that a strong anatomical
possibility of convergence exists does not means that this is
constantly used. A recent modeling study starting from entirely 3-d
reconstructed pallidal neurons showed that their morphology alone is
able to create a center-surround pattern of activity. Physiological analyses have shown a central inhibition/peripheral excitation pattern,
able of focusing the pallidal response in normal conditions. Percheron
and Filion (1991) thus argued for a "dynamically focused convergence". Disease, is able to alter the normal focusing. In monkeys intoxicated by
MPTP, striatal stimulations lead to a large convergence on pallidal neurons and a less precise mapping.
Focusing is not a property of the striatopallidal system. But, the very
particular and contrasted geometry of the connection between striatal
axons and pallidonigral dendrites offers particular conditions (the
possibility for a very large number of combinations through local
additions of simultaneous inputs to one tree or to several distant foci
for instance). The disfocusing of the system is thought to be
responsible for most of the parkinsonian series symptoms. The mechanism
of focusing is not known yet. The structure of the dopaminergic
innervation does not seem to allow it to operate for this function. More
likely focusing is regulated by the upstream striatopallidal and
corticostriatal systems.
Synaptology and combinatory
The
synaptology of the striato- pallidonigral connection is so peculiar as
to be recognized easily. Pallidonigral dendrites are entirely covered
with synapses without any apposition of glia.
This gives in sections characteristic images of "pallissades" or of
"rosettes". More than 90% of these synapses are of striatal origin. The
few other synapses such as the dopaminergic or the cholinergic are
interspersed among the GABAergic striatonigral synapses. The way
striatal axons distribute their synapses is a disputed point. The fact
that striatal axons are seen parallel to dendrites as "woolly fibers"
has led to exaggerate the distances along which dendrites and axons are
parallel. Striatal axons may in fact simply cross the dendrite and give a
single synapse. More frequently the striatal axon curves its course and
follow the dendrite forming "parallel contacts" for a rather short
distance. The average length of parallel contacts was found to be 55
micrometres with 3 to 10 boutons (synapses). In another type of axonal
pattern the afferent axon bifurcates and gives two or more branches,
parallel to the dendrite, thus increasing the number of synapses given
by one striatal axon. The same axon may reach other parts of the same
dendritic arborisation (forming "random cascades")
With this pattern, it is more than likely that 1 or even 5 striatal
axons are not able to influence (to inhibit) the activity of one
pallidal neuron. Certain spatio-temporal conditions would be necessary
for this, implying more afferent axons.
Pallidonigral outmaps
What
is described above concerned the input map or "inmap" (corresponding to
the spatial distribution of the afferent axons from one source to one
target). This does not correspond necessarily to the output map or
outmap (corresponding to the distribution of the neurons in relation to
their axonal targets). Physiological studies and transsynaptic viral
markers have shown that islands of pallidal neurons (only their cell
bodies or somata, or trigger points) sending their axons through their
particular thalamic territories (or nuclei) to one determined cortical
target are organized into radial bands.
These were assested to be totally representative of the pallidal
organisation. This is certainly not the case. Pallidum is precisely one
cerebral place where there is a dramatic change between one afferent
geometry and a completely different efferent one. The inmap and the
outmap are totally different. This is an indication of the fundamental
role of the pallidonigral set: the spatial reorganisation of information
for a particular "function", which is predictably a particular
reorganisation within the thalamus preparing a distribution to the
cortex.
The outmap of the nigra (lateralis reticulata) is less differentiated.
Substantia nigra compacta (SNpc) and nearby dopaminergic elements
In strict sense, the
pars compacta
is a part of the core of basal ganglia core since it directly receives
synapses from striatal axons through the striatopallidonigral bundle.
The long ventral dendrites of the pars compacta indeed plunge deep in
the pars reticulata where they receive synapses from the bundle. However, its constitution, physiology and mediator contrast with the
rest of the nigra. This explains why it is analysed here between the
elements of the core and the regulators. Ageing leads to the blackening
of its cell bodies, by deposit of melanin, visible by naked eye. This
is the origin of the name of the ensemble, first "locus niger" (Vicq
d'Azyr), meaning black place, and then "substantia nigra" (Sömmerring),
meaning black substance.
