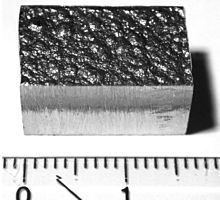
Four common conflict minerals, clockwise from top left: coltan, cassiterite, gold ore, and wolframite.
Conflict resources are natural resources extracted in a conflict zone and sold to perpetuate the fighting.
There is both statistical and anecdotal evidence that belligerent
accessibility to precious commodities can prolong conflicts (a "resource curse"). The most prominent contemporary example has been the eastern provinces of the Democratic Republic of the Congo
(DRC), where various armies, rebel groups, and outside actors have
profited from mining while contributing to violence and exploitation
during wars in the region.
The four most commonly mined conflict minerals (known as 3TGs, from their initials) are cassiterite (for tin), wolframite (for tungsten), coltan (for tantalum), and gold
ore, which are extracted from the eastern Congo, and passed through a
variety of intermediaries before being purchased. These minerals are
essential in the manufacture of a variety of devices, including consumer electronics such as mobile phones, laptops, and MP3 players.
The extraction and sale of blood diamonds, also known as "conflict diamonds", is a better-known phenomenon which occurs under virtually identical conditions. Even petroleum can be a conflict resource; ISIS used oil revenue to finance its military and terrorist activities.
There have been international efforts to reduce trade in conflict
resources, which try to reduce incentives to extract and fight over
them. For example, in the United States, the 2010 Dodd–Frank Wall Street Reform and Consumer Protection Act required manufacturers to audit their supply chains and report use of conflict minerals. In 2015 a US federal appeals court struck down some aspects of the reporting requirements as a violation of corporations’ freedom of speech, but left others in place.
History
The concept of 'conflict resource', or 'conflict commodity' emerged in the late 1990s, initially in relation to the 'conflict diamonds' that were financing rebellions in Angola and Sierra Leone. (The media often called these 'blood diamonds'.) Then 'conflict timber' financed hostilities in Cambodia and Liberia.
Conventions
The concept was first officially discussed by the UN General Assembly in the context of 'conflict diamonds': The UN Security Council has since referred to conflict resources in several resolutions.
Global Witness
has called for an international standardized definition to facilitate a
more systematic application of UN resolutions, the prevention of
complicity in abuses during hostilities by commercial entities
exploiting or trading in conflict resources, and the prosecution of war profiteers suspected of supporting or abetting war criminals."
...natural resources whose systematic exploitation and trade in a context of conflict contribute to, benefit from or result in the commission of serious violations of human rights, violations of international humanitarian law or violations amounting to crimes under international law.
— Global Witness, proposed Definition of conflict resources
Since 1996 the Bonn International Center for Conversion has tracked resource governance and conflict intensity by country. Aside from fossil fuels, metals, diamonds, and timber it tracks the governance of other primary goods that might fund conflicts, including: poppy seeds and talc (Afghanistan), rubber (Côte d'Ivoire), cotton (Zambia), and cocoa (Indonesia).
Conflict minerals
The four most prominent conflict minerals, for example codified in the U.S. Conflict Minerals Law, are:
- Columbite-tantalite (or coltan, the colloquial African term) is the metal ore from which the element tantalum is extracted. Tantalum is used primarily for the production of tantalum capacitors, particularly for applications requiring high performance, a small compact format and high reliability, from hearing aids and pacemakers, to airbags, GPS, ignition systems and anti-lock braking systems in automobiles, through to laptop computers, mobile phones, video game consoles, video cameras and digital cameras. In its carbide form, tantalum possesses significant hardness and wear resistance properties. As a result, it is used in jet engine/turbine blades, drill bits, end mills and other tools.
- Cassiterite is the chief ore needed to produce tin, essential for the production of tin cans and the solder on the circuit boards of electronic equipment. Tin is also commonly a component of biocides, fungicides and as tetrabutyl tin/tetraoctyl tin, an intermediate in polyvinyl chloride (PVC) and high performance paint manufacturing.
