From Wikipedia, the free encyclopedia
Iron, 26Fe
 |
| General properties |
|---|
| Appearance | lustrous metallic with a grayish tinge |
|---|
| Standard atomic weight (Ar, standard) | 55.845(2) |
|---|
| Iron in the periodic table |
|---|
|
|
| Atomic number (Z) | 26 |
|---|
| Group | group 8 |
|---|
| Period | period 4 |
|---|
| Block | d-block |
|---|
| Element category | transition metal |
|---|
| Electron configuration | [Ar] 3d6 4s2 |
|---|
Electrons per shell
| 2, 8, 14, 2 |
|---|
| Physical properties |
|---|
| Phase at STP | solid |
|---|
| Melting point | 1811 K (1538 °C, 2800 °F) |
|---|
| Boiling point | 3134 K (2862 °C, 5182 °F) |
|---|
| Density (near r.t.) | 7.874 g/cm3 |
|---|
| when liquid (at m.p.) | 6.98 g/cm3 |
|---|
| Heat of fusion | 13.81 kJ/mol |
|---|
| Heat of vaporization | 340 kJ/mol |
|---|
| Molar heat capacity | 25.10 J/(mol·K) |
|---|
Vapor pressure
| P (Pa)
|
1
|
10
|
100
|
1 k
|
10 k
|
100 k
|
| at T (K)
|
1728
|
1890
|
2091
|
2346
|
2679
|
3132
|
|
| Atomic properties |
|---|
| Oxidation states | −4, −2, −1, +1, +2, +3, +4, +5, +6, +7 |
|---|
| Electronegativity | Pauling scale: 1.83 |
|---|
| Ionization energies |
- 1st: 762.5 kJ/mol
- 2nd: 1561.9 kJ/mol
- 3rd: 2957 kJ/mol
|
|---|
| Atomic radius | empirical: 126 pm |
|---|
| Covalent radius | Low spin: 132±3 pm
High spin: 152±6 pm |
|---|
|
Spectral lines of iron |
| Other properties |
|---|
| Natural occurrence | primordial |
|---|
| Crystal structure | body-centered cubic (bcc)
a=286.65 pm |
|---|
| Crystal structure | face-centered cubic (fcc)
between 1185–1667 K |
|---|
| Speed of sound thin rod | 5120 m/s (at r.t.) (electrolytic) |
|---|
| Thermal expansion | 11.8 µm/(m·K) (at 25 °C) |
|---|
| Thermal conductivity | 80.4 W/(m·K) |
|---|
| Electrical resistivity | 96.1 nΩ·m (at 20 °C) |
|---|
| Curie point | 1043 K |
|---|
| Magnetic ordering | ferromagnetic |
|---|
| Young's modulus | 211 GPa |
|---|
| Shear modulus | 82 GPa |
|---|
| Bulk modulus | 170 GPa |
|---|
| Poisson ratio | 0.29 |
|---|
| Mohs hardness | 4 |
|---|
| Vickers hardness | 608 MPa |
|---|
| Brinell hardness | 200–1180 MPa |
|---|
| CAS Number | 7439-89-6 |
|---|
| History |
|---|
| Discovery | before 5000 BC |
|---|
| Main isotopes of iron |
|---|
|
|
Iron is a
chemical element with symbol
Fe (from
Latin:
ferrum) and
atomic number 26. It is a
metal in the
first transition series. It is by mass the most common element on
Earth, forming much of Earth's
outer and
inner core. It is the fourth most common
element in the Earth's crust. Its abundance in
rocky planets like Earth is due to its abundant production by
fusion in high-mass
stars, where it is the last element to be produced with release of energy before the violent collapse of a
supernova, which scatters the iron into space.
Like the other
group 8 elements,
ruthenium and
osmium, iron exists in a wide range of
oxidation states, −2 to +7, although +2 and +3 are the most common. Elemental iron occurs in
meteoroids and other low
oxygen environments, but is reactive to oxygen and
water. Fresh iron surfaces appear lustrous silvery-gray, but
oxidize in normal air to give
hydrated iron oxides, commonly known as
rust. Unlike the metals that form
passivating oxide layers, iron oxides occupy more volume than the metal and thus flake off, exposing fresh surfaces for corrosion.
Iron metal has been used since
ancient times, although
copper alloys,
which have lower melting temperatures, were used even earlier in human
history. Pure iron is relatively soft, but is unobtainable by
smelting because it is significantly hardened and strengthened by impurities, in particular
carbon, from the smelting process. A certain proportion of carbon (between 0.002% and 2.1%) produces
steel, which may be up to 1000 times harder than pure iron. Crude iron metal is produced in
blast furnaces, where ore is reduced by
coke to
pig iron,
which has a high carbon content. Further refinement with oxygen reduces
the carbon content to the correct proportion to make steel. Steels and
iron
alloys formed with other metals (
alloy steels)
are by far the most common industrial metals because they have a great
range of desirable properties and iron-bearing rock is abundant.
Iron chemical compounds have many uses. Iron oxide mixed with aluminum powder can be ignited to create a
thermite reaction, used in welding and purifying ores. Iron forms binary compounds with the
halogens and the
chalcogens. Among its organometallic compounds is
ferrocene, the first
sandwich compound discovered.
Iron plays an important role in biology, forming complexes with molecular oxygen in
hemoglobin and
myoglobin; these two compounds are common oxygen-handling proteins in vertebrates (hemoglobin for
oxygen transport, and myoglobin for oxygen storage). Iron is also the metal at the active site of many important
redox enzymes dealing with
cellular respiration and
oxidation and reduction
in plants and animals. Iron is distributed throughout the human body,
and is especially abundant in hemoglobin. Total iron content of the
adult human body is approximately 3.8 grams in males and 2.3 grams in
females. Iron is a critical element in the
metabolism of hundreds of
proteins and
enzymes involved in diverse body functions, such as
oxygen transport,
DNA synthesis, and
cell growth.
Characteristics
Mechanical properties
The mechanical properties of iron and its alloys can be evaluated using a variety of tests, including the
Brinell test,
Rockwell test and the
Vickers hardness test. The data on iron is so consistent that it is often used to calibrate measurements or to compare tests.
However, the mechanical properties of iron are significantly affected
by the sample's purity: pure, single crystals of iron are actually
softer than aluminum, and the purest industrially produced iron (99.99%) has a hardness of 20–30 Brinell.
An increase in the carbon content will cause a significant increase in
the hardness and tensile strength of iron. Maximum hardness of
65 Rc is achieved with a 0.6% carbon content, although the alloy has low tensile strength. Because of the softness of iron, it is much easier to work with than its heavier
congeners ruthenium and
osmium.
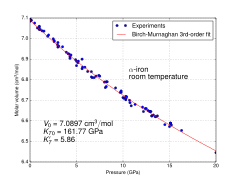
Molar volume vs. pressure for α iron at room temperature
Because of its significance for planetary cores, the physical
properties of iron at high pressures and temperatures have also been
studied extensively. The form of iron that is stable under standard
conditions can be subjected to pressures up to ca. 15 GPa before
transforming into a high-pressure form, as described in the next
section.
Phase diagram and allotropes
Iron represents an example of
allotropy
in a metal. At least four allotropic forms of iron are known as α, γ,
δ, and ε; at very high pressures and temperatures, some controversial
experimental evidence exists for a stable β phase.
