From Wikipedia, the free encyclopedia
Manganese, 25Mn
 |
| Manganese |
|---|
| Pronunciation | (MANG-gə-neez) |
|---|
| Appearance | silvery metallic |
|---|
| Standard atomic weight Ar, std(Mn) | 54.938043(2) |
|---|
| Manganese in the periodic table |
|---|
|
|
| Atomic number (Z) | 25 |
|---|
| Group | group 7 |
|---|
| Period | period 4 |
|---|
| Block | d-block |
|---|
| Element category | transition metal |
|---|
| Electron configuration | [Ar] 3d5 4s2 |
|---|
Electrons per shell
| 2, 8, 13, 2 |
|---|
| Physical properties |
|---|
| Phase at STP | solid |
|---|
| Melting point | 1519 K (1246 °C, 2275 °F) |
|---|
| Boiling point | 2334 K (2061 °C, 3742 °F) |
|---|
| Density (near r.t.) | 7.21 g/cm3 |
|---|
| when liquid (at m.p.) | 5.95 g/cm3 |
|---|
| Heat of fusion | 12.91 kJ/mol |
|---|
| Heat of vaporization | 221 kJ/mol |
|---|
| Molar heat capacity | 26.32 J/(mol·K) |
|---|
Vapor pressure
| P (Pa)
|
1
|
10
|
100
|
1 k
|
10 k
|
100 k
|
| at T (K)
|
1228
|
1347
|
1493
|
1691
|
1955
|
2333
|
|
| Atomic properties |
|---|
| Oxidation states | −3, −2, −1, +1, +2, +3, +4, +5, +6, +7 (depending on the oxidation state, an acidic, basic, or amphoteric oxide) |
|---|
| Electronegativity | Pauling scale: 1.55 |
|---|
| Ionization energies |
- 1st: 717.3 kJ/mol
- 2nd: 1509.0 kJ/mol
- 3rd: 3248 kJ/mol
|
|---|
| Atomic radius | empirical: 127 pm |
|---|
| Covalent radius | Low spin: 139±5 pm
High spin: 161±8 pm |
|---|
|
Spectral lines of manganese |
| Other properties |
|---|
| Natural occurrence | primordial |
|---|
| Crystal structure | body-centered cubic (bcc)
|
|---|
| Speed of sound thin rod | 5150 m/s (at 20 °C) |
|---|
| Thermal expansion | 21.7 µm/(m·K) (at 25 °C) |
|---|
| Thermal conductivity | 7.81 W/(m·K) |
|---|
| Electrical resistivity | 1.44 µΩ·m (at 20 °C) |
|---|
| Magnetic ordering | paramagnetic |
|---|
| Magnetic susceptibility | (α) +529.0·10−6 cm3/mol (293 K) |
|---|
| Young's modulus | 198 GPa |
|---|
| Bulk modulus | 120 GPa |
|---|
| Mohs hardness | 6.0 |
|---|
| Brinell hardness | 196 MPa |
|---|
| CAS Number | 7439-96-5 |
|---|
| History |
|---|
| Discovery | Carl Wilhelm Scheele (1774) |
|---|
| First isolation | Johann Gottlieb Gahn (1774) |
|---|
| Main isotopes of manganese |
|---|
|
|
Manganese is a chemical element with the symbol Mn and atomic number 25. It is not found as a free element in nature; it is often found in minerals in combination with iron. Manganese is a transition metal with important industrial alloy uses, particularly in stainless steels.
Historically, manganese is named for pyrolusite and other black minerals from the region of Magnesia in Greece, which also gave its name to magnesium and the iron ore magnetite. By the mid-18th century, Swedish-German chemist Carl Wilhelm Scheele had used pyrolusite to produce chlorine. Scheele and others were aware that pyrolusite (now known to be manganese dioxide) contained a new element, but they were unable to isolate it. Johan Gottlieb Gahn was the first to isolate an impure sample of manganese metal in 1774, which he did by reducing the dioxide with carbon.
