| Cancer immunotherapy | |
|---|---|

|
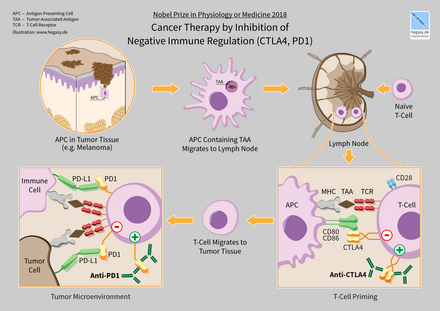
Cancer immunotherapy (sometimes called immuno-oncology) is the artificial stimulation of the immune system to treat cancer, improving on the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology and a growing subspeciality of oncology. It exploits the fact that cancer cells often have tumor antigens, molecules on their surface that can be detected by the antibody proteins of the immune system, binding to them. The tumor antigens are often proteins or other macromolecules (e.g. carbohydrates). Normal antibodies bind to external pathogens, but the modified immunotherapy antibodies bind to the tumor antigens marking and identifying the cancer cells for the immune system to inhibit or kill. In 2018, James P. Allison and Tasuku Honjo received the Nobel Prize in Physiology or Medicine for their discovery of cancer therapy by inhibition of negative immune regulation.
Categories
Immunotherapies can be categorized as active, passive or hybrid (active and passive). Active immunotherapy directs the immune system to attack tumor cells by targeting tumor antigens. Passive immunotherapies enhance existing anti-tumor responses and include the use of monoclonal antibodies, lymphocytes and cytokines.
A wide range of cancers can be treated by various immunotherapy
medicines that have been approved in many jurisdictions around the
world.
Passive antibody therapies commonly involve the targeting of Cell surface receptors and include CD20, CD274 and CD279 antibodies. Once bound to a cancer antigen, the modified antibodies can induce antibody-dependent cell-mediated cytotoxicity, activate the complement system, or prevent a receptor from interacting with its ligand, all of which can lead to cell death. Apart from classical immunomodulatory receptors, cell surface proteoglycans are an emerging class of targets for cancer immunotherapy.
Approved immunotherapy antibodies include alemtuzumab, ipilimumab, nivolumab, ofatumumab, pembrolizumab and rituximab.
Active cellular therapies usually involve the removal of immune
cells from the blood or from a tumor. Those specific for the tumor are
grown in culture and returned to the patient where they attack the
tumor; alternatively, immune cells can be genetically engineered to express a tumor-specific receptor, cultured and returned to the patient. Cell types that can be used in this way are natural killer (NK) cells, lymphokine-activated killer cells, cytotoxic T cells and dendritic cells.
Cellular immunotherapy
Dendritic cell therapy
Blood
cells are removed from the body, incubated with tumour antigen(s) and
activated. Mature dendritic cells are then returned to the original
cancer-bearing donor to induce an immune response.
Dendritic cell therapy provokes anti-tumor responses by causing
dendritic cells to present tumor antigens to lymphocytes, which
activates them, priming them to kill other cells that present the
antigen. Dendritic cells are antigen presenting cells (APCs) in the
mammalian immune system. In cancer treatment they aid cancer antigen targeting. The only approved cellular cancer therapy based on dendritic cells is sipuleucel-T.
One method of inducing dendritic cells to present tumor antigens is by vaccination with autologous tumor lysates
or short peptides (small parts of protein that correspond to the
protein antigens on cancer cells). These peptides are often given in
combination with adjuvants (highly immunogenic
substances) to increase the immune and anti-tumor responses. Other
adjuvants include proteins or other chemicals that attract and/or
activate dendritic cells, such as granulocyte macrophage colony-stimulating factor
(GM-CSF). The most common source of antigens used for dendritic cell
vaccine in Glioblastoma (GBM) as an aggressive brain tumor were whole
tumor lysate, CMV antigen RNA and tumor associated peptides like
EGFRvIII.
Dendritic cells can also be activated in vivo
by making tumor cells express GM-CSF. This can be achieved by either
genetically engineering tumor cells to produce GM-CSF or by infecting
tumor cells with an oncolytic virus that expresses GM-CSF.
Another strategy is to remove dendritic cells from the blood of a
patient and activate them outside the body. The dendritic cells are
activated in the presence of tumor antigens, which may be a single
tumor-specific peptide/protein or a tumor cell lysate (a solution of broken down tumor cells). These cells (with optional adjuvants) are infused and provoke an immune response.
