From Wikipedia, the free encyclopedia
Antibiotic resistance tests:
Bacteria are streaked on dishes with white disks, each impregnated with
a different antibiotic. Clear rings, such as those on the left, show
that bacteria have not grown—indicating that these bacteria are not
resistant. The bacteria on the right are fully resistant to all but two
of the seven antibiotics tested.
Antibiotic
resistance may claim as many as 10 million lives per year by the year
2050. That's more than all deaths caused by cancer combined. Chemist
prof. dr. Nathaniel Martin (
Leiden University) states that it's time for action. He explains how the problem can be fixed.
Antimicrobial resistance (AMR) occurs when microbes evolve mechanisms that protect them from the effects of antimicrobials. Antibiotic resistance is a subset of AMR, that applies specifically to bacteria that become resistant to antibiotics.
Infections caused by resistant microbes are more difficult to treat,
requiring higher doses of antimicrobial drugs, or alternative medications which may prove more toxic. These approaches may also be more expensive. Microbes resistant to multiple antimicrobials are called multidrug resistant (MDR).
All classes of microbes can evolve resistance. Fungi evolve antifungal resistance. Viruses evolve antiviral resistance. Protozoa evolve antiprotozoal resistance, and bacteria evolve antibiotic
resistance. Those bacteria that are considered extensively drug
resistant (XDR) or totally drug-resistant (TDR) are sometimes called
"superbugs". Resistance in bacteria can arise naturally by genetic mutation, or by one species acquiring resistance from another.
Resistance can appear spontaneously because of random mutations.
However, extended use of antimicrobials appears to encourage selection
for mutations which can render antimicrobials ineffective.
The prevention of antibiotic misuse, which can lead to antibiotic resistance, includes taking antibiotics only when prescribed. Narrow-spectrum antibiotics are preferred over broad-spectrum antibiotics
when possible, as effectively and accurately targeting specific
organisms is less likely to cause resistance, as well as side effects.
For people who take these medications at home, education about proper
use is essential. Health care providers can minimize spread of resistant
infections by use of proper sanitation and hygiene, including handwashing and disinfecting between patients, and should encourage the same of the patient, visitors, and family members.
Rising drug resistance is caused mainly by use of antimicrobials
in humans and other animals, and spread of resistant strains between the
two.
Growing resistance has also been linked to releasing inadequately
treated effluents from the pharmaceutical industry, especially in
countries where bulk drugs are manufactured. Antibiotics increase selective pressure
in bacterial populations, causing vulnerable bacteria to die; this
increases the percentage of resistant bacteria which continue growing.
Even at very low levels of antibiotic, resistant bacteria can have a
growth advantage and grow faster than vulnerable bacteria.
As resistance to antibiotics becomes more common there is greater need
for alternative treatments. Calls for new antibiotic therapies have been
issued, but new drug development is becoming rarer.
Antimicrobial resistance is increasing globally due to increased prescription and dispensing of antibiotic drugs in developing countries.
Estimates are that 700,000 to several million deaths result per year
and continues to pose a major public health threat worldwide. Each year in the United States,
at least 2.8 million people become infected with bacteria that are
resistant to antibiotics and at least 35,000 people die and US$55
billion in increased health care costs and lost productivity. According to World Health Organization (WHO) estimates, three hundred and fifty million deaths could be caused by AMR by 2050. By then, the yearly death toll will be ten million, according to a United Nations report.
There are public calls for global collective action to address the threat that include proposals for international treaties on antimicrobial resistance.
Worldwide antibiotic resistance is not completely identified, but
poorer countries with weaker healthcare systems are more affected. During the COVID-19 pandemic, action against antimicrobial resistance slowed due to scientists focusing more on SARS-CoV-2 research.
Definition
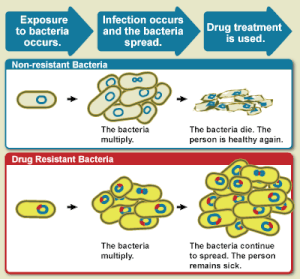
Diagram
showing the difference between non-resistant bacteria and drug
resistant bacteria. Non-resistant bacteria multiply, and upon drug
treatment, the bacteria die. Drug resistant bacteria multiply as well,
but upon drug treatment, the bacteria continue to spread.
The WHO defines antimicrobial resistance as a microorganism's resistance to an antimicrobial drug that was once able to treat an infection by that microorganism.
A person cannot become resistant to antibiotics. Resistance is a
property of the microbe, not a person or other organism infected by a
microbe.
Antibiotic resistance is a subset of antimicrobial
resistance. This more specified resistance is linked to pathogenic
bacteria and thus broken down into two further subsets, microbiological
and clinical. Resistance linked microbiologically is the most common and
occurs from genes, mutated or inherited, that allow the bacteria to
resist the mechanism associated with certain antibiotics. Clinical
resistance is shown through the failure of many therapeutic techniques
where the bacteria that are normally susceptible to a treatment become
resistant after surviving the outcome of the treatment. In both cases of
acquired resistance, the bacteria can pass the genetic catalyst for
resistance through conjugation, transduction, or transformation. This
allows the resistance to spread across the same pathogen or even similar
bacterial pathogens.
Overview
WHO
report released April 2014 stated, "this serious threat is no longer a
prediction for the future, it is happening right now in every region of
the world and has the potential to affect anyone, of any age, in any
country. Antibiotic resistance—when bacteria change so antibiotics no
longer work in people who need them to treat infections—is now a major
threat to public health." In 2018, WHO considered antibiotic resistance to be one of the biggest threats to global health, food security and development. The European Centre for Disease Prevention and Control
calculated that in 2015 there were 671,689 infections in the EU and
European Economic Area caused by antibiotic-resistant bacteria,
resulting in 33,110 deaths. Most were acquired in healthcare settings.
