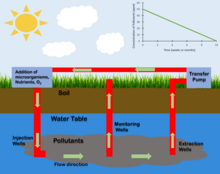
Bioremediation is a process used to treat contaminated media, including water, soil and subsurface material, by altering environmental conditions to stimulate growth of microorganisms and degrade the target pollutants. Cases where bioremediation is commonly seen is oil spills, soils contaminated with acidic mining drainage, underground pipe leaks, and crime scene cleanups. These toxic compounds are metabolized by enzymes present in microorganisms. Most bioremediation processes involve oxidation-reduction reactions where either an electron acceptor (commonly oxygen) is added to stimulate oxidation of a reduced pollutant (e.g. hydrocarbons) or an electron donor (commonly an organic substrate) is added to reduce oxidized pollutants (nitrate, perchlorate, oxidized metals, chlorinated solvents, explosives and propellants). Bioremediation is used to reduce the impact of byproducts created from anthropogenic activities, such as industrialization and agricultural processes. In many cases, bioremediation is less expensive and more sustainable than other remediation alternatives. Other remediation techniques include, thermal desorption, vitrification, air stripping, bioleaching, rhizofiltration, and soil washing. Biological treatment, bioremediation, is a similar approach used to treat wastes including wastewater, industrial waste and solid waste. The end goal of bioremediation is to remove or reduce harmful compounds to improve soil and water quality.
Contaminants can be removed or reduced with varying bioremediation techniques that are in-situ or ex-situ. Bioremediation techniques are classified based on the treatment locality. In-situ techniques treats polluted sites in a non-destructive manner and cost-effective. Whereas, ex-situ techniques commonly require the contaminated site to be excavated which increases costs. In both these approaches, additional nutrients, vitamins, minerals, and pH buffers may be added to optimize conditions for the microorganisms. In some cases, specialized microbial cultures are added (biostimulation) to further enhance biodegradation. Some examples of bioremediation related technologies are phytoremediation, bioventing, bioattenuation, biosparging, composting (biopiles and windrows), and landfarming.
Chemistry
Most bioremediation processes involve oxidation-reduction (redox) reactions where a chemical species donates an electron (electron donor) to a different species that accepts the electron (electron acceptor). During this process, the electron donor is oxidized while the electron acceptor is reduced. Common electron acceptors in bioremediation processes include oxygen, nitrate, manganese (III and IV), iron (III), sulfate, carbon dioxide and some pollutants (chlorinated solvents, explosives, oxidized metals, and radionuclides). Electron donors include sugars, fats, alcohols, natural organic material, fuel hydrocarbons and a variety of reduced organic pollutants. The redox potential for common biotransformation reactions is shown in the table.
| Process | Reaction | Redox potential (Eh in mV) |
|---|---|---|
| aerobic | O2 + 4e− + 4H+ → 2H2O | 600 ~ 400 |
| anaerobic |
|
|
| denitrification | 2NO3− + 10e− + 12H+ → N2 + 6H2O | 500 ~ 200 |
| manganese IV reduction | MnO2 + 2e− + 4H+ → Mn2+ + 2H2O | 400 ~ 200 |
| iron III reduction | Fe(OH)3 + e− + 3H+ → Fe2+ + 3H2O | 300 ~ 100 |
| sulfate reduction | SO42− + 8e− +10 H+ → H2S + 4H2O | 0 ~ −150 |
| fermentation | 2CH2O → CO2 + CH4 | −150 ~ −220 |
In-situ techniques
Bioventing
Bioventing is a process that increases the oxygen or air flow into the unsaturated zone of the soil, this in turn increases the rate of natural in-situ degradation of the targeted hydrocarbon contaminant. Bioventing, an aerobic bioremediation, is the most common form of oxidative bioremediation process where oxygen is provided as the electron acceptor for oxidation of petroleum, polyaromatic hydrocarbons (PAHs), phenols, and other reduced pollutants. Oxygen is generally the preferred electron acceptor because of the higher energy yield and because oxygen is required for some enzyme systems to initiate the degradation process. Microorganisms can degrade a wide variety of hydrocarbons, including components of gasoline, kerosene, diesel, and jet fuel. Under ideal aerobic conditions, the biodegradation rates of the low- to moderate-weight aliphatic, alicyclic, and aromatic compounds can be very high. As molecular weight of the compound increases, the resistance to biodegradation increases simultaneously. This results in higher contaminated volatile compounds due to their high molecular weight and an increased difficulty to remove from the environment.
Most bioremediation processes involve oxidation-reduction reactions where either an electron acceptor (commonly oxygen) is added to stimulate oxidation of a reduced pollutant (e.g. hydrocarbons) or an electron donor (commonly an organic substrate) is added to reduce oxidized pollutants (nitrate, perchlorate, oxidized metals, chlorinated solvents, explosives and propellants). In both these approaches, additional nutrients, vitamins, minerals, and pH buffers may be added to optimize conditions for the microorganisms. In some cases, specialized microbial cultures are added (bioaugmentation) to further enhance biodegradation.