Dendritic cell therapies include the use of antibodies that bind
to receptors on the surface of dendritic cells. Antigens can be added to
the antibody and can induce the dendritic cells to mature and provide
immunity to the tumor. Dendritic cell receptors such as TLR3, TLR7, TLR8 or CD40 have been used as antibody targets.
Dendritic cell-NK cell interface also has an important role in
immunotherapy. The design of new dendritic cell-based vaccination
strategies should also encompass NK cell-stimulating potency. It is
critical to systematically incorporate NK cells monitoring as an outcome
in antitumor DC-based clinical trials.
Approved drugs
Sipuleucel-T (Provenge) was approved for treatment of asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer in 2010. The treatment consists of removal of antigen-presenting cells from blood by leukapheresis and growing them with the fusion protein PA2024 made from GM-CSF and prostate-specific prostatic acid phosphatase (PAP) and reinfused. This process is repeated three times.
CAR-T cell therapy
The premise of CAR-T immunotherapy is to modify T cells to recognize
cancer cells in order to more effectively target and destroy them.
Scientists harvest T cells from people, genetically alter them to add a
chimeric antigen receptor (CAR) that specifically recognizes cancer
cells, then infuse the resulting CAR-T cells into patients to attack
their tumors.
Approved drugs
Tisagenlecleucel (Kymriah), a chimeric antigen receptor (CAR-T) therapy, was approved by FDA in 2017 to treat acute lymphoblastic leukemia (ALL). This treatment removes CD19 positive cells (B-cells) from the body (including the diseased cells, but also normal antibody producing cells).
Axicabtagene ciloleucel (Yescarta) is another CAR-T therapeutic, approved in 2017 for treatment of diffuse large B-cell lymphoma (DLBCL).
Antibody therapy
Many forms of antibodies can be engineered.
Antibodies are a key component of the adaptive immune response,
playing a central role in both recognizing foreign antigens and
stimulating an immune response. Antibodies are Y-shaped proteins
produced by some B cells and are composed of two regions: an antigen-binding fragment (Fab), which binds to antigens, and a Fragment crystallizable (Fc) region, which interacts with so-called Fc receptors that are expressed on the surface of different immune cell types including macrophages, neutrophils and NK cells. Many immunotherapeutic regimens involve antibodies. Monoclonal antibody
technology engineers and generates antibodies against specific
antigens, such as those present on tumor surfaces. These antibodies that
are specific to the antigens of the tumor, can then be injected into a
tumor.
Antibody types
Conjugation
Two types are used in cancer treatments:
- Naked monoclonal antibodies are antibodies without added elements. Most antibody therapies use this antibody type.
- Conjugated monoclonal antibodies are joined to another molecule, which is either cytotoxic or radioactive. The toxic chemicals are those typically used as chemotherapy drugs, but other toxins can be used. The antibody binds to specific antigens on cancer cell surfaces, directing the therapy to the tumor. Radioactive compound-linked antibodies are referred to as radiolabelled. Chemolabelled or immunotoxins antibodies are tagged with chemotherapeutic molecules or toxins, respectively. Research has also demonstrated conjugation of a TLR agonist to an anti-tumor monoclonal antibody.
Fc regions
Fc's ability to bind Fc receptors
is important because it allows antibodies to activate the immune
system. Fc regions are varied: they exist in numerous subtypes and can
be further modified, for example with the addition of sugars in a
process called glycosylation. Changes in the Fc region
can alter an antibody's ability to engage Fc receptors and, by
extension, will determine the type of immune response that the antibody
triggers. Many cancer immunotherapy drugs, including PD-1 and PD-L1 inhibitors, are antibodies. For example, immune checkpoint blockers targeting PD-1 are antibodies designed to bind PD-1 expressed by T cells and reactivate these cells to eliminate tumors. Anti-PD-1 drugs
contain not only an Fab region that binds PD-1 but also an Fc region.
Experimental work indicates that the Fc portion of cancer immunotherapy
drugs can affect the outcome of treatment. For example, anti-PD-1 drugs
with Fc regions that bind inhibitory Fc receptors can have decreased
therapeutic efficacy.