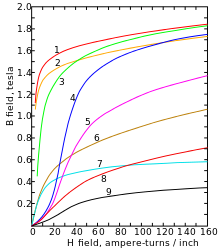
Magnetization curves of 9 ferromagnetic materials, showing saturation. 1. Sheet steel, 2. Silicon steel, 3. Cast steel, 4. Tungsten steel, 5. Magnet steel, 6. Cast iron, 7. Nickel, 8. Cobalt, 9. Magnetite
As molten iron cools past its freezing point of 1538 °C, it crystallizes into its δ allotrope, which has a
body-centered cubic (bcc)
crystal structure. As it cools further to 1394 °C, it changes to its γ-iron allotrope, a
face-centered cubic (fcc) crystal structure, or
austenite. At 912 °C and below, the crystal structure again becomes the bcc α-iron allotrope. Finally, at 770 °C (the
Curie point, T
c) iron's magnetic ordering changes from
paramagnetic to
ferromagnetic.
As it passes through the Curie temperature, iron does not change its
structure, but "magnetic domains" appear, where each domain contains
iron atoms with a particular electronic spin. In unmagnetized iron, all
the electronic spins of the atoms within one domain have the same axis
orientation; however, the electrons of neighboring domains have other
orientations with the result of mutual cancellation and no magnetic
field. In magnetized iron, the electronic spins of the domains are
aligned and the magnetic effects are reinforced. Although each domain
contains billions of atoms, they are very small, about 10 micrometres
across. This happens because the two unpaired electrons on each iron atom are in the d
z2 and d
x2 − y2
orbitals, which do not point directly at the nearest neighbors in the
body-centered cubic lattice and therefore do not participate in metallic
bonding; thus, they can interact magnetically with each other so that
their spins align.
At pressures above approximately 10 GPa and temperatures of a few hundred kelvin or less, α-iron changes into a
hexagonal close-packed (hcp) structure, which is also known as
ε-iron;
the higher-temperature γ-phase also changes into ε-iron, but does so at
higher pressure. The β-phase, if it exists, would appear at pressures
of at least 50 GPa and temperatures of at least 1500 K and have an
orthorhombic or a double hcp structure. These high-pressure phases of iron are important as endmember models for the solid parts of planetary cores. The
inner core of the
Earth is generally presumed to be an iron-
nickel alloy with ε (or β) structure.
Somewhat confusingly, the term "β-iron" is sometimes also used to refer
to α-iron above its Curie point, when it changes from being
ferromagnetic to paramagnetic, even though its crystal structure has not
changed.
The melting point of iron is experimentally well defined for
pressures less than 50 GPa. For greater pressures, studies put the
γ-ε-liquid
triple point at pressures that differ by tens of gigapascals and 1000 K in the melting point. Generally speaking,
molecular dynamics
computer simulations of iron melting and shock wave experiments suggest
higher melting points and a much steeper slope of the melting curve
than static experiments carried out in
diamond anvil cells. The melting and boiling points of iron, along with its
enthalpy of atomization, are lower than those of the earlier 3d elements from
scandium to
chromium,
showing the lessened contribution of the 3d electrons to metallic
bonding as they are attracted more and more into the inert core by the
nucleus; however, they are higher than the values for the previous element
manganese
because that element has a half-filled 3d subshell and consequently its
d-electrons are not easily delocalized. This same trend appears for
ruthenium but not osmium.
Isotopes
Naturally occurring iron consists of four stable
isotopes: 5.845% of
54Fe, 91.754% of
56Fe, 2.119% of
57Fe and 0.282% of
58Fe. Of these stable isotopes, only
57Fe has a nuclear
spin (−
1⁄2). The
nuclide 54Fe theoretically can undergo
double electron capture to
54Cr, but the process has never been observed and only a lower limit on the half-life of 3.1×10
22 years has been established.
60Fe is an
extinct radionuclide of long
half-life (2.6 million years). It is not found on Earth, but its ultimate decay product is its granddaughter, the stable nuclide
60Ni. Much of the past work on isotopic composition of iron has focused on the
nucleosynthesis of
60Fe through studies of
meteorites and ore formation. In the last decade, advances in
mass spectrometry have allowed the detection and quantification of minute, naturally occurring variations in the ratios of the
stable isotopes of iron. Much of this work is driven by the
Earth and
planetary science communities, although applications to biological and industrial systems are emerging.
In phases of the meteorites
Semarkona and
Chervony Kut, a correlation between the concentration of
60Ni, the
granddaughter of
60Fe, and the abundance of the stable iron isotopes provided evidence for the existence of
60Fe at the time of
formation of the Solar System. Possibly the energy released by the decay of
60Fe, along with that released by
26Al, contributed to the remelting and
differentiation of
asteroids after their formation 4.6 billion years ago. The abundance of
60Ni present in
extraterrestrial material may bring further insight into the origin and early history of the
Solar System.
The most abundant iron isotope
56Fe is of particular interest to nuclear scientists because it represents the most common endpoint of
nucleosynthesis. Since
56Ni (14
alpha particles) is easily produced from lighter nuclei in the
alpha process in
nuclear reactions in supernovae (see
silicon burning process), it is the endpoint of fusion chains inside
extremely massive stars, since addition of another alpha particle, resulting in
60Zn, requires a great deal more energy. This
56Ni,
which has a half-life of about 6 days, is created in quantity in these
stars, but soon decays by two successive positron emissions within
supernova decay products in the
supernova remnant gas cloud, first to radioactive
56Co, and then to stable
56Fe. As such, iron is the most abundant element in the core of
red giants, and is the most abundant metal in
iron meteorites and in the dense metal
cores of planets such as
Earth. It is also very common in the universe, relative to other stable
metals of approximately the same
atomic weight. Iron is the sixth most
abundant element in the
Universe, and the most common
refractory element.
Although a further tiny energy gain could be extracted by synthesizing
62Ni, which has a marginally higher binding energy than
56Fe,
conditions in stars are unsuitable for this process. Element production
in supernovas and distribution on Earth greatly favor iron over nickel,
and in any case,
56Fe still has a lower mass per nucleon than
62Ni due to its higher fraction of lighter protons. Hence, elements heavier than iron require a
supernova for their formation, involving
rapid neutron capture by starting
56Fe nuclei.
In the
far future of the universe, assuming that
proton decay does not occur, cold
fusion occurring via
quantum tunneling would cause the light nuclei in ordinary matter to fuse into
56Fe nuclei. Fission and
alpha-particle emission would then make heavy nuclei decay into iron, converting all stellar-mass objects to cold spheres of pure iron.
Iron meteorites, similar in composition to the Earth's inner- and outer core
Occurrence
Metallic or
native iron
is rarely found on the surface of the Earth because it tends to
oxidize, but its oxides are pervasive and represent the primary ores.
While it makes up about 5% of the
Earth's crust, both the Earth's
inner and
outer core are believed to consist largely of an iron-
nickel
alloy constituting 35% of the mass of the Earth as a whole. Iron is
consequently the most abundant element on Earth, but only the fourth
most abundant element in the Earth's crust, after
oxygen,
silicon, and
aluminium. Most of the iron in the crust is found combined with oxygen as
iron oxide minerals such as
hematite (Fe
2O
3),
magnetite (Fe
3O
4), and
siderite (FeCO
3). Many
igneous rocks also contain the sulfide minerals
pyrrhotite and
pentlandite.