Structure
The densely distributed neurons of the
pars compacta have larger and thicker dendritic arborizations than those of the
pars reticulata
and lateralis.
The ventral dendrites descending in the pars reticulata receives
inhibitory synapses from the initial axonal collaterals of pars
reticulata neurons (Hajos and Greefield, 1994). Groups of dopaminergic
neurons located more dorsally and posteriorly in the tegmentum are of
the same type without forming true nuclei. The "cell groups A8 and A10"
are spread inside the cerebral peduncule.
They are not known to receive striatal afferences and are not in a
topographical position to do so. The dopaminergic ensemble is thus also
on this point inhomogeneous. This is another major difference with the
pallidonigral ensemble. The axons of the dopaminergic neurons, that are
thin and varicose, leave the nigra dorsally. They turn round the medial
border of the subthalamic nucleus, enter the H2 field above the
subthalamic nucleus, then cross the internal capsule to reach the upper
part of the medial pallidum where they enter the pallidal laminae, from
which they enter the striatum. They end intensively but inhomogeneously in the
striatum, rather in the matrix of the anterior part and rather in the striosomes dorsalwards. These authors insit on the extrastriatal dopaminergic innervation of other elements of the basal ganglia system:
pallidum and
subthalamic nucleus.
Physiology
Contrarily to the neurons of the pars reticulata-lateralis,
dopaminergic neurons are "low-spiking pacemakers",
spiking at low frequency (0,2 to 10 Hz) (below 8, Schultz). The role of
the dopaminergic neurons has been the source of a considerable
literature. As the pathological disappearance of the black neurons was
linked to the appearance of
Parkinson's disease,
their activity was thought to be "motor" . A major discovery has been
that the stimulation of the black neurons had no motor effect. Their
activity is in fact linked to
reward and prediction of reward. In a recent review (Schultz 2007), it is demonstrated that
phasic
responses to reward-related events, notably reward-prediction errors,
...lead to ..dopamine release..." While it is thought that there could
be different behavioral processes including long time regulation. Due to
its widespread distribution, the dopaminergic system may regulate the
basal ganglia system in many places.
Regulators of the basal ganglia core
Subthalamic nucleus, or corpus Luysi
As indicated by its name, the
subthalamic nucleus is located below the
thalamus; dorsally to the
substantia nigra and medial to the
internal capsule.
The subthalamic nucleus is lenticular in form and of homogeneous
aspect. It is made up of a particular neuronal species having rather
long ellipsoid dendritic arborisations, devoid of spines, mimicking the
shape of the whole nucleus. The subthalamic neurons are "fast-spiking pacemakers" spiking at 80 to 90 Hz. There are also about 7,5% of GABA microneurons participating in the local circuitry.
The subthalamic nucleus receives its main afference from the lateral
pallidum. Another afference comes from the cerebral cortex
(glutamatergic), particularly from the motor cortex, which is too much
neglected in models. A cortical excitation, via the subthalamic nucleus
provokes an early short latency excitation leading to an inhibition in
pallidal neurons.
Subthalamic axons leave the nucleus dorsally. Except for the connection
to the striatum (17.3% in macaques), most of the principal neurons are
multitargets and ffed axons to the other elements of the core of the
basal ganglia.
Some send axons to the substantia nigra medially and the medial and
lateral nuclei of the pallidum laterally (3-target 21.3%). Some are
2-target with the lateral pallidum and the substantia nigra (2.7%) or
the lateral pallidum and the medial(48%). Fewer are single target for
the lateral pallidum. If one adds all those reaching this target, the
main afference of the subthalamic nucleus is, in 82.7% of the cases, the
lateral pallidum (external segment of the
globus pallidus.