Imaging studies have further shown that the Fc region of anti-PD-1
drugs can bind Fc receptors expressed by tumor-associated macrophages.
This process removes the drugs from their intended targets (i.e. PD-1
molecules expressed on the surface of T cells) and limits therapeutic
efficacy. Furthermore, antibodies targeting the co-stimulatory protein CD40 require engagement with selective Fc receptors for optimal therapeutic efficacy. Together, these studies underscore the importance of Fc status in antibody-based immune checkpoint targeting strategies.
Human/non-human balance
Antibodies may also referred to as murine, chimeric, humanized or human. Murine antibodies are from mice and carry a risk of immune reaction. Chimeric antibodies attempt to reduce murine antibodies' immunogenicity
by replacing part of the antibody with the corresponding human
counterpart, known as the constant region. Humanized antibodies are
almost completely human; only the complementarity determining regions of the variable regions are derived from murine sources. Human antibodies have been produced using unmodified human DNA.
Antibody-dependent
cell-mediated cytotoxicity. When the Fc receptors on natural killer
(NK) cells interact with Fc regions of antibodies bound to cancer cells,
the NK cell releases perforin and granzyme, leading to cancer cell
apoptosis.
Cell death mechanisms
Antibody-dependent cell-mediated cytotoxicity (ADCC)
Antibody-dependent cell-mediated cytotoxicity
(ADCC) requires antibodies to bind to target cell surfaces. Antibodies
are formed of a binding region (Fab) and the Fc region that can be
detected by immune system cells via their Fc surface receptors.
Fc receptors are found on many immune system cells, including NK cells.
When NK cells encounter antibody-coated cells, the latter's Fc regions
interact with their Fc receptors, releasing perforin and granzyme B to kill the tumor cell. Examples include Rituximab, Ofatumumab, Elotuzumab,
and Alemtuzumab. Antibodies under development have altered Fc regions
that have higher affinity for a specific type of Fc receptor, FcγRIIIA,
which can dramatically increase effectiveness.
Complement
The complement system includes blood proteins that can cause cell death after an antibody binds to the cell surface (the classical complement pathway,
among the ways of complement activation). Generally the system deals
with foreign pathogens, but can be activated with therapeutic antibodies
in cancer. The system can be triggered if the antibody is chimeric,
humanized or human; as long as it contains the IgG1 Fc region. Complement can lead to cell death by activation of the membrane attack complex, known as complement-dependent cytotoxicity; enhancement of antibody-dependent cell-mediated cytotoxicity;
and CR3-dependent cellular cytotoxicity. Complement-dependent
cytotoxicity occurs when antibodies bind to the cancer cell surface, the
C1 complex binds to these antibodies and subsequently protein pores are
formed in the cancer cell membrane.
FDA-approved antibodies
| Antibody | Brand name | Type | Target | Approval date | Approved treatment(s) |
|---|---|---|---|---|---|
| Alemtuzumab | Campath | humanized | CD52 | 2001 | B-cell chronic lymphocytic leukemia (CLL) |
| Atezolizumab | Tecentriq | humanized | PD-L1 | 2016 | bladder cancer |
| Avelumab | Bavencio | human | PD-L1 | 2017 | metastatic Merkel cell carcinoma |
| Ipilimumab | Yervoy | human | CTLA4 | 2011 | metastatic melanoma |
| Elotuzumab | Empliciti | humanized | SLAMF7 | 2015 | Multiple myeloma |
| Ofatumumab | Arzerra | human | CD20 | 2009 | refractory CLL |
| Nivolumab | Opdivo | human | PD-1 | 2014 | unresectable or metastatic melanoma, squamous non-small cell lung cancer, Renal cell carcinoma, colorectal cancer, hepatocellular carcinoma, classical hodgkin lymphoma |
| Pembrolizumab | Keytruda | humanized | PD-1 | 2014 | unresectable or metastatic melanoma, squamous non-small cell lung cancer (NSCLC) , Hodgkin's lymphoma, Merkel-cell carcinoma (MCC), primary mediastinal B-cell lymphoma (PMBCL), stomach cancer, cervical cancer |
| Rituximab | Rituxan, Mabthera | chimeric | CD20 | 1997 | non-Hodgkin lymphoma |
| Durvalumab | Imfinzi | human | PD-L1 | 2017 | bladder cancer non-small cell lung cancer |
Alemtuzumab
Alemtuzumab (Campath-1H) is an anti-CD52 humanized IgG1 monoclonal antibody indicated for the treatment of fludarabine-refractory chronic lymphocytic leukemia (CLL), cutaneous T-cell lymphoma, peripheral T-cell lymphoma and T-cell prolymphocytic leukemia. CD52 is found on >95% of peripheral blood lymphocytes (both T-cells and B-cells) and monocytes,
but its function in lymphocytes is unknown. It binds to CD52 and
initiates its cytotoxic effect by complement fixation and ADCC
mechanisms. Due to the antibody target (cells of the immune system)
common complications of alemtuzumab therapy are infection, toxicity and myelosuppression.