Ferropericlase (Mg,Fe)O, a solid solution of
periclase (MgO) and
wüstite (FeO), makes up about 20% of the volume of the
lower mantle of the Earth, which makes it the second most abundant mineral phase in that region after
silicate perovskite (Mg,Fe)SiO
3; it also is the major host for iron in the lower mantle. At the bottom of the
transition zone of the mantle, the reaction γ-(Mg,Fe)
2[SiO
4] ↔ (Mg,Fe)[SiO
3] + (Mg,Fe)O transforms
γ-olivine
into a mixture of silicate perovskite and ferropericlase and vice
versa. In the literature, this mineral phase of the lower mantle is also
often called magnesiowüstite.
Silicate perovskite may form up to 93% of the lower mantle, and the magnesium iron form, (Mg,Fe)SiO
3, is considered to be the most abundant
mineral in the Earth, making up 38% of its volume.
Large deposits of iron are found in
banded iron formations.
These geological formations are a type of rock consisting of repeated
thin layers of iron oxides alternating with bands of iron-poor
shale and
chert. The banded iron formations were laid down in the time between
3,700 million years ago and
1,800 million years ago.
The mentioned iron compounds have been used as
pigments (compare
ochre) since historical time and contribute as well to the color of various geological formations, e.g. the
Buntsandstein (British
Bunter, colored sandstein). In the case of the Eisensandstein (a jurassic 'iron sandstone', e.g. from
Donzdorf) in Germany and
Bath stone in the UK, iron pigments contribute to the yellowish color of large amounts of historical buildings and sculptures. The proverbial
red color of the surface of Mars is derived from an iron oxide-rich
regolith.
Significant amounts of iron occur in the iron sulfide mineral
pyrite (FeS
2),
but it is difficult to extract iron from it and it is therefore not
used. In fact, iron is so common that production generally focuses only
on ores with very high quantities of it. During
weathering,
iron tends to leach from sulfide deposits as the sulfate and from
silicate deposits as the bicarbonate. Both of these are oxidized in
aqueous solution and precipitate in even mildly elevated pH as
iron(III) oxide.
Chemistry and compounds
Iron shows the characteristic chemical properties of the
transition metals,
namely the ability to form variable oxidation states differing by steps
of one and a very large coordination and organometallic chemistry:
indeed, it was the discovery of an iron compound,
ferrocene, that revolutionalized the latter field in the 1950s.
Iron is sometimes considered as a prototype for the entire block of
transition metals, due to its abundance and the immense role it has
played in the technological progress of humanity. Its 26 electrons are arranged in the
configuration [Ar]3d
64s
2,
of which the 3d and 4s electrons are relatively close in energy, and
thus it can lose a variable number of electrons and there is no clear
point where further ionization becomes unprofitable.
Iron forms compounds mainly in the +2 and +3
oxidation states. Traditionally, iron(II) compounds are called
ferrous, and iron(III) compounds
ferric. Iron also occurs in
higher oxidation states, e.g. the purple
potassium ferrate (K
2FeO
4), which contains iron in its +6 oxidation state. Although iron(VIII) oxide (FeO
4)
has been claimed, the report could not be reproduced and such a species
(at least with iron in its +8 oxidation state) has been found to be
improbable computationally. However, one form of anionic [FeO
4]
–
with iron in its +7 oxidation state, along with an iron(V)-peroxo
isomer, has been detected by infrared spectroscopy at 4 K after
cocondensation of laser-ablated Fe atoms with a mixture of O
2/Ar. Iron(IV) is a common intermediate in many biochemical oxidation reactions. Numerous
organoiron
compounds contain formal oxidation states of +1, 0, −1, or even −2. The
oxidation states and other bonding properties are often assessed using
the technique of
Mössbauer spectroscopy.
Many
mixed valence compounds contain both iron(II) and iron(III) centers, such as
magnetite and
Prussian blue (Fe
4(Fe[CN]
6)
3). The latter is used as the traditional "blue" in
blueprints.
Iron is the first of the transition metals that cannot reach its
group oxidation state of +8, although its heavier congeners ruthenium
and osmium can, with ruthenium having more difficulty than osmium.
Ruthenium exhibits an aqueous cationic chemistry in its low oxidation
states similar to that of iron, but osmium does not, favoring high
oxidation states in which it forms anionic complexes.
In the second half of the 3d transition series, vertical similarities
down the groups compete with the horizontal similarities of iron with
its neighbors
cobalt and
nickel in the periodic table, which are also ferromagnetic at
room temperature and share similar chemistry. As such, iron, cobalt, and nickel are sometimes grouped together as the
iron triad.
The iron compounds produced on the largest scale in industry are
iron(II) sulfate (FeSO
4·7
H2O) and
iron(III) chloride (FeCl
3). The former is one of the most readily available sources of iron(II), but is less stable to aerial oxidation than
Mohr's salt ((NH
4)
2Fe(SO
4)
2·6H
2O). Iron(II) compounds tend to be oxidized to iron(III) compounds in the air.
Unlike many other metals, iron does not form amalgams with
mercury. As a result, mercury is traded in standardized 76 pound flasks (34 kg) made of iron.
Iron is by far the most reactive element in its group; it is
pyrophoric when finely divided and dissolves easily in dilute acids,
giving Fe
2+. However, it does not react with concentrated
nitric acid and other oxidizing acids due to the formation of an impervious oxide layer, which can nevertheless react with
hydrochloric acid.
Binary compounds
The binary ferrous and ferric halides are well-known, with the
exception of ferric iodide. The ferrous halides typically arise from
treating iron metal with the corresponding
hydrohalic acid to give the corresponding hydrated salts.
- Fe + 2 HX → FeX2 + H2 (X = F, Cl, Br, I)
Iron reacts with fluorine, chlorine, and bromine to give the corresponding ferric halides,
ferric chloride being the most common.
- 2 Fe + 3 X2 → 2 FeX3 (X = F, Cl, Br)
Ferric iodide is an exception, being thermodynamically unstable due to the oxidizing power of Fe3+ and the high reducing power of I−:
- 2 I− + 2 Fe3+ → I2 + 2 Fe2+ (E0 = +0.23 V)
Nevertheless, milligram amounts of ferric iodide, a black solid, may still be prepared through the reaction of
iron pentacarbonyl with
iodine and
carbon monoxide in the presence of
hexane and light at the temperature of −20 °C, making sure that the system is well sealed off from air and water.
Solution chemistry
Comparison of colors of solutions of ferrate (left) and permanganate (right)
| Fe2+ + 2 e− |
⇌ Fe |
E0 = −0.447 V
|
| Fe3+ + 3 e− |
⇌ Fe |
E0 = −0.037 V
|
FeO2−
4 + 8 H+ + 3 e− |
⇌ Fe3+ + 4 H2O |
E0 = +2.20 V
|
The red-purple tetrahedral
ferrate(VI)
anion is such a strong oxidizing agent that it oxidizes nitrogen and
ammonia at room temperature, and even water itself in acidic or neutral
solutions:
- 4 FeO2−
4 + 10 H
2O → 4 Fe3+ + 20 OH− + 3 O2
The Fe3+ ion has a large simple cationic chemistry, although the pale-violet hexaquo ion [Fe(H2O)6]3+ is very readily hydrolyzed when pH increases above 0 as follows:
| [Fe(H2O)6]3+ |
⇌ [Fe(H2O)5(OH)]2+ + H+ |
K = 10−3.05 mol dm−3
|
| [Fe(H2O)5(OH)]2+ |
⇌ [Fe(H2O)4(OH)2]+ + H+ |
K = 10−3.26 mol dm−3
|
| 2 [Fe(H2O)6]3+ |
⇌ [Fe(H
2O)
4(OH)]4+
2 + 2 H+ + 2 H2O |
K = 10−2.91 mol dm−3
|
As pH rises above 0 the above yellow hydrolyzed species form and as it rises above 2–3, reddish-brown hydrous
iron(III) oxide precipitates out of solution. Although Fe
3+ has an d
5 configuration, its absorption spectrum is not like that of Mn
2+ with its weak, spin-forbidden d–d bands, because Fe
3+ has higher positive charge and is more polarizing, lowering the energy of its ligand-to-metal
charge transfer
absorptions. Thus, all the above complexes are rather strongly colored,
with the single exception of the hexaquo ion – and even that has a
spectrum dominated by charge transfer in the near ultraviolet region. On the other hand, the pale green iron(II) hexaquo ion [Fe(H
2O)
6]
2+ does not undergo appreciable hydrolysis. Carbon dioxide is not evolved when
carbonate anions are added, which instead results in white
iron(II) carbonate
being precipitated out. In excess carbon dioxide this forms the
slightly soluble bicarbonate, which occurs commonly in groundwater, but
it oxidizes quickly in air to form
iron(III) oxide that accounts for the brown deposits present in a sizeable number of streams.