Manganese phosphating is used for rust and corrosion prevention on steel. Ionized manganese is used industrially as pigments of various colors, which depend on the oxidation state of the ions. The permanganates of alkali and alkaline earth metals are powerful oxidizers. Manganese dioxide is used as the cathode (electron acceptor) material in zinc-carbon and alkaline batteries.
In biology, manganese(II) ions function as cofactors for a large variety of enzymes with many functions. Manganese enzymes are particularly essential in detoxification of superoxide free radicals in organisms that must deal with elemental oxygen. Manganese also functions in the oxygen-evolving complex of photosynthetic plants. While the element is a required trace mineral for all known living organisms, it also acts as a neurotoxin in larger amounts. Especially through inhalation, it can cause manganism, a condition in mammals leading to neurological damage that is sometimes irreversible.
Characteristics
Physical properties
Electrolytically refined manganese chips and a 1 cm3 cube
Manganese is a silvery-gray
metal that resembles iron. It is hard and very brittle, difficult to fuse, but easy to oxidize. Manganese metal and its common ions are
paramagnetic. Manganese tarnishes slowly in air and oxidizes ("rusts") like iron in water containing dissolved oxygen.
Isotopes
Naturally occurring manganese is composed of one stable
isotope,
55Mn. Eighteen
radioisotopes have been isolated and described, ranging in
atomic weight from 46
u (
46Mn) to 65 u (
65Mn). The most stable are
53Mn with a
half-life of 3.7 million years,
54Mn with a half-life of 312.3 days, and
52Mn with a half-life of 5.591 days. All of the remaining
radioactive isotopes have half-lives of less than three hours, and the majority of less than one minute. The primary
decay mode before the most abundant stable isotope,
55Mn, is
electron capture and the primary mode after is
beta decay. Manganese also has three
meta states.
Manganese is part of the
iron group of elements, which are thought to be synthesized in large
stars shortly before the
supernova explosion.
53Mn decays to
53Cr with a
half-life of 3.7 million years. Because of its relatively short half-life,
53Mn is relatively rare, produced by
cosmic rays impact on
iron. Manganese isotopic contents are typically combined with
chromium isotopic contents and have found application in
isotope geology and
radiometric dating. Mn–Cr isotopic ratios reinforce the evidence from
26Al and
107Pd for the early history of the
solar system. Variations in
53Cr/
52Cr and Mn/Cr ratios from several
meteorites suggest an initial
53Mn/
55Mn ratio, which indicates that Mn–Cr isotopic composition must result from
in situ decay of
53Mn in differentiated planetary bodies. Hence,
53Mn provides additional evidence for
nucleosynthetic processes immediately before coalescence of the
solar system.
Oxidation States
Manganese(II) chloride crystals – the pale pink color of Mn(II) salts is due to a spin-forbidden 3d transition.
The most common
oxidation states of manganese are +2, +3, +4, +6, and +7, though all oxidation states from −3 to +7 have been observed. Mn
2+ often competes with Mg
2+
in biological systems. Manganese compounds where manganese is in
oxidation state +7, which are mostly restricted to the unstable oxide Mn
2O
7, compounds of the intensely purple permanganate anion MnO
4−, and a few oxyhalides (MnO
3F and MnO
3Cl), are powerful
oxidizing agents. Compounds with oxidation states +5 (blue) and +6 (green) are strong oxidizing agents and are vulnerable to
disproportionation.
Aqueous solution of KMnO4 illustrating the deep purple of Mn(VII) as it occurs in permanganate
The most stable oxidation state for manganese is +2, which has a pale
pink color, and many manganese(II) compounds are known, such as
manganese(II) sulfate (MnSO
4) and
manganese(II) chloride (MnCl
2). This oxidation state is also seen in the mineral rhodochrosite (
manganese(II) carbonate).