Durvalumab
Durvalumab
(Imfinzi) is a human immunoglobulin G1 kappa (IgG1κ) monoclonal
antibody that blocks the interaction of programmed cell death ligand 1
(PD-L1) with the PD-1 and CD80 (B7.1) molecules. Durvalumab is approved
for the treatment of patients with locally advanced or metastatic
urothelial carcinoma who:
- have disease progression during or following platinum-containing chemotherapy.
- have disease progression within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy.
On 16 February 2018, the Food and Drug Administration approved
durvalumab for patients with unresectable stage III non-small cell lung
cancer (NSCLC) whose disease has not progressed following concurrent
platinum-based chemotherapy and radiation therapy.
Ipilimumab
Ipilimumab (Yervoy) is a human IgG1 antibody that binds the surface protein CTLA4. In normal physiology T-cells are activated by two signals: the T-cell receptor binding to an antigen-MHC complex and T-cell surface receptor CD28 binding to CD80 or CD86
proteins. CTLA4 binds to CD80 or CD86, preventing the binding of CD28
to these surface proteins and therefore negatively regulates the
activation of T-cells.
Active cytotoxic T-cells
are required for the immune system to attack melanoma cells. Normally
inhibited active melanoma-specific cytotoxic T-cells can produce an
effective anti-tumor response. Ipilumumab can cause a shift in the ratio
of regulatory T-cells
to cytotoxic T-cells to increase the anti-tumor response. Regulatory
T-cells inhibit other T-cells, which may benefit the tumor.
Nivolumab
Ofatumumab
Ofatumumab is a second generation human IgG1 antibody that binds to CD20. It is used in the treatment of chronic lymphocytic leukemia (CLL) because the cancerous cells of CLL are usually CD20-expressing B-cells. Unlike rituximab,
which binds to a large loop of the CD20 protein, ofatumumab binds to a
separate, small loop. This may explain their different characteristics.
Compared to rituximab, ofatumumab induces complement-dependent
cytotoxicity at a lower dose with less immunogenicity.
Pembrolizumab
As of 2019, pembrolizumab, which blocks PD-1, programmed cell death protein 1, has been used via intravenous infusion to treat inoperable or metastatic melanoma, metastatic non-small cell lung cancer (NSCLC) in certain situations, as a second-line treatment for head and neck squamous cell carcinoma (HNSCC), after platinum-based chemotherapy, and for the treatment of adult and pediatric patients with refractory classic Hodgkin's lymphoma (cHL). It is also indicated for certain patients with urothelial carcinoma, stomach cancer and cervical cancer.
Rituximab
Rituximab is a chimeric monoclonal IgG1 antibody specific for CD20, developed from its parent antibody Ibritumomab.
As with ibritumomab, rituximab targets CD20, making it effective in
treating certain B-cell malignancies. These include aggressive and
indolent lymphomas such as diffuse large B-cell lymphoma and follicular lymphoma and leukemias such as B-cell chronic lymphocytic leukemia. Although the function of CD20 is relatively unknown, CD20 may be a calcium channel involved in B-cell activation. The antibody's mode of action is primarily through the induction of ADCC and complement-mediated cytotoxicity. Other mechanisms include apoptosis and cellular growth arrest. Rituximab also increases the sensitivity of cancerous B-cells to chemotherapy.
Cytokine therapy
Cytokines
are proteins produced by many types of cells present within a tumor.
They can modulate immune responses. The tumor often employs them to
allow it to grow and reduce the immune response. These immune-modulating
effects allow them to be used as drugs to provoke an immune response.
Two commonly used cytokines are interferons and interleukins.