Coordination compounds
Ball-and-stick model of a chain in the crystal structure of iron(II) oxalate dihydrate
Prussian blue, Fe
4[Fe(CN)
6]
3,
is the most famous of the cyanide complexes of iron. Its formation can
be used as a simple wet chemistry test to distinguish between aqueous
solutions of Fe
2+ and Fe
3+ as they react (respectively) with
potassium ferricyanide and
potassium ferrocyanide to form Prussian blue.
Blood-red positive thiocyanate test for iron(III)
Iron(III) complexes are quite similar to those of
chromium(III) with the exception of iron(III)'s preference for
O-donor instead of
N-donor
ligands. The latter tend to be rather more unstable than iron(II)
complexes and often dissociate in water. Many Fe–O complexes show
intense colors and are used as tests for
phenols or
enols. For example, in the
ferric chloride test, used to determine the presence of phenols,
iron(III) chloride reacts with a phenol to form a deep violet complex:
- 3 ArOH + FeCl3 → Fe(OAr)3 + 3 HCl (Ar = aryl)
Among the halide and pseudohalide complexes, fluoro complexes of iron(III) are the most stable, with the colorless [FeF
5(H
2O)]
2− being the most stable in aqueous solution. Chloro complexes are less stable and favor tetrahedral coordination as in [FeCl
4]
−; [FeBr
4]
− and [FeI
4]
− are reduced easily to iron(II).
Thiocyanate is a common test for the presence of iron(III) as it forms the blood-red [Fe(SCN)(H
2O)
5]
2+. Like manganese(II), most iron(III) complexes are high-spin, the exceptions being those with ligands that are high in the
spectrochemical series such as
cyanide. An example of a low-spin iron(III) complex is [Fe(CN)
6]
3−. The cyanide ligands may easily be detached in [Fe(CN)
6]
3−, and hence this complex is poisonous, unlike the iron(II) complex [Fe(CN)
6]
4− found in Prussian blue, which does not release
hydrogen cyanide except when dilute acids are added. Iron shows a great variety of electronic
spin states, including every possible spin quantum number value for a d-block element from 0 (diamagnetic) to
5⁄2
(5 unpaired electrons). This value is always half the number of
unpaired electrons. Complexes with zero to two unpaired electrons are
considered low-spin and those with four or five are considered
high-spin.
Iron(II) complexes are less stable than iron(III) complexes but the preference for O-donor ligands is less marked, so that for example [Fe(NH3)6]2+ is known while [Fe(NH3)6]3+ is not. They have a tendency to be oxidized to iron(III) but this can be moderated by low pH and the specific ligands used.
Organometallic compounds
Fulvalene, which Pauson and Kealy sought to prepare
The (incorrect) structure for ferrocene that Pauson and Kealy proposed
Ferrocene was an extremely important compound in the early history of the branch of
organometallic chemistry, and to this day iron is still one of the most important metals in this field. It was first synthesized in 1951 during an attempt to prepare the
fulvalene (C
10H
8) by oxidative dimerization of
cyclopentadiene; the resultant product was found to have
molecular formula C
10H
10Fe and reported to exhibit "remarkable stability". The discovery sparked substantial interest in the field of organometallic chemistry,
in part because the structure proposed by Pauson and Kealy (shown at
right) was inconsistent with then-existing bonding models and did not
explain its unexpected stability. Consequently, the initial challenge
was to definitively determine the structure of ferrocene in the hope
that its bonding and properties would then be understood. The shockingly
novel sandwich structure, [Fe(η
5-C
5H
5)
2], was deduced and reported independently by three groups in 1952:
Robert Burns Woodward and
Geoffrey Wilkinson investigated the reactivity in order to determine the structure and demonstrated that ferrocene undergoes similar reactions to a typical aromatic molecule (such as
benzene),
Ernst Otto Fischer deduced the sandwich structure and also began synthesising other
metallocenes including
cobaltocene; Eiland and Pepinsky provided
X-ray crystallographic confirmation of the sandwich structure.
Applying
valence bond theory to ferrocene by considering an Fe
2+ center and two cyclopentadienide anions (C
5H
5−), which are known to be
aromatic according to
Hückel's rule and hence highly stable, allowed correct prediction of the geometry of the molecule. Once
molecular orbital theory was successfully applied and the
Dewar-Chatt-Duncanson model proposed, the reasons for ferrocene's remarkable stability became clear. Ferrocene was not the first organometallic compound known –
Zeise's salt, K[PtCl
3(C
2H
4)]·H
2O was reported in 1831 and
Mond's discovery of Ni(CO)
4 occurred in 1888, but it was ferrocene's discovery that began
organometallic chemistry as a separate area of chemistry. It was so important that Wilkinson and Fischer shared the 1973
Nobel Prize for Chemistry "for their pioneering work, performed independently, on the chemistry of the organometallic, so called
sandwich compounds". Ferrocene itself can be used as the backbone of a ligand, e.g.
1,1'-bis(diphenylphosphino)ferrocene (dppf). Ferrocene can itself be oxidized to the
ferrocenium cation (Fc
+); the ferrocene/ferrocenium couple is often used as a reference in electrochemistry.
Etymology
"iren," an Old English word for 'iron'
As iron has been in use for such a long time, it has many different
names in different languages. The source of its chemical symbol
Fe is the Latin word
ferrum, and its descendants are the names of the element in the
Romance languages (for example,
French fer,
Spanish hierro, and
Italian and
Portuguese ferro). The word
ferrum itself possibly comes from the
Semitic languages, via
Etruscan, from a root that also gave rise to
Old English bræs "
brass". The English word
iron derives ultimately from
Proto-Germanic *isarnan, which is also the source of the German name
Eisen. It was most likely borrowed from
Celtic *isarnon, which ultimately comes from
Proto-Indo-European *is-(e)ro- "powerful, holy" and finally
*eis "strong", referencing iron's strength as a metal. Kluge relates
*isarnon to Illyric and Latin
ira, 'wrath'). The
Balto-Slavic names for iron (e.g.
Russian железо [
zhelezo],
Polish żelazo,
Lithuanian geležis) are the only ones to come directly from the Proto-Indo-European
*ghelgh- "iron". In many of these languages, the word for
iron
may also be used to denote other objects made of iron or steel, or
figuratively because of the hardness and strength of the metal. The
Chinese tiě (
traditional 鐵;
simplified 铁) derives from
Proto-Sino-Tibetan *hliek, and was borrowed into
Japanese as 鉄
tetsu, which also has the native reading
kurogane "black metal" (similar to how iron is referenced in the English word
blacksmith).