- Wolframite is an important source of the element tungsten. Tungsten is a very dense metal and is frequently used for this property, such as in fishing weights, dart tips and golf club heads. Like tantalum carbide, tungsten carbide possesses hardness and wear resistance properties and is frequently used in applications like metalworking tools, drill bits and milling. Smaller amounts are used to substitute lead in "green ammunition". Minimal amounts are used in electronic devices, including the vibration mechanism of cell phones.
- Gold is used in jewelry, investments, electronics, and dental products. It is also present in some chemical compounds used in certain semiconductor manufacturing processes.
These are sometimes referred to as "the 3T's and gold", 3TG, or even simply the "3T's". Under the US Conflict Minerals Law, additional minerals may be added to this list in the future.
Democratic Republic of the Congo
As of 2010, the conflict resource fueling the world's deadliest war is gold in the Congo. Gold bars are less traceable than diamonds, and gold is abundant in the Kivu conflict region. In any case, no jewellery
industry standard exists for verifying gold origination, as it does for
diamonds (though jeweler's total outlay on gold is five times that on
diamonds). Other conflict minerals being illicitly exported from the Congo include cobalt, tungsten, cassiterite, and coltan (which provides the tantalum for mobile phones, and is also said to be directly sustaining the conflict).
Armed conflict and mineral resource looting by the Congolese National Army and various armed rebel groups, including the Democratic Forces for the Liberation of Rwanda (FDLR) and the National Congress for the Defense of the People
(CNDP), a proxy Rwandan militia group, has occurred throughout the late
20th century and the early 21st century. Additionally, the looting of
the Congo's natural resources is not limited to domestic actors. During
the Congo Wars (First Congo War (1996–1997) and Second Congo War
(1998–2003)), Rwanda, Uganda and Burundi particularly profited from the
Congo's resources. These governments continued to smuggle resources out
of the Congo to this day.
The profits from the sale of these minerals have financed
fighting in the Second Congo War and ongoing follow-on conflicts.
Control of lucrative mines has also itself become a military objective.
Mines
Mines, in
eastern Congo, are often located far from populated areas in remote and
dangerous regions. A recent study by International Peace Information
Service (IPIS) indicates that armed groups are present at more than 50%
of mining sites. At many sites, armed groups illegally tax, extort, and
coerce civilians to work. Miners, including children, work up to
48-hour shifts amidst mudslides and tunnel collapses that kill many.
The groups are often affiliated with rebel groups, or with the
Congolese National Army, but both use rape and violence to control the
local population.
United States law
In April 2009, Senator Sam Brownback (R-KS) introduced the Congo Conflict Minerals Act of 2009 (S. 891)
to require electronics companies to verify and disclose their sources
of cassiterite, wolframite, and tantalum. This legislation died in
committee. However, Brownback added similar language as Section 1502 of
the Dodd–Frank Wall Street Reform and Consumer Protection Act, which passed Congress and was signed into law by President Barack Obama on July 21, 2010.
The U.S. Securities and Exchange Commission (SEC) draft regulations to implement the Conflict Mineral Law, published in the Federal Register of December 23, 2010.
would have required U.S. and certain foreign companies to report and
make public their use of so-called "conflict minerals" from the
Democratic Republic of the Congo or adjoining countries in their
products. Comments on this proposal were extended until March 2, 2011. The comments on the proposal were reviewable by the public.
One report on the proposal stated the following statistics for the submitted comments:
- Slightly more than 700 comment letters were submitted to SEC on the proposal;
- Approximately 65% of those were form letters or basic letters from the general public supporting the rule's intent;
- The remaining 35% (roughly 270) represent views of businesses, trade/industry associations, the investment/financial community, professional auditing firms, and other relevant governmental entities; and
- Of those 270 comments, an estimated 200 contained substantive and/or technical comments.
That report also contained what it calls a "preview of the final SEC
regulations" synthesized from their detailed research and analysis of a
large body of documents, reports and other information on the law,
proposed regulation and the current budget/political setting facing the
SEC in the current administration.
The final rule went into effect 13 November 2012.