Manganese(II) most commonly exists with a high spin, S = 5/2 ground
state because of the high pairing energy for manganese(II). However,
there are a few examples of low-spin, S =1/2 manganese(II).
There are no spin-allowed d–d transitions in manganese(II), explaining
why manganese(II) compounds are typically pale to colorless.
The +3 oxidation state is known in compounds like
manganese(III) acetate, but these are quite powerful
oxidizing agents and also prone to
disproportionation
in solution, forming manganese(II) and manganese(IV). Solid compounds
of manganese(III) are characterized by its strong purple-red color and a
preference for distorted octahedral coordination resulting from the
Jahn-Teller effect.
The oxidation state +5 can be produced by dissolving manganese dioxide in molten
sodium nitrite. Manganate (VI) salts can be produced by dissolving Mn compounds, such as
manganese dioxide,
in molten alkali while exposed to air. Permanganate (+7 oxidation
state) compounds are purple, and can give glass a violet color.
Potassium permanganate,
sodium permanganate, and
barium permanganate are all potent oxidizers. Potassium permanganate, also called Condy's crystals, is a commonly used laboratory
reagent
because of its oxidizing properties; it is used as a topical medicine
(for example, in the treatment of fish diseases). Solutions of potassium
permanganate were among the first stains and fixatives to be used in
the preparation of biological cells and tissues for electron microscopy.
History
The origin of the name manganese is complex. In ancient times, two black minerals from
Magnesia (located within modern Greece) were both called
magnes from their place of origin, but were considered to differ in gender. The masculine
magnes attracted iron, and was the iron ore now known as
lodestone or
magnetite, and which probably gave us the term
magnet. The feminine
magnes ore did not attract iron, but was used to decolorize glass. This feminine
magnes was later called
magnesia, known now in modern times as
pyrolusite or
manganese dioxide. Neither this mineral nor elemental manganese is magnetic. In the 16th century, manganese dioxide was called
manganesum
(note the two Ns instead of one) by glassmakers, possibly as a
corruption and concatenation of two words, since alchemists and
glassmakers eventually had to differentiate a
magnesia negra (the black ore) from
magnesia alba (a white ore, also from Magnesia, also useful in glassmaking).
Michele Mercati called magnesia negra
manganesa, and finally the metal isolated from it became known as
manganese (German:
Mangan). The name
magnesia eventually was then used to refer only to the white
magnesia alba (magnesium oxide), which provided the name
magnesium for the free element when it was isolated much later.
Some of the cave paintings in Lascaux, France, use manganese-based pigments.
Several colorful oxides of manganese, for example
manganese dioxide, are abundant in nature and have been used as pigments since the
Stone Age. The cave paintings in
Gargas that are 30,000 to 24,000 years old contain manganese pigments.
Manganese compounds were used by Egyptian and Roman glassmakers, either to add to, or remove color from glass. Use as "glassmakers soap" continued through the
Middle Ages until modern times and is evident in 14th-century glass from
Venice.
Scheele and other chemists were aware that manganese dioxide contained a new element, but they were not able to isolate it.
Johan Gottlieb Gahn was the first to isolate an impure sample of manganese metal in 1774, by
reducing the dioxide with
carbon.
The manganese content of some iron ores used in Greece led to
speculations that steel produced from that ore contains additional
manganese, making the
Spartan steel exceptionally hard.
Around the beginning of the 19th century, manganese was used in
steelmaking and several patents were granted. In 1816, it was documented
that iron alloyed with manganese was harder but not more brittle. In
1837, British academic
James Couper noted an association between miners' heavy exposure to manganese with a form of
Parkinson's disease.
In 1912, United States patents were granted for protecting firearms
against rust and corrosion with manganese phosphate electrochemical
conversion coatings, and the process has seen widespread use ever since.