History
Wrought iron
The symbol for Mars has been used since antiquity to represent iron.
The iron pillar of Delhi is an example of the iron extraction and processing methodologies of early India.
Iron is one of the elements undoubtedly known to the ancient world. It has been worked, or
wrought,
for millennia. However, iron objects of great age are much rarer than
objects made of gold or silver due to the ease with which iron corrodes.
Beads made from
meteoric iron in 3500 BC or earlier were found in Gerzah, Egypt by G.A. Wainwright.
The beads contain 7.5% nickel, which is a signature of meteoric origin
since iron found in the Earth's crust generally has only minuscule
nickel impurities. Meteoric iron was highly regarded due to its origin
in the heavens and was often used to forge weapons and tools. For example, a
dagger made of meteoric iron was found in the tomb of
Tutankhamun,
containing similar proportions of iron, cobalt, and nickel to a
meteorite discovered in the area, deposited by an ancient meteor shower. Items that were likely made of iron by Egyptians date from 3000 to 2500 BC. Meteoritic iron is comparably soft and ductile and easily forged by
cold working but may get brittle when heated because of the
nickel content.
The first iron production started in the
Middle Bronze Age but it took several centuries before iron displaced bronze. Samples of
smelted iron from
Asmar, Mesopotamia and Tall Chagar Bazaar in northern Syria were made sometime between 3000 and 2700 BC. The
Hittites established an empire in north-central
Anatolia
around 1600 BC. They appear to be the first to understand the
production of iron from its ores and regard it highly in their society. The
Hittites
began to smelt iron between 1500 and 1200 BC and the practice spread to
the rest of the Near East after their empire fell in 1180 BC. The subsequent period is called the
Iron Age.
Artifacts of smelted iron are found in
India dating from 1800 to 1200 BC, and in the
Levant from about 1500 BC (suggesting smelting in
Anatolia or the
Caucasus). Alleged references (compare
history of metallurgy in South Asia) to iron in the Indian
Vedas have been used for claims of a very early usage of iron in India respectively to date the texts as such. The
rigveda term
ayas (metal) probably refers to copper and bronze, while iron or
śyāma ayas, literally "black metal", first is mentioned in the post-rigvedic
Atharvaveda.
Some archaeological evidence suggests iron was smelted in
Zimbabwe and southeast Africa as early as the eighth century BC. Iron working was introduced to
Greece in the late 11th century BC, from which it spread quickly throughout Europe.
The spread of ironworking in Central and Western Europe is associated with
Celtic expansion. According to
Pliny the Elder, iron use was common in the
Roman era. The annual iron output of the
Roman Empire is estimated at
84750 tonnes, while the similarly populous and contemporary Han China produced around
5000 tonnes. In China, iron only appears circa 700–500 BC. Iron smelting may have been introduced into China through Central Asia. The earliest evidence of the use of a
blast furnace in China dates to the 1st century AD, and cupola furnaces were used as early as the
Warring States period (403–221 BC). Usage of the blast and cupola furnace remained widespread during the
Song and
Tang Dynasties.
Cast iron
Cast iron was first produced in
China during 5th century BC, but was hardly in Europe until the medieval period. The earliest
cast iron artifacts were discovered by archaeologists in what is now modern
Luhe County,
Jiangsu in China. Cast iron was used in
ancient China for warfare, agriculture, and architecture. During the
medieval period, means were found in Europe of producing wrought iron from cast iron (in this context known as
pig iron) using
finery forges. For all these processes,
charcoal was required as fuel.
Medieval
blast furnaces were about 10 feet (3.0 m) tall and made of fireproof brick; forced air was usually provided by hand-operated bellows.
Modern blast furnaces have grown much bigger, with hearths fourteen
meters in diameter that allow them to produce thousands of tons of iron
each day, but essentially operate in much the same way as they did
during medieval times.
In 1709,
Abraham Darby I established a
coke-fired
blast furnace to produce cast iron, replacing charcoal, although
continuing to use blast furnaces. The ensuing availability of
inexpensive iron was one of the factors leading to the
Industrial Revolution.
Toward the end of the 18th century, cast iron began to replace wrought
iron for certain purposes, because it was cheaper. Carbon content in
iron was not implicated as the reason for the differences in properties
of wrought iron, cast iron, and steel until the 18th century.
Since iron was becoming cheaper and more plentiful, it also
became a major structural material following the building of the
innovative
first iron bridge
in 1778. This bridge still stands today as a monument to the role iron
played in the Industrial Revolution. Following this, iron was used in
rails, boats, ships, aqueducts, and buildings, as well as in iron
cylinders in
steam engines. Railways have been central to the formation of modernity and ideas of progress and various languages (e.g. French, Spanish, Italian and German) refer to railways as
iron road.
Steel
Steel (with smaller carbon content than pig iron but more than wrought iron) was first produced in antiquity by using a
bloomery. Blacksmiths in
Luristan in western Persia were making good steel by 1000 BC. Then improved versions,
Wootz steel by India and
Damascus steel
were developed around 300 BC and AD 500 respectively. These methods
were specialized, and so steel did not become a major commodity until
the 1850s.
New methods of producing it by
carburizing bars of iron in the
cementation process were devised in the 17th century. In the
Industrial Revolution,
new methods of producing bar iron without charcoal were devised and
these were later applied to produce steel. In the late 1850s,
Henry Bessemer
invented a new steel making process, involving blowing air through
molten pig iron, to produce mild steel. This made steel much more
economical, thereby leading to wrought iron no longer being produced in
large quantities.
Foundations of modern chemistry
In 1774,
Antoine Lavoisier used the reaction of water steam with metallic iron inside an incandescent iron tube to produce
hydrogen in his experiments leading to the demonstration of the
conservation of mass, which was instrumental in changing chemistry from a qualitative science to a quantitative one.
Symbolic role
"Gold gab ich für Eisen" – "I gave gold for iron". German-American brooch from WWI.
Iron plays a certain role in mythology and has found various usage
as a metaphor and in
folklore. The
Greek poet
Hesiod's
Works and Days (lines 109–201) lists different
ages of man named after metals like gold, silver, bronze and iron to account for successive ages of humanity. The Iron Age was closely related with Rome, and in Ovid's
Metamorphoses
The Virtues, in despair, quit the earth; and the depravity of man becomes universal and complete. Hard steel succeeded then.
An example of the importance of iron's symbolic role may be found in the
German Campaign of 1813.
Frederick William III commissioned then the first
Iron Cross as military decoration.
Berlin iron jewellery reached its peak production between 1813 and 1815, when the Prussian
royal family urged citizens to donate gold and silver jewellery for military funding. The inscription
Gold gab ich für Eisen (I gave gold for iron) was used as well in later war efforts.
Production of metallic iron
Industrial routes
The production of iron or steel is a process consisting of two main
stages. In the first stage pig iron is produced in a blast furnace.
Alternatively, it may be directly reduced. In the second stage, pig iron
is converted to wrought iron, steel, or cast iron.
The fining process of smelting iron ore to make wrought iron from pig iron, with the right illustration displaying men working a blast furnace, from the Tiangong Kaiwu encyclopedia, published in 1637 by Song Yingxing.
How iron was extracted in the 19th century
For a few limited purposes when it is needed, pure iron is produced
in the laboratory in small quantities by reducing the pure oxide or
hydroxide with hydrogen, or forming iron pentacarbonyl and heating it to
250 °C so that it decomposes to form pure iron powder. Another method is electrolysis of ferrous chloride onto an iron cathode.