The SEC rule did not go unnoticed by the international community,
including entities seeking to undermine traceability efforts. A report
published by a metals trading publication illustrated one DRC
ore/mineral flow method that has apparently been devised to thwart
detection.
On July 15, 2011, the US State Department issued a statement on the subject. Section 1502(c) of the Law mandates that the State Department work in conjunction with SEC on certain elements of conflict minerals policy development and support.
On October 23, 2012 U.S. State Dept Officials asserted that
ultimately, it falls on the U.S. State Dept. to determine when this rule
would no longer apply.
In April 2014, the United States Court of Appeals for the
District of Columbia Circuit struck down several parts of the SEC Rules
as unconstitutional.
Auditing and reporting requirements
US Conflict Minerals Law contains two requirements that are closely connected:
- independent third party supply chain traceability audits
- reporting of audit information to the public and SEC.
Even companies not directly regulated by the SEC will be impacted by
the audit requirements because they will be pushed down through entire
supply chains, including privately held and foreign-owned companies.
SEC estimated that 1,199 "issuers" (i.e., companies subject to
filing other SEC reports) will be required to submit full conflict
mineral reports. This estimate was developed by finding the amount of
tantalum produced by the DRC in comparison to global production (15% –
20%). The Commission selected the higher figure of 20% and multiplied
that by 6,000 (the total number of "issuers" SEC will be required to do
initial product/process evaluations).
This estimate does not account for the companies who supply materials
to the "issuers" (but are not themselves SEC-regulated) but who will
almost certainly be required to conduct conflict minerals audits to meet
the demands of those customers. Other estimates indicate that the
total number of US companies likely impacted may exceed 12,000.
A study of the potential impact of the regulation in early 2011 by the IPC – Association Connecting Electronic Industries trade association. was submitted with the association's comments to the SEC.
The study states that the IPC survey respondents had a median of 163
direct suppliers. Applying that number to the SEC's estimated number of
impacted issuers results in the possibility of over 195,000 businesses
that could be subject to some level of supply chain traceability effort.
Applicability in general
Under the law, companies have to submit an annual conflict minerals report to the SEC if:
- (a) they are required to file reports with the SEC under the Exchange Act of 1934
- (b) conflict minerals are necessary to the functionality or production of a product that they manufacture or contract to be manufactured. That statement contains two separate – but critical concepts: the purpose of the conflict mineral in the product/process, and the control that the company exerts over the manufacturing process/specifications.
A company would be deemed to contract an item to be manufactured if it:
- Exerts any influence over the manufacturing process; or,
- Offers a generic product under its own brand name or a separate brand name (regardless of whether the company has any influence over the manufacturing process) and the company contracted to have the product manufactured specifically for itself.
This language implied that some retailers who are not manufacturers might be subject to the audit and disclosure requirements.
"Contracting to manufacture" a product requires some actual
influence over the manufacturing of process that product, a
determination based on facts and circumstances. A company is not to be deemed to have influence over the manufacturing process if it merely:
- Affixes its brand, marks, logo, or label to a generic product manufactured by a third party.
- Services, maintains, or repairs a product manufactured by a third party.
- Specifies or negotiates contractual terms with a manufacturer that do not directly relate to the manufacturing of the product.
The proposed regulations attempted to clarify that tools used in assembly and manufacturing will not trigger the law.
The intent was to cover minerals/metals in the final product only.
Nothing specifically addresses intermediate chemical processes that use
chemicals that contain conflict minerals. Additionally, neither the law nor the proposed regulation established a de minimis quantity or other form of materiality threshold that would preclude the applicability of the auditing/reporting requirements.
Supply chain traceability auditing
The
law mandates the use of an "independent private sector auditor" to
conduct the audits. SEC has proposed two different standards for the
audits: the "reasonable inquiry" and the "due diligence". Should the final rule include this structure, the reasonable inquiry
would be the first step to determine if the company can on its own,
using reasonable efforts and trustworthy information, make a reliable
determination as to the source/origin of its tin, tantalum, tungsten
and/or gold. Where companies are unable to make such a determination
for any reason, they would then be required to take the next step of the
"due diligence", which is the independent private sector audit.