Interleukin-2 and interferon-α
are cytokines, proteins that regulate and coordinate the behavior of
the immune system. They have the ability to enhance anti-tumor activity
and thus can be used as passive cancer treatments. Interferon-α is used
in the treatment of hairy-cell leukaemia, AIDS-related Kaposi's sarcoma, follicular lymphoma, chronic myeloid leukaemia and malignant melanoma. Interleukin-2 is used in the treatment of malignant melanoma and renal cell carcinoma.
Interferon
Interferons
are produced by the immune system. They are usually involved in
anti-viral response, but also have use for cancer. They fall in three
groups: type I (IFNα and IFNβ), type II (IFNγ) and type III (IFNλ). IFNα has been approved for use in hairy-cell leukaemia, AIDS-related Kaposi's sarcoma, follicular lymphoma, chronic myeloid leukaemia
and melanoma. Type I and II IFNs have been researched extensively and
although both types promote anti-tumor immune system effects, only type I
IFNs have been shown to be clinically effective. IFNλ shows promise for
its anti-tumor effects in animal models.
Unlike type I IFNs, Interferon gamma is not approved yet for the treatment of any cancer. However, improved survival was observed when Interferon gamma was administrated to patients with bladder carcinoma and melanoma cancers. The most promising result was achieved in patients with stage 2 and 3 of ovarian carcinoma. The in vitro
study of IFN-gamma in cancer cells is more extensive and results
indicate anti-proliferative activity of IFN-gamma leading to the growth
inhibition or cell death, generally induced by apoptosis but sometimes by autophagy.
Interleukin
Interleukins have an array of immune system effects. Interleukin-2 is used in the treatment of malignant melanoma and renal cell carcinoma. In normal physiology it promotes both effector T cells and T-regulatory cells, but its exact mechanism of action is unknown.
Combination immunotherapy
Combining various immunotherapies such as PD1 and CTLA4 inhibitors can enhance anti-tumor response leading to durable responses.
Combining ablation therapy of tumors with immunotherapy enhances the immunostimulating response and has synergistic effects for curative metastatic cancer treatment.
Combining checkpoint immunotherapies with pharmaceutical agents
has the potential to improve response, and such combination therapies
are a highly investigated area of clinical investigation. Immunostimulatory drugs such as CSF-1R inhibitors and TLR agonists have been particularly effective in this setting.
Polysaccharide-K
Japan's Ministry of Health, Labour and Welfare approved the use of polysaccharide-K extracted from the mushroom, Coriolus versicolor, in the 1980s, to stimulate the immune systems of patients undergoing chemotherapy. It is a dietary supplement in the US and other jurisdictions.
Genetic pre-testing for therapeutic significance
Because
of the high cost of many of the immunotherapy medications and the
reluctance of medical insurance companies to prepay for their
prescriptions various test methods have been proposed, to attempt to
forecast the effectiveness of these medications. The detection of PD-L1
protein seemed to be an indication of cancer susceptible to several
immunotherapy medications, but research found that both the lack of this
protein or its inclusion in the cancerous tissue was inconclusive, due
to the little-understood varying quantities of the protein during
different times and locations within the infected cells and tissue.
In 2018 some genetic indications such as Tumor Mutational Burden (TMB, the number of mutations within a targeted genetic region in the cancerous cell's DNA), and Microsatellite instability
(MSI, the quantity of impaired DNA mismatch leading to probable
mutations), have been approved by the FDA as good indicators for the
probability of effective treatment of immunotherapy medication for
certain cancers, but research is still in progress.
In some cases the FDA has approved genetic tests for medication
that is specific to certain genetic markers. For example, the FDA
approved BRAF associated medication for metastatic melanoma, to be administered to patients after testing for the BRAF genetic mutation.
Tests of this sort are being widely advertised for general cancer
treatment and are expensive. In the past, some genetic testing for
cancer treatment has been involved in scams such as the Duke University
Cancer Fraud scandal, or claimed to be hoaxes.
Research
Adoptive T-cell therapy
Cancer
specific T-cells can be obtained by fragmentation and isolation of
tumour infiltrating lymphocytes, or by genetically engineering cells
from peripheral blood. The cells are activated and grown prior to
transfusion into the recipient (tumor bearer).