The invention of the
Leclanché cell in 1866 and the subsequent improvement of batteries containing manganese dioxide as cathodic
depolarizer increased the demand for manganese dioxide. Until the development of batteries with
nickel-cadmium and lithium, most batteries contained manganese. The
zinc-carbon battery and the
alkaline battery
normally use industrially produced manganese dioxide because naturally
occurring manganese dioxide contains impurities. In the 20th century,
manganese dioxide was widely used as the cathodic for commercial disposable dry batteries of both the standard (zinc-carbon) and alkaline types.
Occurrence and production
Manganese comprises about 1000
ppm (0.1%) of the
Earth's crust, the 12th most abundant of the crust's elements. Soil contains 7–9000 ppm of manganese with an average of 440 ppm. Seawater has only 10
ppm manganese and the atmosphere contains 0.01 µg/m
3. Manganese occurs principally as
pyrolusite (
MnO2),
braunite, (Mn
2+Mn
3+6)(SiO
12),
psilomelane (Ba,H
2O)
2Mn
5O
10, and to a lesser extent as
rhodochrosite (
MnCO3).
|
|
|
|
|
|
| Manganese ore
|
Psilomelane (manganese ore)
|
Spiegeleisen is an iron alloy with a manganese content of approximately 15%
|
|
|
Percentage of manganese output in 2006 by countries
The most important manganese ore is pyrolusite (
MnO2). Other economically important manganese ores usually show a close spatial relation to the iron ores.
Land-based resources are large but irregularly distributed. About 80%
of the known world manganese resources are in South Africa; other
important manganese deposits are in Ukraine, Australia, India, China,
Gabon and Brazil.
According to 1978 estimate, the
ocean floor has 500 billion tons of
manganese nodules. Attempts to find economically viable methods of harvesting manganese nodules were abandoned in the 1970s. The CIA once used mining manganese modules on the ocean floor as a cover story for recovering a sunken Soviet submarine.
In South Africa, most identified deposits are located near
Hotazel in the
Northern Cape Province, with a 2011 estimate of 15 billion tons. In 2011 South Africa produced 3.4 million tons, topping all other nations.
Manganese is mainly mined in South Africa, Australia, China, Gabon, Brazil, India, Kazakhstan, Ghana, Ukraine and Malaysia.
US Import Sources (1998–2001): Manganese ore: Gabon, 70%; South Africa,
10%; Australia, 9%; Mexico, 5%; and other, 6%. Ferromanganese: South
Africa, 47%; France, 22%; Mexico, 8%; Australia, 8%; and other, 15%.
Manganese contained in all manganese imports: South Africa, 31%; Gabon,
21%; Australia, 13%; Mexico, 8%; and other, 27%.
For the production of
ferromanganese, the manganese ore is mixed with iron ore and carbon, and then reduced either in a blast furnace or in an electric arc furnace. The resulting
ferromanganese has a manganese content of 30 to 80%. Pure manganese used for the production of iron-free alloys is produced by
leaching manganese ore with
sulfuric acid and a subsequent
electrowinning process.
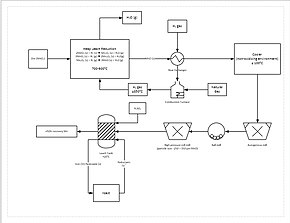
Process flow diagram for a manganese refining circuit.
A more progressive extraction process involves directly reducing
manganese ore in a heap leach. This is done by percolating natural gas
through the bottom of the heap; the natural gas provides the heat (needs
to be at least 850 °C) and the reducing agent (carbon monoxide). This
reduces all of the manganese ore to manganese oxide (MnO), which is a
leachable form. The ore then travels through a grinding circuit to
reduce the particle size of the ore to between 150–250 μm, increasing
the surface area to aid leaching. The ore is then added to a leach tank
of
sulfuric acid and ferrous iron (Fe
2+) in a 1.6:1 ratio. The iron reacts with the manganese dioxide to form
iron hydroxide
and elemental manganese. This process yields approximately 92% recovery
of the manganese. For further purification, the manganese can then be
sent to an electrowinning facility.