Blast furnace processing
Industrial iron production starts with iron ores, principally
hematite, which has a nominal formula Fe
2O
3, and
magnetite, with the formula Fe
3O
4. These ores are reduced to the metal in a
carbothermic
reaction, i.e. by treatment with carbon. The conversion is typically
conducted in a blast furnace at temperatures of about 2000 °C. Carbon is
provided in the form of coke. The process also contains a
flux such as
limestone,
which is used to remove silicaceous minerals in the ore, which would
otherwise clog the furnace. The coke and limestone are fed into the top
of the furnace, while a massive blast of
air heated to 900 °C, about 4 tons per ton of iron, is forced into the furnace at the bottom.
In the furnace, the coke reacts with oxygen in the air blast to produce
carbon monoxide:
- 2 C + O2 → 2 CO
- Fe2O3 + 3 CO → 2 Fe + 3 CO2
Some iron in the high-temperature lower region of the furnace reacts directly with the coke:
- 2 Fe2O3 + 3 C → 4 Fe + 3 CO2
The flux present to melt impurities in the ore is principally
limestone (
calcium carbonate) and
dolomite
(calcium-magnesium carbonate). Other specialized fluxes are used
depending on the details of the ore. In the heat of the furnace the
limestone flux decomposes to
calcium oxide (also known as quicklime):
- CaCO3 → CaO + CO2
Then calcium oxide combines with silicon dioxide to form a liquid
slag.
- CaO + SiO2 → CaSiO3
The slag melts in the heat of the furnace. In the bottom of the
furnace, the molten slag floats on top of the denser molten iron, and
apertures in the side of the furnace are opened to run off the iron and
the slag separately. The iron, once cooled, is called pig iron, while
the slag can be used as a material in
road construction or to improve mineral-poor soils for
agriculture.
This heap of iron ore pellets will be used in steel production.
Direct iron reduction
Owing to environmental concerns, alternative methods of processing iron have been developed. "
Direct iron reduction"
reduces iron ore to a ferrous lump called
"sponge" iron or "direct" iron that is suitable for steelmaking. Two main reactions comprise the direct reduction process:
Natural gas is partially oxidized (with heat and a catalyst):
- 2 CH4 + O2 → 2 CO + 4 H2
Iron ore is then treated with these gases in a furnace, producing solid sponge iron:
- Fe2O3 + CO + 2 H2 → 2 Fe + CO2 + 2 H2O
Thermite
Iron is a byproduct of burning a mixture of aluminium powder and rust powder.
- Fe2O3 + 2 Al → 2 Fe + Al2O3
Further processes
A pot of molten iron being used to make steel
Pig iron is not pure iron, but has 4–5% carbon dissolved in it with
small amounts of other impurities like sulfur, magnesium, phosphorus,
and manganese. As the carbon is the major impurity, the iron (pig iron)
becomes brittle and hard. Removing the other impurities results in cast iron, which is used to cast articles in
foundries; for example stoves, pipes, radiators, lamp-posts, and rails.
Annealing
involves the heating of a piece of steel to 700–800 °C for several
hours and then gradual cooling. It makes the steel softer and more
workable.
Applications
Iron-carbon phase diagram
Metallurgical
Iron is the most widely used of all the metals, accounting for over
90% of worldwide metal production. Its low cost and high strength make
it indispensable in engineering applications such as the construction of
machinery and
machine tools,
automobiles, the
hulls of large
ships,
and structural components for buildings. Since pure iron is quite soft,
it is most commonly combined with alloying elements to make steel.
α-Iron is a fairly soft metal that can dissolve only a small concentration of carbon (no more than 0.021% by mass at 910 °C).
Austenite
(γ-iron) is similarly soft and metallic but can dissolve considerably
more carbon (as much as 2.04% by mass at 1146 °C). This form of iron is
used in the type of
stainless steel used for making cutlery, and hospital and food-service equipment.
Commercially available iron is classified based on purity and the abundance of additives.
Pig iron has 3.5–4.5% carbon and contains varying amounts of contaminants such as
sulfur, silicon and
phosphorus.
Pig iron is not a saleable product, but rather an intermediate step in
the production of cast iron and steel. The reduction of contaminants in
pig iron that negatively affect material properties, such as sulfur and
phosphorus, yields cast iron containing 2–4% carbon, 1–6% silicon, and
small amounts of
manganese. Pig iron has a
melting point
in the range of 1420–1470 K, which is lower than either of its two main
components, and makes it the first product to be melted when carbon and
iron are heated together. Its mechanical properties vary greatly and depend on the form the carbon takes in the alloy.
"White" cast irons contain their carbon in the form of
cementite, or iron carbide (Fe
3C).
This hard, brittle compound dominates the mechanical properties of
white cast irons, rendering them hard, but unresistant to shock. The
broken surface of a white cast iron is full of fine facets of the broken
iron carbide, a very pale, silvery, shiny material, hence the
appellation. Cooling a mixture of iron with 0.8% carbon slowly below
723 °C to room temperature results in separate, alternating layers of
cementite and α-iron, which is soft and malleable and is called
pearlite for its appearance. Rapid cooling, on the other hand, does not allow time for this separation and creates hard and brittle
martensite.
The steel can then be tempered by reheating to a temperature in
between, changing the proportions of pearlite and martensite. The end
product below 0.8% carbon content is a pearlite-αFe mixture, and that
above 0.8% carbon content is a pearlite-cementite mixture.
In
gray iron the carbon exists as separate, fine flakes of
graphite, and also renders the material brittle due to the sharp edged flakes of graphite that produce
stress concentration sites within the material. A newer variant of gray iron, referred to as
ductile iron, is specially treated with trace amounts of
magnesium
to alter the shape of graphite to spheroids, or nodules, reducing the
stress concentrations and vastly increasing the toughness and strength
of the material.
Iron production 2009 (million tonnes)
| Country |
Iron ore |
Pig iron |
Direct iron |
Steel
|
| China |
1,114.9 |
549.4 |
|
573.6
|
| Australia |
393.9 |
4.4 |
|
5.2
|
| Brazil |
305.0 |
25.1 |
0.011 |
26.5
|
| Japan |
|
66.9 |
|
87.5
|
| India |
257.4 |
38.2 |
23.4 |
63.5
|
| Russia |
92.1 |
43.9 |
4.7 |
60.0
|
| Ukraine |
65.8 |
25.7 |
|
29.9
|
| South Korea |
0.1 |
27.3 |
|
48.6
|
| Germany |
0.4 |
20.1 |
0.38 |
32.7
|
| World |
1,594.9 |
914.0 |
64.5 |
1,232.4
|
Wrought iron contains less than 0.25% carbon but large amounts of slag that give it a fibrous characteristic.
It is a tough, malleable product, but not as fusible as pig iron. If
honed to an edge, it loses it quickly. Wrought iron is characterized by
the presence of fine fibers of
slag entrapped within the metal. Wrought iron is more corrosion resistant than steel. It has been almost completely replaced by
mild steel for traditional "wrought iron" products and
blacksmithing.
Mild steel corrodes more readily than wrought iron, but is cheaper and more widely available.
Carbon steel contains 2.0% carbon or less, with small amounts of
manganese,
sulfur,
phosphorus, and silicon.