Approaches for oxygen addition below the water table include recirculating aerated water through the treatment zone, addition of pure oxygen or peroxides, and air sparging. Recirculation systems typically consist of a combination of injection wells or galleries and one or more recovery wells where the extracted groundwater is treated, oxygenated, amended with nutrients and re-injected. However, the amount of oxygen that can be provided by this method is limited by the low solubility of oxygen in water (8 to 10 mg/L for water in equilibrium with air at typical temperatures). Greater amounts of oxygen can be provided by contacting the water with pure oxygen or addition of hydrogen peroxide (H2O2) to the water. In some cases, slurries of solid calcium or magnesium peroxide are injected under pressure through soil borings. These solid peroxides react with water releasing H2O2 which then decomposes releasing oxygen. Air sparging involves the injection of air under pressure below the water table. The air injection pressure must be great enough to overcome the hydrostatic pressure of the water and resistance to air flow through the soil.
Biostimulation
Bioremediation can be carried out by bacteria that is naturally present in the environment or adding nutrients, this process is called biostimulation.
Bacteria, also known as microbia, are naturally occurring in the environment and are used to degrade hydrocarbons. Many biological processes are sensitive to pH and function most efficiently in near neutral conditions. Low pH can interfere with pH homeostasis or increase the solubility of toxic metals. Microorganisms can expend cellular energy to maintain homeostasis or cytoplasmic conditions may change in response to external changes in pH. Anaerobes have adapted to low pH conditions through alterations in carbon and electron flow, cellular morphology, membrane structure, and protein synthesis.
Bioremediation utilizing microbes works through the use of a microbial consortium. In this context, a microbial consortium is a symbiotically associated population of microbes that survive by utilizing the secondary metabolites of the species around them. An individual species of microbes is generally incapable of fully breaking down complex molecules, but may be able to partially degrade a compound. Another part of that partially digested molecule may be broken down by another species in the consortia, a pattern that can be repeated until the environmental contaminant is broken down into harmless byproducts.
In the event of biostimulation, adding nutrients that are limited to make the environment more suitable for bioremediation, nutrients such as nitrogen, phosphorus, oxygen, and carbon may be added to the system to improve effectiveness of the treatment. Nutrients are required for the biodegradation of oil pollution and can be used to reduce the negative output on the environment. Specific to marine oil spills, nitrogen and phosphorus have been key nutrients in biodegradation.
Many biological processes are sensitive to pH and function most efficiently in near neutral conditions. Low pH can interfere with pH homeostasis or increase the solubility of toxic metals. Microorganisms can expend cellular energy to maintain homeostasis or cytoplasmic conditions may change in response to external changes in pH. Some anaerobes have adapted to low pH conditions through alterations in carbon and electron flow, cellular morphology, membrane structure, and protein synthesis.
Anaerobic bioremediation can be employed to treat a broad range of oxidized contaminants including chlorinated ethylenes (PCE, TCE, DCE, VC), chlorinated ethanes (TCA, DCA), chloromethanes (CT, CF), chlorinated cyclic hydrocarbons, various energetics (e.g., perchlorate, RDX, TNT), and nitrate. This process involves the addition of an electron donor to: 1) deplete background electron acceptors including oxygen, nitrate, oxidized iron and manganese and sulfate; and 2) stimulate the biological and/or chemical reduction of the oxidized pollutants. Hexavalent chromium (Cr[VI]) and uranium (U[VI]) can be reduced to less mobile and/or less toxic forms (e.g., Cr[III], U[IV]). Similarly, reduction of sulfate to sulfide (sulfidogenesis) can be used to precipitate certain metals (e.g., zinc, cadmium). The choice of substrate and the method of injection depend on the contaminant type and distribution in the aquifer, hydrogeology, and remediation objectives. Substrate can be added using conventional well installations, by direct-push technology, or by excavation and backfill such as permeable reactive barriers (PRB) or biowalls. Slow-release products composed of edible oils or solid substrates tend to stay in place for an extended treatment period. Soluble substrates or soluble fermentation products of slow-release substrates can potentially migrate via advection and diffusion, providing broader but shorter-lived treatment zones. The added organic substrates are first fermented to hydrogen (H2) and volatile fatty acids (VFAs). The VFAs, including acetate, lactate, propionate and butyrate, provide carbon and energy for bacterial metabolism.
Bioattenuation
During bioattenuation, biodegradation occurs naturally with the addition of nutrients or bacteria. The indigenous microbes present will determine the metabolic activity and act as a natural attenuation. While there is no anthropogenic involvement in bioattenuation, the contaminated site must still be monitored.
Biosparging
Biosparging is the process of groundwater remediation as oxygen, and possible nutrients, is injected. When oxygen is injected, indigenous bacteria are stimulated to increase rate of degradation. However, biosparging focuses on saturated contaminated zones, specifically related to ground water remediation.
Ex Situ techniques
Biopiles
Biopiles, similar to bioventing, are used to reduce petroleum pollutants by introducing aerobic hydrocarbons to contaminated soils. However, the soil is excavated and piled with an aeration system. This aeration system enhances microbial activity by introducing oxygen under positive pressure or removes oxygen under negative pressure.