In 1972 the
CIA's
Project Azorian, through billionaire
Howard Hughes, commissioned the ship
Hughes Glomar Explorer
with the cover story of harvesting manganese nodules from the sea
floor. That triggered a rush of activity to collect manganese nodules,
which was not actually practical. The real mission of
Hughes Glomar Explorer was to raise a sunken
Soviet submarine, the
K-129, with the goal of retrieving Soviet code books.
Applications
Manganese has no satisfactory substitute in its major applications in metallurgy. In minor applications, (e.g., manganese phosphating),
zinc and sometimes
vanadium are viable substitutes.
Steel
Manganese is essential to iron and
steel production by virtue of its sulfur-fixing,
deoxidizing, and
alloying properties, as first recognized by the British metallurgist
Robert Forester Mushet (1811–1891) who, in 1856, introduced the element, in the form of
Spiegeleisen,
into steel for the specific purpose of removing excess dissolved
oxygen, sulfur, and phosphorus in order to improve its malleability.
Steelmaking,
including its ironmaking component, has accounted for most manganese
demand, presently in the range of 85% to 90% of the total demand. Manganese is a key component of low-cost
stainless steel. Often
ferromanganese (usually about 80% manganese) is the intermediate in modern processes.
Small amounts of manganese improve the workability of steel at
high temperatures by forming a high-melting sulfide and preventing the
formation of a liquid
iron sulfide
at the grain boundaries. If the manganese content reaches 4%, the
embrittlement of the steel becomes a dominant feature. The embrittlement
decreases at higher manganese concentrations and reaches an acceptable
level at 8%. Steel containing 8 to 15% of manganese has a high
tensile strength of up to 863 MPa. Steel with 12% manganese was discovered in 1882 by
Robert Hadfield and is still known as
Hadfield steel (mangalloy). It was used for British military
steel helmets and later by the U.S. military.
Aluminium alloys
The second largest application for manganese is in aluminium alloys.
Aluminium with roughly 1.5% manganese has increased resistance to
corrosion through grains that absorb impurities which would lead to
galvanic corrosion. The corrosion-resistant
aluminium alloys 3004 and 3104 (0.8 to 1.5% manganese) are used for most
beverage cans. Before 2000, more than 1.6 million
tonnes of those alloys were used; at 1% manganese, this consumed 16,000 tonnes of manganese.
Other uses
Manganese(IV) oxide (manganese dioxide, MnO
2) is used as a reagent in
organic chemistry for the
oxidation of benzylic
alcohols (where the
hydroxyl group is adjacent to an
aromatic ring).
Manganese dioxide has been used since antiquity to oxidize and
neutralize the greenish tinge in glass from trace amounts of iron
contamination. MnO
2 is also used in the manufacture of oxygen and chlorine and in drying black paints. In some preparations, it is a brown
pigment for
paint and is a constituent of natural
umber.
Manganese(IV) oxide was used in the original type of dry cell
battery
as an electron acceptor from zinc, and is the blackish material in
carbon–zinc type flashlight cells. The manganese dioxide is reduced to
the manganese oxide-hydroxide MnO(OH) during discharging, preventing the
formation of hydrogen at the anode of the battery.
- MnO2 + H2O + e− → MnO(OH) + OH−
The same material also functions in newer
alkaline batteries
(usually battery cells), which use the same basic reaction, but a
different electrolyte mixture. In 2002, more than 230,000 tons of
manganese dioxide was used for this purpose.
World-War-II-era
5-cent coin (1942-5 identified by mint mark P, D or S above dome) made
from a 56% copper-35% silver-9% manganese alloy
The metal is occasionally used in coins; until 2000, the only United States coin to use manganese was the
"wartime" nickel from 1942 to 1945.