The statute specified that the audits be "conducted in accordance with standards established by the Comptroller General of the United States,
in accordance with rules promulgated by the Commission." This means
that the same auditing standards that apply to other SEC auditing
requirements will apply to conflict minerals audits
Because of this language, SEC will have little discretion to allow
companies to issue self-generated statements or certifications to
satisfy the law.
Third party audits for conflict minerals supply chain
traceability began in summer 2010 under the Electronic Industry
Citizenship Coalition (EICC), a US-based electronics manufacturing trade
association.
Under this program, EICC selected three audit firms to conduct the
actual audits, with two of the three participating in the pilot audits
in 2010. After concluding the pilot, one of the two firms involved in
2010 withdrew from the program specifically in response to the SEC's
proposal and to reduce potential legal risks to the audited entities.
Neither the law nor the proposed regulations provide guidance on
what will be considered an acceptable audit scope or process, preferring
to allow companies the flexibility meeting the requirement in a manner
that is responsive to their own individual business and supply chain.
At the same time, the law contains a provision that preserves the
government's rights to deem any report, audit or other due diligence
processes as being unreliable, and in such cases, the report shall not
satisfy the requirements of the regulations,
further emphasizing the need for such audits to conform to established
SEC auditing standards. Comments on the proposed regulation pointed out
that, should SEC not specify an applicable audit standard, it cannot
also be silent or ambiguous on the auditor standards as well, or the
Commission will violate the plain language of the Law mandating
"standards established by the Comptroller General of the United States".
It is generally expected that SEC will provide specificity on both the
audit standard and the auditor standard. SEC's proposal attempted to
clarify its position on auditor requirements.
The Organisation for Economic Co-operation and Development (OECD) published its Guidance on conflict minerals supply chain traceability.
This guidance is gaining much momentum as "the" standard within US
policy. However, a recent critical analysis of the standard in
comparison to existing US auditing standards under SEC highlighted a
number of significant inconsistencies and conflict with relevant US
standards.
Companies subject to the US law who implement the OECD Guidance
without regard for the SEC auditing standards may face legal compliance
risks.
Reporting and disclosure
Companies
subject to the SEC reporting requirement would be required to disclose
whether the minerals used in their products originated in the DRC or
adjoining countries (as defined above). The law mandates that this
reporting be submitted/made available annually. Many comments to the
proposed regulation asked SEC to clarify whether the report must be
"furnished"—meaning it is made available to SEC but not directly
incorporated within the company's formal financial report—or
"submitted"—meaning the report is directly incorporated into the
financial report.
At first glance, this may appear to be a minor point; however, this
difference is very important in determining the audit/auditor standards
and related liabilities.
If it is determined that none of the minerals originated in the
DRC or adjoining countries, the report must include a statement to that
effect and provide an explanation of the country of origin analysis that
was used to arrive at the ultimate conclusion. On the other hand, if
conflict minerals originating in the DRC or adjoining countries were
used (or if it is not possible to determine the country of origin of the
conflict minerals used), companies would be required to state as such
in the annual report. In either case, companies would also be required
to make this information public by posting their annual conflict
minerals report on their websites, and providing the SEC with the
internet addresses where the reports may be found. Further, the proposed
regulations would require companies to maintain records relating to the
country of origin of conflict minerals used in their products.
Media outlets have reported that many companies required to file Specialized Disclosure Reports to the U.S. Securities and Exchange Commission
(SEC) and any necessary conflict minerals reports for 2013 under the
SEC’s conflict minerals rule are struggling to meet the June 2, 2014
report filing deadline. Many impacted companies were hoping for clarification regarding filing requirements, from the United States Court of Appeals for the District of Columbia Circuit from a lawsuit filed by the National Association of Manufacturers.
The appellate court’s ruling left the necessary conflict minerals
reporting requirements largely intact and it has been suggested that
impacted companies should review the SEC’s Division of Corporation
Finance’s response to the court’s ruling which provides guidance regarding the effect of the appellate court’s ruling.