Adoptive T cell therapy is a form of passive immunization by the transfusion of T-cells (adoptive cell transfer). They are found in blood and tissue and usually activate when they find foreign pathogens.
Specifically they activate when the T-cell's surface receptors
encounter cells that display parts of foreign proteins on their surface
antigens. These can be either infected cells, or Antigen-presenting cells (APCs). They are found in normal tissue and in tumor tissue, where they are known as tumor infiltrating lymphocytes (TILs). They are activated by the presence of APCs such as dendritic cells that present tumor antigens.
Although these cells can attack the tumor, the environment within the
tumor is highly immunosuppressive, preventing immune-mediated tumour
death.
Multiple ways of producing and obtaining tumour targeted T-cells
have been developed. T-cells specific to a tumor antigen can be removed
from a tumor sample (TILs) or filtered from blood. Subsequent activation
and culturing is performed ex vivo, with the results reinfused. Activation can take place through gene therapy, or by exposing the T cells to tumor antigens.
As of 2014, multiple ACT clinical trials were underway.
Importantly, one study from 2018 showed that clinical responses can be
obtained in patients with metastatic melanoma resistant to multiple
previous immunotherapies.
The first 2 adoptive T-cell therapies, tisagenlecleucel and axicabtagene ciloleucel, were approved by the FDA in 2017.
Another approach is adoptive transfer of haploidentical γδ T cells or NK cells from a healthy donor. The major advantage of this approach is that these cells do not cause GVHD. The disadvantage is frequently impaired function of the transferred cells.
Anti-CD47 therapy
Many tumor cells overexpress CD47 to escape immunosurveilance of host immune system. CD47 binds to its receptor signal regulatory protein alpha (SIRPα) and downregulate phagocytosis of tumor cell.
Therefore, anti-CD47 therapy aims to restore clearance of tumor cells.
Additionally, growing evidence supports the employment of tumor
antigen-specific T cell response in response to anti-CD47 therapy. A number of therapeutics is being developed, including anti-CD47 antibodies, engineered decoy receptors, anti-SIRPα antibodies and bispecific agents. As of 2017, wide range of solid and hematologic malignancies were being clinically tested.
Anti-GD2 antibodies
The GD2 ganglioside
Carbohydrate antigens on the surface of cells can be used as targets for immunotherapy. GD2 is a ganglioside found on the surface of many types of cancer cell including neuroblastoma, retinoblastoma, melanoma, small cell lung cancer, brain tumors, osteosarcoma, rhabdomyosarcoma, Ewing's sarcoma, liposarcoma, fibrosarcoma, leiomyosarcoma and other soft tissue sarcomas.
It is not usually expressed on the surface of normal tissues, making it
a good target for immunotherapy. As of 2014, clinical trials were
underway.
Immune checkpoints
Cancer therapy by inhibition of negative immune regulation (CTLA4, PD1)
Immune checkpoints
affect immune system function. Immune checkpoints can be stimulatory or
inhibitory. Tumors can use these checkpoints to protect themselves from
immune system attacks. Currently approved checkpoint therapies block
inhibitory checkpoint receptors. Blockade of negative feedback signaling
to immune cells thus results in an enhanced immune response against
tumors.
One ligand-receptor interaction under investigation is the interaction between the transmembrane programmed cell death 1 protein (PDCD1, PD-1; also known as CD279) and its ligand, PD-1 ligand 1
(PD-L1, CD274). PD-L1 on the cell surface binds to PD1 on an immune
cell surface, which inhibits immune cell activity. Among PD-L1 functions
is a key regulatory role on T cell activities. It appears that
(cancer-mediated) upregulation of PD-L1 on the cell surface may inhibit T
cells that might otherwise attack. PD-L1 on cancer cells also inhibits
FAS- and interferon-dependent apoptosis, protecting cells from cytotoxic
molecules produced by T cells. Antibodies that bind to either PD-1 or
PD-L1 and therefore block the interaction may allow the T-cells to
attack the tumor.
CTLA-4 blockade
The first checkpoint antibody approved by the FDA was ipilimumab, approved in 2011 for treatment of melanoma. It blocks the immune checkpoint molecule CTLA-4.
Clinical trials have also shown some benefits of anti-CTLA-4 therapy on
lung cancer or pancreatic cancer, specifically in combination with
other drugs. In on-going trials the combination of CTLA-4 blockade with PD-1 or PD-L1 inhibitors is tested on different types of cancer.