An alloy of 75% copper and 25% nickel was traditionally used for the
production of nickel coins. However, because of shortage of nickel metal
during the war, it was substituted by more available silver and
manganese, thus resulting in an alloy of 56% copper, 35% silver and 9%
manganese. Since 2000,
dollar coins, for example the
Sacagawea dollar and the
Presidential $1 coins, are made from a brass containing 7% of manganese with a pure copper core.
In both cases of nickel and dollar, the use of manganese in the coin
was to duplicate the electromagnetic properties of a previous
identically sized and valued coin in the mechanisms of vending machines.
In the case of the later U.S. dollar coins, the manganese alloy was
intended to duplicate the properties of the copper/nickel alloy used in
the previous
Susan B. Anthony dollar.
Manganese compounds have been used as pigments and for the
coloring of ceramics and glass. The brown color of ceramic is sometimes
the result of manganese compounds.
In the glass industry, manganese compounds are used for two effects.
Manganese(III) reacts with iron(II) to induce a strong green color in
glass by forming less-colored iron(III) and slightly pink manganese(II),
compensating for the residual color of the iron(III). Larger quantities of manganese are used to produce pink colored glass.
Tetravalent manganese is used as an
activator in red-emitting
phosphors. While many compounds are known which show
luminescence, the majority are not used in commercial application due to low efficiency or deep red emission. However, several Mn
4+ activated fluorides were reported as potential red-emitting phosphors for warm-white LEDs. But to this day, only K
2SiF
6:Mn
4+ is commercially available for use in warm-white
LEDs.
Biological role
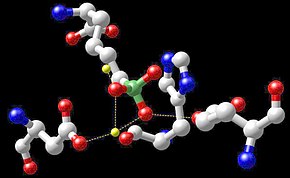
Reactive center of arginase with boronic acid inhibitor – the manganese atoms are shown in yellow.
Manganese is an essential human dietary element. It is present as a
coenzyme in several biological processes, which include macronutrient
metabolism, bone formation, free radical defense systems. It is a
critical component in dozens of proteins and enzymes.
The human body contains about 12 mg of manganese, mostly in the bones.
The soft tissue remainder is concentrated in the liver and kidneys. In the human brain, the manganese is bound to manganese
metalloproteins, most notably
glutamine synthetase in
astrocytes.
Excessive exposure or intake may lead to a condition known as
manganism, a
neurodegenerative disorder that causes dopaminergic neuronal death and symptoms similar to
Parkinson's disease. The classes of enzymes that have manganese
cofactors is large and includes
oxidoreductases,
transferases,
hydrolases,
lyases,
isomerases,
ligases,
lectins, and
integrins. The
reverse transcriptases of many
retroviruses (though not
lentiviruses such as
HIV) contain manganese. The best-known manganese-containing
polypeptides may be
arginase, the
diphtheria toxin, and Mn-containing
superoxide dismutase (
Mn-SOD).
Mn-SOD is the type of SOD present in eukaryotic
mitochondria,
and also in most bacteria (this fact is in keeping with the
bacterial-origin theory of mitochondria). The Mn-SOD enzyme is probably
one of the most ancient, for nearly all organisms living in the presence
of oxygen use it to deal with the toxic effects of
superoxide (
O−
2), formed from the 1-electron reduction of dioxygen. The exceptions, which are all bacteria, include
Lactobacillus plantarum and related
lactobacilli, which use a different nonenzymatic mechanism with manganese (Mn
2+) ions complexed with polyphosphate, suggesting a path of evolution for this function in aerobic life.