On August 18, 2015 the divided D.C. Circuit Court again held the SEC's conflict materials rule violates the First Amendment. Senior Circuit Judge A. Raymond Randolph, joined by Senior Circuit Judge David B. Sentelle, weighed if the required disclosures were effective and uncontroversial. Citing news reports and a Congressional hearing, the court decided the policy was ineffective.
The court next found the required label was controversial because it
"is a metaphor that conveys moral responsibility for the Congo war." As such, the court struck down the conflict materials rule’s disclosure requirements as a violation of corporations’ freedom of speech. Circuit Judge Sri Srinivasan dissented, writing that the required disclosures were not controversial because they were truthful.
Criticism of the law
The
law has been criticised for not addressing the root causes of the
conflict, leaving to the Congolese government the responsibility for
providing an environment in which companies can practice due diligence
and legitimately purchase the minerals they need, when the reality is
that mechanisms for transparency do not exist.
The effect has been to halt legitimate mining ventures that provided
livelihoods for people, reducing the Congo's legal exports of tantalum
by 90%.
An investigation by the U.S. Government Accountability Office (GAO) found that most companies were unable to determine the source of their conflict minerals.
Technology manufacturers criticized a law which required them to label a product as not "DRC Conflict Free" as compelled speech, and in violation of the First Amendment.
Proposed law in Europe
The
European Parliament passed legislation in 2015; negotiations are
currently underway among member states as to specific wording details.
On 16 June 2016 the European Parliament confirmed that "mandatory
due diligence" would be required for "all but the smallest EU firms
importing tin, tungsten, tantalum, gold and their ores".
On May 17, 2017 the EU passed Regulation (EU) 2017/821 of the
Parliament and of the Council on the supply chain due diligence
obligations for importers of tin, tantalum, tungsten, their ores, and
gold from conflict-affected and high risk areas. The regulation will
take effect in January 2021, and will directly apply to companies that
import 3TG metals into the EU, no matter where they originate.
On August 10, 2018 The European Commission published their
non-binding guidelines for the identification of conflict-affected and
high-risk areas and other supply chain risks under Regulation (EU)
2017/821 of the European Parliament and of the Council.
Conflict resources in supply chains
Increases
in business process outsourcing to globally dispersed production
facilities means that social problems and human rights violations are no
longer only an organization matter, but also often occur in companies’
supply chains, and challenge for supply chain managers.
Besides the harm conflict minerals do where they are produced, human
rights violations also raise an enormous risk to corporate reputations.
Consumers, mass media and employees expect companies to behave
responsibly and have become intolerant of those who don't.
Consequently, firms that are located downstream in the supply
chain and that are more visible to stakeholders are particularly
threatened by social supply chain problems. The recent debate concerning
conflict minerals illustrates the importance of social and human rights
issues in supply chain management practice as well as the emerging need
to react to social conflicts. Conflict minerals are processed in many
different components throughout various industries and hence have a high
overall impact on business.
Initiatives like the Dodd–Frank Wall Street Reform and Consumer Protection Act or the OECD Due Diligence Guidance for Responsible Supply Chains of Minerals from Conflict-Affected and High-Risk Areas
demand that supply chain managers verify purchased goods as
‘‘conflict-free’’ or implement measures to better manage any inability
to do so.
Minerals mined in Eastern Congo pass through the hands of
numerous middlemen as they are shipped out of Congo, through neighboring
countries such as Rwanda or Burundi, to East Asian processing plants.
Because of this, the US Conflict Minerals Law applies to materials
originating (or claimed to originate) from the DRC as well as the nine
adjoining countries: Angola, Burundi, Central African Republic, Congo Republic, Rwanda, South Sudan, Zimbabwe, Uganda, and Zambia.