Alloy steels contain varying amounts of carbon as well as other metals, such as
chromium,
vanadium,
molybdenum, nickel,
tungsten,
etc. Their alloy content raises their cost, and so they are usually
only employed for specialist uses. One common alloy steel, though, is
stainless steel. Recent developments in ferrous metallurgy have produced a growing range of microalloyed steels, also termed '
HSLA'
or high-strength, low alloy steels, containing tiny additions to
produce high strengths and often spectacular toughness at minimal cost.
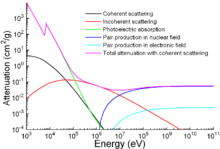
Apart from traditional applications, iron is also used for protection
from ionizing radiation. Although it is lighter than another
traditional protection material,
lead, it is much stronger mechanically. The attenuation of radiation as a function of energy is shown in the graph.
The main disadvantage of iron and steel is that pure iron, and most of its alloys, suffer badly from
rust if not protected in some way, a cost amounting to over 1% of the world's economy.
Painting,
galvanization,
passivation, plastic coating and
bluing are all used to protect iron from rust by excluding
water and oxygen or by
cathodic protection. The mechanism of the rusting of iron is as follows:
- Cathode: 3 O2 + 6 H2O + 12 e− → 12 OH−
- Anode: 4 Fe → 4 Fe2+ + 8 e−; 4 Fe2+ → 4 Fe3+ + 4 e−
- Overall: 4 Fe + 3 O2 + 6 H2O → 4 Fe3+ + 12 OH− → 4 Fe(OH)3 or 4 FeO(OH) + 4 H2O
The electrolyte is usually
iron(II) sulfate in urban areas (formed when atmospheric
sulfur dioxide attacks iron), and salt particles in the atmosphere in seaside areas.
Iron compounds
Iron(II) sulfate is used as a precursor to other iron compounds. It is also used to
reduce chromate in cement. It is used to fortify foods and treat
iron deficiency anemia.
Iron(III) sulfate is used in settling minute sewage particles in tank water.
Iron(II) chloride
is used as a reducing flocculating agent, in the formation of iron
complexes and magnetic iron oxides, and as a reducing agent in organic
synthesis.
Biological and pathological role
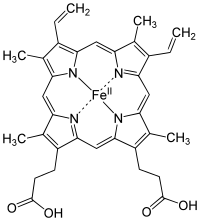
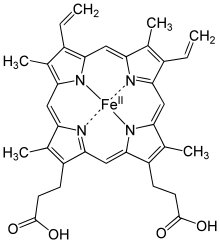
Structure of Heme b; in the protein additional ligand(s) would be attached to Fe.
Examples of iron-containing proteins in higher organisms include hemoglobin,
cytochrome (see
high-valent iron), and
catalase.
The average adult human contains about 0.005% body weight of iron, or
about four grams, of which three quarters is in hemoglobin – a level
that remains constant despite only about one milligram of iron being
absorbed each day, because the human body recycles its hemoglobin for the iron content.
Biochemistry
Iron acquisition poses a problem for aerobic organisms because ferric
iron is poorly soluble near neutral pH. Thus, these organisms have
developed means to absorb iron as complexes, sometimes taking up ferrous
iron before oxidising it back to ferric iron. In particular, bacteria have evolved very high-affinity
sequestering agents called
siderophores.
After uptake in human
cells, iron storage is precisely regulated. A major component of this regulation is the protein
transferrin, which binds iron ions absorbed from the
duodenum and carries it in the
blood to cells. Transferrin contains Fe
3+ in the middle of a distorted octahedron, bonded to one nitrogen, three oxygens and a chelating
carbonate anion that traps the Fe
3+ ion: it has such a high
stability constant that it is very effective at taking up Fe
3+ ions even from the most stable complexes. At the bone marrow, transferrin is reduced from Fe
3+ and Fe
2+ and stored as
ferritin to be incorporated into hemoglobin.
Hemoglobin is an oxygen carrier that occurs in
red blood cells and contributes their color, transporting oxygen in the arteries from the lungs to the muscles where it is transferred to
myoglobin, which stores it until it is needed for the metabolic oxidation of
glucose, generating energy. Here the hemoglobin binds to
carbon dioxide, produced when glucose is oxidized, which is transported through the veins by hemoglobin (predominantly as
bicarbonate anions) back to the lungs where it is exhaled. In hemoglobin, the iron is in one of four
heme groups and has six possible coordination sites; four are occupied by nitrogen atoms in a
porphyrin ring, the fifth by an
imidazole nitrogen in a
histidine
residue of one of the protein chains attached to the heme group, and
the sixth is reserved for the oxygen molecule it can reversibly bind to. When hemoglobin is not attached to oxygen (and is then called deoxyhemoglobin), the Fe
2+ ion at the center of the
heme
group (in the hydrophobic protein interior) is in a high-spin
configuration. It is thus too large to fit inside the porphyrin ring,
which bends instead into a dome with the Fe
2+ ion about
55 picometers above it. In this configuration, the sixth coordination
site reserved for the oxygen is blocked by another histidine residue.
When deoxyhemoglobin picks up an oxygen molecule, this histidine
residue moves away and returns once the oxygen is securely attached to
form a
hydrogen bond with it. This results in the Fe
2+
ion switching to a low-spin configuration, resulting in a 20% decrease
in ionic radius so that now it can fit into the porphyrin ring, which
becomes planar.
(Additionally, this hydrogen bonding results in the tilting of the
oxygen molecule, resulting in a Fe–O–O bond angle of around 120° that
avoids the formation of Fe–O–Fe or Fe–O
2–Fe bridges that would lead to electron transfer, the oxidation of Fe
2+ to Fe
3+,
and the destruction of hemoglobin.) This results in a movement of all
the protein chains that leads to the other subunits of hemoglobin
changing shape to a form with larger oxygen affinity. Thus, when
deoxyhemoglobin takes up oxygen, its affinity for more oxygen increases,
and vice versa.
Myoglobin, on the other hand, contains only one heme group and hence
this cooperative effect cannot occur. Thus, while hemoglobin is almost
saturated with oxygen in the high partial pressures of oxygen found in
the lungs, its affinity for oxygen is much lower than that of myoglobin,
which oxygenates even at low partial pressures of oxygen found in
muscle tissue. As described by the
Bohr effect (named after
Christian Bohr, the father of
Niels Bohr), the oxygen affinity of hemoglobin diminishes in the presence of carbon dioxide.
Carbon monoxide and
phosphorus trifluoride
are poisonous to humans because they bind to hemoglobin similarly to
oxygen, but with much more strength, so that oxygen can no longer be
transported throughout the body. Hemoglobin bound to carbon monoxide is
known as
carboxyhemoglobin. This effect also plays a minor role in the toxicity of
cyanide,
but there the major effect is by far its interference with the proper
functioning of the electron transport protein cytochrome a.
The cytochrome proteins also involve heme groups and are involved in
the metabolic oxidation of glucose by oxygen. The sixth coordination
site is then occupied by either another imidazole nitrogen or a
methionine
sulfur, so that these proteins are largely inert to oxygen – with the
exception of cytochrome a, which bonds directly to oxygen and thus is
very easily poisoned by cyanide.
Here, the electron transfer takes place as the iron remains in low spin
but changes between the +2 and +3 oxidation states. Since the reduction
potential of each step is slightly greater than the previous one, the
energy is released step-by-step and can thus be stored in
adenosine triphosphate.