Windrows
Windrow systems are similar to compost techniques where soil is periodically turned in order to enhance aeration. This periodic turning also allows contaminants present in the soil to be uniformly distributed which accelerates the process of bioremediation.
Landfarming
Landfarming, or land treatment, is a method commonly used for sludge spills. This method disperses contaminated soil and aerates the soil by cyclically rotating. This process is an above land application and contaminated soils are required to be shallow in order for microbial activity to be stimulated. However, if the contamination is deeper than 5 feet, then the soil is required to be excavated to above ground.
Heavy metals
Heavy metals become present in the environment due to anthropogenic activities or natural factors. Anthropogenic activities include industrial emissions, electronic waste, and ore mining. Natural factors include mineral weathering, soil erosion and forest fires. Heavy metals including cadmium, chromium, lead and uranium are unlike organic compounds and cannot be biodegraded. However, bioremediation processes can potentially be used to reduce the mobility of these material in the subsurface, reducing the potential for human and environmental exposure. Heavy metals from these factors are predominantly present in water sources due to runoff where it is uptake by marine fauna and flora.
The mobility of certain metals including chromium (Cr) and uranium (U) varies depending on the oxidation state of the material. Microorganisms can be used to reduce the toxicity and mobility of chromium by reducing hexavalent chromium, Cr(VI) to trivalent Cr (III). Uranium can be reduced from the more mobile U(VI) oxidation state to the less mobile U(IV) oxidation state. Microorganisms are used in this process because the reduction rate of these metals is often slow unless catalyzed by microbial interactions Research is also underway to develop methods to remove metals from water by enhancing the sorption of the metal to cell walls. This approach has been evaluated for treatment of cadmium, chromium, and lead. Phytoextraction processes concentrate contaminants in the biomass for subsequent removal.
Limitations of bioremediation
Bioremediation can be used to completely mineralize organic pollutants, to partially transform the pollutants, or alter their mobility. Heavy metals and radionuclides are elements that cannot be biodegraded, but can be bio-transformed to less mobile forms. In some cases, microbes do not fully mineralize the pollutant, potentially producing a more toxic compound. For example, under anaerobic conditions, the reductive dehalogenation of TCE may produce dichloroethylene (DCE) and vinyl chloride (VC), which are suspected or known carcinogens. However, the microorganism Dehalococcoides can further reduce DCE and VC to the non-toxic product ethene. Additional research is required to develop methods to ensure that the products from biodegradation are less persistent and less toxic than the original contaminant. Thus, the metabolic and chemical pathways of the microorganisms of interest must be known. In addition, knowing these pathways will help develop new technologies that can deal with sites that have uneven distributions of a mixture of contaminants.
Also, for biodegradation to occur, there must be a microbial population with the metabolic capacity to degrade the pollutant, an environment with the right growing conditions for the microbes, and the right amount of nutrients and contaminants. The biological processes used by these microbes are highly specific, therefore, many environmental factors must be taken into account and regulated as well. Thus, bioremediation processes must be specifically made in accordance to the conditions at the contaminated site. Many factors are interdependent, such as small-scale tests which are usually performed before carrying out the procedure at the contaminated site. However, it can be difficult to extrapolate the results from the small-scale test studies into big field operations. In many cases, bioremediation takes more time than other alternatives such as land filling and incineration. Another example is bioventing, which is inexpensive to bioremediate contaminated sites, however this process is extensive and can take a few years to decontaminate a site.
In agricultural industries, the use of pesticides is a top factor in direct soil contamination and runoff water contamination. The limitation or remediation of pesticides is the low bioavailability. Altering the pH and temperature of the contaminated soil is a resolution to increase bioavailability which, in turn, increased degradation of harmful compounds. The compoundacrylonitrile is commonly produced in industrial setting but adversely contaminates soils. Microorganisms containing nitrile hydratases (NHase) degraded harmful acrylonitrile compounds into non-polluting substances.
Since the experience with harmful contaminants are limited, laboratory practices are required to evaluate effectiveness, treatment designs, and estimate treatment times. Bioremediation processes may take several months to several years depending on the size of the contaminated area.
Genetic engineering
The use of genetic engineering to create organisms specifically designed for bioremediation is under preliminary research. Two category of genes can be inserted in the organism: degradative genes which encode proteins required for the degradation of pollutants, and reporter genes that are able to monitor pollution levels. Numerous members of Pseudomonas have also been modified with the lux gene, but for the detection of the polyaromatic hydrocarbon naphthalene. A field test for the release of the modified organism has been successful on a moderately large scale.
There are concerns surrounding release and containment of genetically modified organisms into the environment due to the potential of horizontal gene transfer. Genetically modified organisms are classified and controlled under the Toxic Substances Control Act of 1976 under United States Environmental Protection Agency. Measures have been created to address these concerns. Organisms can be modified such that they can only survive and grow under specific sets of environmental conditions. In addition, the tracking of modified organisms can be made easier with the insertion of bioluminescence genes for visual identification.
Genetically modified organisms have been created to treat oil spills and break down certain plastics (PET).