Dietary recommendations
Current AIs of Mn by age group and sex
| Males
|
Females
|
| Age
|
AI (mg/day)
|
Age
|
AI (mg/day)
|
| 1–3
|
1.2
|
1–3
|
1.2
|
| 4–8
|
1.5
|
4–8
|
1.5
|
| 9–13
|
1.9
|
9–13
|
1.6
|
| 14–18
|
2.2
|
14–18
|
1.6
|
| 19+
|
2.3
|
19+
|
1.8
|
| pregnant: 2
|
| lactating: 2.6
|
The U.S. Institute of Medicine (IOM) updated Estimated Average
Requirements (EARs) and Recommended Dietary Allowances (RDAs) for
minerals in 2001. For manganese there was not sufficient information to
set EARs and RDAs, so needs are described as estimates for
Adequate Intakes (AIs). As for safety, the IOM sets
Tolerable upper intake levels
(ULs) for vitamins and minerals when evidence is sufficient. In the
case of manganese the adult UL is set at 11 mg/day. Collectively the
EARs, RDAs, AIs and ULs are referred to as
Dietary Reference Intakes (DRIs). Manganese deficiency is rare.
The
European Food Safety Authority
(EFSA) refers to the collective set of information as Dietary Reference
Values, with Population Reference Intake (PRI) instead of RDA, and
Average Requirement instead of EAR. AI and UL defined the same as in
United States. For people ages 15 and older the AI is set at 3.0 mg/day.
AIs for pregnancy and lactation is 3.0 mg/day. For children ages 1–14
years the AIs increase with age from 0.5 to 2.0 mg/day. The adult AIs
are higher than the U.S. RDAs. The EFSA reviewed the same safety question and decided that there was insufficient information to set a UL.
For U.S. food and dietary supplement labeling purposes the amount
in a serving is expressed as a percent of Daily Value (%DV). For
manganese labeling purposes 100% of the Daily Value was 2.0 mg, but as
of May 27, 2016 it was revised to 2.3 mg to bring it into agreement with
the RDA. A table of the old and new adult Daily Values is provided at
Reference Daily Intake. Food and supplement companies have until January 1, 2020 to comply with the change.
Precautions
Manganese compounds are less toxic than those of other widespread metals, such as
nickel and
copper. However, exposure to manganese dusts and fumes should not exceed the ceiling value of 5 mg/m
3 even for short periods because of its toxicity level. Manganese poisoning has been linked to impaired motor skills and cognitive disorders.
Permanganate exhibits a higher toxicity than manganese(II)
compounds. The fatal dose is about 10 g, and several fatal intoxications
have occurred. The strong oxidative effect leads to
necrosis of the
mucous membrane. For example, the
esophagus
is affected if the permanganate is swallowed. Only a limited amount is
absorbed by the intestines, but this small amount shows severe effects
on the kidneys and on the liver.
Generally, exposure to ambient Mn air concentrations in excess of 5 μg Mn/m3 can lead to Mn-induced symptoms. Increased
ferroportin
protein expression in human embryonic kidney (HEK293) cells is
associated with decreased intracellular Mn concentration and attenuated
cytotoxicity, characterized by the reversal of Mn-reduced
glutamate uptake and diminished
lactate dehydrogenase leakage.
Environmental health concerns
Manganese in drinking water
Waterborne manganese has a greater
bioavailability than dietary manganese. According to results from a 2010 study, higher levels of exposure to manganese in
drinking water are associated with increased
intellectual impairment and reduced
intelligence quotients
in school-age children. It is hypothesized that long-term exposure due
to inhaling the naturally occurring manganese in shower water puts up to
8.7 million Americans at risk.
However, data indicates that the human body can recover from certain
adverse effects of overexposure to manganese if the exposure is stopped
and the body can clear the excess.
Manganese in gasoline
Methylcyclopentadienyl manganese tricarbonyl (MMT) is a
gasoline additive used to replace lead compounds for unleaded gasolines to improve the
octane rating of low octane petroleum distillates. It reduces
engine knock agent through the action of the
carbonyl groups. Fuels containing manganese tend to form manganese carbides, which damage
exhaust valves. Compared to 1953, levels of manganese in air have dropped.
Many racing competitions specifically ban manganese compounds in racing
fuel for carts and minibikes. MMT contains 24.4–25.2% manganese.