Cytochrome a is slightly distinct, as it occurs at the mitochondrial
membrane, binds directly to oxygen, and transports protons as well as
electrons, as follows:
- 4 Cytc2+ + O2 + 8H+
inside → 4 Cytc3+ + 2 H2O + 4H+
outside
Although the heme proteins are the most important class of iron-containing proteins, the
iron-sulfur proteins
are also very important, being involved in electron transfer, which is
possible since iron can exist stably in either the +2 or +3 oxidation
states. These have one, two, four, or eight iron atoms that are each
approximately tetrahedrally coordinated to four sulfur atoms; because of
this tetrahedral coordination, they always have high-spin iron. The
simplest of such compounds is
rubredoxin, which has only one iron atom coordinated to four sulfur atoms from
cysteine residues in the surrounding peptide chains. Another important class of iron-sulfur proteins is the
ferredoxins, which have multiple iron atoms. Transferrin does not belong to either of these classes.
The ability of sea
mussels to maintain their grip on rocks in the ocean is facilitated by their use of
organometallic iron-based bonds in their protein-rich
cuticles. Based on synthetic replicas, the presence of iron in these structures increased
elastic modulus 770 times,
tensile strength 58 times, and
toughness 92 times. The amount of stress required to permanently damage them increased 76 times.
Health and diet
Iron is pervasive, but particularly rich sources of dietary iron include
red meat,
oysters,
lentils,
beans,
poultry,
fish,
leaf vegetables,
watercress,
tofu,
chickpeas,
black-eyed peas, and
blackstrap molasses.
Bread and
breakfast cereals are sometimes specifically fortified with iron.
Iron provided by
dietary supplements is often found as
iron(II) fumarate, although
iron(II) sulfate is cheaper and is absorbed equally well.
Elemental iron, or reduced iron, despite being absorbed at only
one-third to two-thirds the efficiency (relative to iron sulfate), is often added to foods such as breakfast cereals or enriched wheat flour. Iron is most available to the body when
chelated to amino acids and is also available for use as a common
iron supplement.
Glycine, the least expensive amino acid, is most often used to produce iron glycinate supplements.
Dietary recommendations
The U.S. Institute of Medicine (IOM) updated Estimated Average
Requirements (EARs) and Recommended Dietary Allowances (RDAs) for iron
in 2001.
The current EAR for iron for women ages 14–18 is 7.9 mg/day, 8.1 for
ages 19–50 and 5.0 thereafter (post menopause). For men the EAR is
6.0 mg/day for ages 19 and up. The RDA is 15.0 mg/day for women ages
15–18, 18.0 for 19–50 and 8.0 thereafter. For men, 8.0 mg/day for ages
19 and up. RDAs are higher than EARs so as to identify amounts that will
cover people with higher than average requirements. RDA for pregnancy
is 27 mg/day and, for lactation, 9 mg/day. For children ages 1–3 years 7 mg/day, 10 for ages 4–8 and 8 for ages 9–13. As for safety, the IOM also sets
Tolerable upper intake levels
(ULs) for vitamins and minerals when evidence is sufficient. In the
case of iron the UL is set at 45 mg/day. Collectively the EARs, RDAs and
ULs are referred to as
Dietary Reference Intakes.
The
European Food Safety Authority
(EFSA) refers to the collective set of information as Dietary Reference
Values, with Population Reference Intake (PRI) instead of RDA, and
Average Requirement instead of EAR. AI and UL defined the same as in
United States. For women the PRI is 13 mg/day ages 15–17 years,
16 mg/day for women ages 18 and up who are premenopausal and 11 mg/day
postmenopausal. For pregnancy and lactation, 16 mg/day. For men the PRI
is 11 mg/day ages 15 and older. For children ages 1 to 14 the PRI
increases from 7 to 11 mg/day. The PRIs are higher than the U.S. RDAs,
with the exception of pregnancy. The EFSA reviewed the same safety question did not establish a UL.
Infants may require iron supplements if they are bottle-fed cow's milk. Frequent
blood donors are at risk of low iron levels and are often advised to supplement their iron intake.
For U.S. food and dietary supplement labeling purposes the amount
in a serving is expressed as a percent of Daily Value (%DV). For iron
labeling purposes 100% of the Daily Value was 18 mg, and as of May 27,
2016 remained unchanged at 18 mg. A table of all of the old and new adult Daily Values is provided at
Reference Daily Intake. The original deadline to be in compliance was July 28, 2018, but on September 29, 2017 the U.S.
Food and Drug Administration
released a proposed rule that extended the deadline to January 1, 2020
for large companies and January 1, 2021 for small companies.
Deficiency
Iron deficiency is the most common
nutritional deficiency in the world. When loss of iron is not adequately compensated by adequate dietary iron intake, a state of
latent iron deficiency occurs, which over time leads to
iron-deficiency anemia
if left untreated, which is characterised by an insufficient number of
red blood cells and an insufficient amount of hemoglobin. Children,
pre-menopausal
women (women of child-bearing age), and people with poor diet are most
susceptible to the disease. Most cases of iron-deficiency anemia are
mild, but if not treated can cause problems like fast or irregular
heartbeat, complications during pregnancy, and delayed growth in infants
and children.
Excess
Iron uptake
is tightly regulated by the human body, which has no regulated
physiological means of excreting iron. Only small amounts of iron are
lost daily due to mucosal and skin epithelial cell sloughing, so control
of iron levels is primarily accomplished by regulating uptake. Regulation of iron uptake is impaired in some people as a result of a
genetic defect that maps to the HLA-H gene region on
chromosome 6 and leads to abnormally low levels of
hepcidin, a key regulator of the entry of iron into the circulatory system in mammals. In these people, excessive iron intake can result in
iron overload disorders, known medically as
hemochromatosis.
Many people have an undiagnosed genetic susceptibility to iron
overload, and are not aware of a family history of the problem. For this
reason, people should not take iron supplements unless they suffer from
iron deficiency
and have consulted a doctor. Hemochromatosis is estimated to be the
cause of 0.3 to 0.8% of all metabolic diseases of Caucasians.
Overdoses of ingested iron can cause excessive levels of free
iron in the blood. High blood levels of free ferrous iron react with
peroxides to produce highly reactive
free radicals that can damage
DNA,
proteins,
lipids,
and other cellular components. Iron toxicity occurs when the cell
contains free iron, which generally occurs when iron levels exceed the
availability of
transferrin to bind the iron. Damage to the cells of the
gastrointestinal tract
can also prevent them from regulating iron absorption, leading to
further increases in blood levels. Iron typically damages cells in the
heart,
liver and elsewhere, causing adverse effects that include
coma,
metabolic acidosis,
shock,
liver failure,
coagulopathy,
adult respiratory distress syndrome, long-term organ damage, and even death.
Humans experience iron toxicity when the iron exceeds 20 milligrams for
every kilogram of body mass; 60 milligrams per kilogram is considered a
lethal dose. Overconsumption of iron, often the result of children eating large quantities of
ferrous sulfate tablets intended for adult consumption, is one of the most common toxicological causes of death in children under six. The
Dietary Reference Intake
(DRI) sets the Tolerable Upper Intake Level (UL) for adults at
45 mg/day. For children under fourteen years old the UL is 40 mg/day.
The medical management of iron toxicity is complicated, and can include use of a specific
chelating agent called
deferoxamine to bind and expel excess iron from the body.
Cancer
The role of iron in cancer defense can be described as a
"double-edged sword" because of its pervasive presence in
non-pathological processes. People having
chemotherapy may develop iron deficiency and
anemia, for which
intravenous iron therapy is used to restore iron levels. Iron overload, which may occur from high consumption of red meat, may initiate
tumor growth and increase susceptibility to cancer onset, particularly for
colorectal cancer.