While striatopallidal and the pallido-subthalamic connections are
inhibitory (GABA), the subthalamic nucleus utilises the excitatory
neurotransmitter glutamate.
Its lesion resulting in
hemiballismus is known for long.
Deep brain stimulation of the nucleus suppress most of the symptoms of the Parkinson' syndrome, particularly
dyskinesia induced by
dopamine therapy.
Subthalamo-lateropallidal pacemaker
As
said before, the lateral pallidum has purely intrinsic basal ganglia
targets. It is particularly linked to the subthalamic nucleus by two-way
connections. Contrary to the two output sources (medial pallidum and
nigra reticulata), neither the lateral pallidum nor the subthalmic
nucleus send axons to the thalamus. The
subthalamic nucleus and lateral pallidum are both fast-firing pacemakers. Together they constitute the "central pacemaker of the basal ganglia"
with synchronous bursts. The pallido-subthalamic connection is
inhibitory, the subthalamo-pallidal is excitatory. They are coupled
regulators or coupled autonomous oscillators, the analysis of which has
been insufficiently deepened. The lateral pallidum receives a lot of
striatal axons, the subthalamic nucleus not. The subthalamic nucleus
receives cortical axons, the pallidum not. The subsystem they make with
their inputs and outputs corresponds to a classical systemic feedback
circuit but it is evidently more complex.
Central region of the thalamus
The
centromedian nucleus
is in the central region of the thalamus. In upper primates it has
three parts instead of two, with their own types of neuron. Output from
here goes to the subthalamic nucleus and the putamen. Its input includes
fibers from the cortex and globus pallidus.
Pedunculopontine complex
The
pedunculopontine nucleus is a part of the
reticular formation in the brainstem and a main component of the
reticular activating system,
and gives a major input to the basal ganglia. As indicated by its name,
it is located at the junction between the pons and the cerebral
peduncle, and near the substantia nigra. The axons are either excitatory
or inhibitory and mainly target the substantia nigra. Another strong
input is to the subthalamic nucleus.
Other targets are the GPi and the striatum. The complex receives direct
afferences from the cortex and above all abundant direct afferences
from the medial pallidum (inhibitory). It sends axons to the pallidal territory of the VL. The activity of the neurons is modified by movement, and precede it.
All this led Mena-Segovia et al. (2004) to propose that the complex be
linked in a way or another to the basal ganglia system. A review on its
role in the system and in diseases is given by Pahapill and Lozano
(2000).
It plays an important role in awakeness and sleep. It has a dual role
as a regulator of, and of being regulated by the basal ganglia.
Outputs of the basal ganglia system
In the
cortico-basal ganglia-thalamo-cortical loop the basal ganglia are interconnected, with little output to external targets. One target is the
superior colliculus, from the
pars reticulata.
The two other major output subsystems are to the thalamus and from
there to the cortex. In the thalamus the GPimedial fibers are separated
from the nigral as their terminal arborisations do not mix. The thalamus relays the nigral output to the premotor and to the frontal cortices.
Medial pallidum to thalamic VL and from there to cortex
The
thalamic fasciculus (
H1 field) consists of fibers from the
ansa lenticularis and from the
lenticular fasciculus (
H2 field), coming from different portions of the
GPi. These tracts are collectively the
pallidothalamic tracts and join before they enter the
ventral anterior nucleus of the
thalamus.
Pallidal axons have their own territory in the
ventral lateral nucleus (VL); separated from the cerebellar and nigral territories. The VL is stained for
calbindin and
acetylcholinesterase. The axons ascend in the nucleus where they branch profusely. The VL output goes preferentially to the
supplementary motor cortex (SMA), to the preSMA and to a lesser extent to the
motor cortex.
The pallidothalamic axons give branches to the pars media of the
central complex which sends axons to the premotor and accessory motor
cortex.
SNpr to thalamic VA and from there to cortex
The
ventral anterior nucleus (VA) output targets the premotor cortex, the
anterior cingulate cortex and the oculomotor cortex, without significant connection to the motor cortex.