Firms have begun to apply governance mechanisms to avoid adverse
effects of conflict mineral sourcing. However, the mere transfer of
responsibilities upstream in the supply chain apparently will not stop
the trade with conflict minerals, notably due to two reasons:
- On the one hand, globalization has created governance gaps in a sense that companies are able to abuse human rights without being sanctioned by independent third parties. This gap results in a non-allocation of responsibility that makes the problem of human rights abuses and social conflicts within dispersed supply chains very likely to endure, particularly without collaborative approaches to remedy these deficiencies.
- On the other hand, conflict minerals usually originate from globally diverse deposits and are difficult to track within components and manufactured products. This is the case because they are mixed with minerals of different origin and added to metal alloys. Consequently, although the share of these minerals in single end products may be negligible, they are prevalent in numerous products and commodities. Together, these circumstances leave downstream firms nearly incapable of detecting risks associated with conflict minerals. Hence, the topic of conflict minerals becomes one of supply chain management rather than of individual companies’ legal or compliance divisions alone. What is needed is effective and supply-chain wide-mechanisms of traceability and due diligence that allow firms to take individual and collective responsibility as parts of supply chains.
In the context of mineral supply chains, due diligence represents a
holistic concept that aims at providing a chain of custody tracking from
mine to export at country level, regional tracking of mineral flows
through the creation of a database on their purchases, independent
audits on all actors in the supply chain, and a monitoring of the whole
mineral chain by a mineral chain auditor. In this sense, due diligence
transcends conventional risk management approaches that usually focus on
the prevention of direct impacts on the core business activities of
companies. Moreover, due diligence focuses on a maximum of transparency
as an end itself while risk management is always directed towards the
end of averting direct damages. However, besides the Dodd–Frank Wall Street Reform and Consumer Protection Act
and the OECD Guidance, there is still a gap in due diligence practices
as international norms are just emerging. Studies found that the
motivation for supply chain due diligence as well as expected outcomes
of these processes vary among firms.
Furthermore, different barriers, drivers, and implementation patterns
of supply chain due diligence have been identified in scholarly
research.
Organizations and activists involved
A number of organizations and celebrities working to find solutions and raise awareness of conflict minerals. These include:
- Save the Congo
- The Enough Project
- Partnership Africa Canada
- The Conflict Free Tin Initiative
- Solutions for Hope
- Raise Hope for Congo
- Stand Canada
- Conflictminerals.org
- Congo Siasa
- ReliefWeb
- Ashley Judd
- Ryan Gosling
- Southern Africa Resource Watch
Moreover, FairPhone
Foundation raises awareness of conflict minerals in the mobile industry
and is a company which tries to produce a smart phone with 'fair'
conditions along the supply chain. Various industry and trade
associations are also monitoring developments in conflict minerals laws
and traceability frameworks. Some of these represent electronics,
retailers, jewelry, mining, electronics components, and general
manufacturing sectors. One organization – ITRI (a UK-based
international non-profit organization representing the tin industry and
sponsored/supported by its members, principally miners and smelters.)
had spearheaded efforts for the development and implementation of a
"bag and tag" scheme at the mine as a key element of credible
traceability.
The program and related efforts were initially not likely to extend
beyond the pilot phase due to a variety of implementation and funding
problems that occurred. In the end however, the device did enter the market.
In late March 2011, the UK government launched an informational
section on its Foreign & Commonwealth Office website dedicated to
conflict minerals.
This information resource is intended to assist British companies in
understanding the issues and, specifically, the US requirements.
On Jan 6th 2014, the semiconductor giant Intel announced that it
would distance itself from conflict minerals. As a result, all Intel
microprocessors henceforth will be conflict-free.
Commercial reporting solutions
Manufacturers
and supply chain partners needing to comply with the ever-increasing
reporting regulations have a few commercial options available.
A major research report from November 2012 by the Southern Africa Resource Watch revealed that gold miners in the east of the Democratic Republic of Congo
were being exploited by corrupt government officials, bureaucrats and
security personnel, who all demand illegal tax, fees and levies from the
miners without delivering any services in return. Despite the alleged
gold rush in regions of the country, none of the population and
workforce is benefiting from this highly lucrative industry.