However, patients treated with check-point blockade (specifically
CTLA-4 blocking antibodies), or a combination of check-point blocking
antibodies, are at high risk of suffering from immune-related adverse
events such as dermatologic, gastrointestinal, endocrine, or hepatic autoimmune reactions.
These are most likely due to the breadth of the induced T-cell
activation when anti-CTLA-4 antibodies are administered by injection in
the blood stream.
Using a mouse model of bladder cancer, researchers have found
that a local injection of a low dose anti-CTLA-4 in the tumour area had
the same tumour inhibiting capacity as when the antibody was delivered
in the blood.
At the same time the levels of circulating antibodies were lower,
suggesting that local administration of the anti-CTLA-4 therapy might
result in fewer adverse events.
PD-1 inhibitors
Initial clinical trial results with IgG4 PD1 antibody Nivolumab were published in 2010.
It was approved in 2014. Nivolumab is approved to treat melanoma, lung
cancer, kidney cancer, bladder cancer, head and neck cancer, and Hodgkin's lymphoma.
A 2016 clinical trial for non-small cell lung cancer failed to meet its
primary endpoint for treatment in the first line setting, but is FDA
approved in subsequent lines of therapy.
Pembrolizumab is another PD1 inhibitor that was approved by the FDA in 2014.
Keytruda (Pembrolizumab) is approved to treat melanoma and lung cancer.
Antibody BGB-A317 is a PD-1 inhibitor (designed to not bind Fc gamma receptor I) in early clinical trials.
PD-L1 inhibitors
In May 2016, PD-L1 inhibitor atezolizumab was approved for treating bladder cancer.
Anti-PD-L1 antibodies currently in development include avelumab and durvalumab, in addition to an affimer biotherapeutic.
Other
Other modes of enhancing [adoptive] immuno-therapy include targeting so-called intrinsic checkpoint blockades e.g. CISH.
A number of cancer patients do not respond to immune checkpoint
blockade. Response rate may be improved by combining immune checkpoint
blockade with additional rationally selected anticancer therapies (out
of which some may stimulate T cell infiltration into tumors). For
example, targeted therapies such, radiotherapy, vasculature targeting
agents, and immunogenic chemotherapy can improve immune checkpoint blockade response in animal models of cancer.
Oncolytic virus
An oncolytic virus is a virus that preferentially infects and kills cancer cells. As the infected cancer cells are destroyed by oncolysis,
they release new infectious virus particles or virions to help destroy
the remaining tumour. Oncolytic viruses are thought not only to cause
direct destruction of the tumour cells, but also to stimulate host
anti-tumour immune responses for long-term immunotherapy.
The potential of viruses as anti-cancer agents was first realized
in the early twentieth century, although coordinated research efforts
did not begin until the 1960s. A number of viruses including adenovirus,
reovirus, measles, herpes simplex, Newcastle disease virus and vaccinia
have now been clinically tested as oncolytic agents. T-Vec is the first
FDA-approved oncolytic virus for the treatment of melanoma. A number of other oncolytic viruses are in Phase II-III development.
Polysaccharides
Certain compounds found in mushrooms, primarily polysaccharides, can up-regulate the immune system and may have anti-cancer properties. For example, beta-glucans such as lentinan have been shown in laboratory studies to stimulate macrophage, NK cells, T cells and immune system cytokines and have been investigated in clinical trials as immunologic adjuvants.
Neoantigens
Many
tumors express mutations. These mutations potentially create new
targetable antigens (neoantigens) for use in T cell immunotherapy. The
presence of CD8+ T cells in cancer lesions, as identified using RNA
sequencing data, is higher in tumors with a high mutational burden.
The level of transcripts associated with cytolytic activity of natural
killer cells and T cells positively correlates with mutational load in
many human tumors. In non–small cell lung cancer patients treated with
lambrolizumab, mutational load shows a strong correlation with clinical
response. In melanoma patients treated with ipilimumab, long-term
benefit is also associated with a higher mutational load, although less
significantly. The predicted MHC binding neoantigens in patients with a
long-term clinical benefit were enriched for a series of tetrapeptide motifs that were not found in tumors of patients with no or minimal clinical benefit. However, human neoantigens identified in other studies do not show the bias toward tetrapeptide signatures.