Elevated atmospheric manganese concentrations are strongly correlated
with automobile traffic density. The level of manganese emitted by MMT
fuels has been found to be safe for the general population and
vulnerable groups such as infants and the elderly by the EPA and
European and Canadian environmental agencies.
Manganese in tobacco smoke
The
tobacco plant readily absorbs and accumulates
heavy metals such as manganese from the surrounding soil into its leaves. These are subsequently inhaled during
tobacco smoking. While manganese is a constituent of
tobacco smoke, studies have largely concluded that concentrations are not hazardous for human health.
Role in neurological disorders
Manganism
Manganese overexposure is most frequently associated with
manganism,
a rare neurological disorder associated with excessive manganese
ingestion or inhalation. Historically, persons employed in the
production or processing of manganese alloys
have been at risk for developing manganism; however, current health and
safety regulations protect workers in developed nations. The disorder was first described in 1837 by British academic John Couper, who studied two patients who were manganese grinders.
Manganism is a biphasic disorder. In its early stages, an
intoxicated person may experience depression, mood swings, compulsive
behaviors, and psychosis. Early neurological symptoms give way to
late-stage manganism, which resembles
Parkinson's disease.
Symptoms include weakness, monotone and slowed speech, an
expressionless face, tremor, forward-leaning gait, inability to walk
backwards without falling, rigidity, and general problems with
dexterity, gait and balance. Unlike
Parkinson's disease, manganism is not associated with loss of the sense of smell and patients are typically unresponsive to treatment with
L-DOPA.
Symptoms of late-stage manganism become more severe over time even if
the source of exposure is removed and brain manganese levels return to
normal.
Chronic manganese exposure has been shown to produce a parkinsonism-like illness characterized by movement abnormalities. This condition is not responsive to
typical therapies used in the treatment of PD, suggesting an alternative pathway than the typical
dopaminergic loss within the
substantia nigra. Manganese may accumulate in the
basal ganglia, leading to the abnormal movements.
A mutation of the SLC30A10 gene, a manganese efflux transporter
necessary for decreasing intracellular Mn, has been linked with the
development of this Parkinsonism-like disease. The
Lewy bodies typical to PD are not seen in Mn-induced parkinsonism.
Animal experiments have given the opportunity to examine the
consequences of manganese overexposure under controlled conditions. In
(non-aggressive) rats, manganese induces mouse-killing behavior.
Childhood developmental disorders
Several recent studies attempt to examine the effects of chronic low-dose manganese overexposure on
child development.
The earliest study was conducted in the Chinese province of Shanxi.
Drinking water there had been contaminated through improper sewage
irrigation and contained 240–350 µg Mn/L. Although Mn concentrations at
or below 300 µg Mn/L were considered safe at the time of the study by
the US EPA and 400 µg Mn/L by the
World Health Organization,
the 92 children sampled (between 11 and 13 years of age) from this
province displayed lower performance on tests of manual dexterity and
rapidity, short-term memory, and visual identification, compared to
children from an uncontaminated area. More recently, a study of
10-year-old children in Bangladesh showed a relationship between Mn
concentration in well water and diminished IQ scores. A third study
conducted in Quebec examined school children between the ages of 6 and
15 living in homes that received water from a well containing 610 µg
Mn/L; controls lived in homes that received water from a 160 µg Mn/L
well. Children in the experimental group showed increased hyperactive
and oppositional behavior.
The current maximum safe concentration under EPA rules is 50 µg Mn/L.
Neurodegenerative diseases
A protein called
DMT1 is the major transporter in manganese absorption from the intestine, and may be the major transporter of manganese across the
blood–brain barrier.
DMT1
also transports inhaled manganese across the nasal epithelium. The
proposed mechanism for manganese toxicity is that dysregulation leads to
oxidative stress, mitochondrial dysfunction, glutamate-mediated
excitoxicity, and aggregation of proteins.