Causes
Antimicrobial
resistance is mainly caused by the overuse of antimicrobials. This
leads to microbes either evolving a defense against drugs used to treat
them, or certain strains of microbes that have a natural resistance to
antimicrobials becoming much more prevalent than the ones that are
easily defeated with medication.
While antimicrobial resistance does occur naturally over time, the use
of antimicrobial agents in a variety of settings both within the
healthcare industry and outside of has led to antimicrobial resistance
becoming increasingly more prevalent.
Natural occurrence
A CDC infographic on how antibiotic resistance (a major type of antimicrobial resistance) happens and spreads.
Antimicrobial resistance can evolve naturally due to continued exposure to antimicrobials. Natural selection means that organisms that are able to adapt to their environment, survive, and continue to produce offspring.
As a result, the types of microorganisms that are able to survive over
time with continued attack by certain antimicrobial agents will
naturally become more prevalent in the environment, and those without
this resistance will become obsolete.
Over time most of the strains of bacteria and infections present will
be the type resistant to the antimicrobial agent being used to treat
them, making this agent now ineffective to defeat most microbes. With
the increased use of antimicrobial agents, there is a speeding up of
this natural process.
Self-medication
Self-medication
by consumers is defined as "the taking of medicines on one's own
initiative or on another person's suggestion, who is not a certified
medical professional", and it has been identified as one of the primary
reasons for the evolution of antimicrobial resistance.
In an effort to manage their own illness, patients take the advice of
false media sources, friends, and family causing them to take
antimicrobials unnecessarily or in excess. Many people resort to this
out of necessity, when they have a limited amount of money to see a
doctor, or in many developing countries a poorly developed economy and
lack of doctors are the cause of self-medication. In these developing
countries, governments resort to allowing the sale of antimicrobials as
over the counter medications so people could have access to them without
having to find or pay to see a medical professional.
This increased access makes it extremely easy to obtain antimicrobials
without the advice of a physician, and as a result many antimicrobials
are taken incorrectly leading to resistant microbial strains. One major
example of a place that faces these challenges is India, where in the
state of Punjab 73% of the population resorted to treating their minor
health issues and chronic illnesses through self-medication.
The major issue with self-medication is the lack of knowledge of
the public on the dangerous effects of antimicrobial resistance, and how
they can contribute to it through mistreating or misdiagnosing
themselves. In order to determine the public's knowledge and
preconceived notions on antibiotic resistance, a major type of
antimicrobial resistance, a screening of 3537 articles published in
Europe, Asia, and North America was done. Of the 55,225 total people
surveyed, 70% had heard of antibiotic resistance previously, but 88% of
those people thought it referred to some type of physical change in the
body.
With so many people around the world with the ability to self-medicate
using antibiotics, and a vast majority unaware of what antimicrobial
resistance is, it makes the increase of antimicrobial resistance much
more likely.
Clinical misuse
Clinical misuse by healthcare professionals is another cause leading to increased antimicrobial resistance. Studies done by the CDC
show that the indication for treatment of antibiotics, choice of the
agent used, and the duration of therapy was incorrect in up to 50% of
the cases studied. In another study done in an intensive care unit in a
major hospital in France, it was shown that 30% to 60% of prescribed
antibiotics were unnecessary.
These inappropriate uses of antimicrobial agents promote the evolution
of antimicrobial resistance by supporting the bacteria in developing
genetic alterations that lead to resistance.
In a study done by the American Journal of Infection Control aimed to
evaluate physicians’ attitudes and knowledge on antimicrobial resistance
in ambulatory settings, only 63% of those surveyed reported antibiotic
resistance as a problem in their local practices, while 23% reported the
aggressive prescription of antibiotics as necessary to avoid failing to
provide adequate care.
This demonstrates how a majority of doctors underestimate the impact
that their own prescribing habits have on antimicrobial resistance as a
whole. It also confirms that some physicians may be overly cautious when
it comes to prescribing antibiotics for both medical or legal reasons,
even when indication for use for these medications is not always
confirmed. This can lead to unnecessary antimicrobial use.
Studies have shown that common misconceptions about the effectiveness and necessity of antibiotics to treat common mild illnesses contribute to their overuse.
Environmental pollution
Untreated effluents from pharmaceutical manufacturing industries,
hospitals and clinics, and inappropriate disposal of unused or expired
medication can expose microbes in the environment to antibiotics and
trigger the evolution of resistance.
Food production
Livestock
A CDC infographic on how antibiotic resistance spreads through farm animals.
The antimicrobial resistance crisis also extends to the food
industry, specifically with food producing animals. Antibiotics are fed
to livestock to act as growth supplements, and a preventative measure
to decrease the likelihood of infections. This results in the transfer
of resistant bacterial strains into the food that humans eat, causing
potentially fatal transfer of disease. While this practice does result
in better yields and meat products, it is a major issue in terms of preventing antimicrobial resistance.
Though the evidence linking antimicrobial usage in livestock to
antimicrobial resistance is limited, the World Health Organization
Advisory Group on Integrated Surveillance of Antimicrobial Resistance
strongly recommended the reduction of use of medically important
antimicrobials in livestock. Additionally, the Advisory Group stated
that such antimicrobials should be expressly prohibited for both growth
promotion and disease prevention.
In a study published by the National Academy of Sciences
mapping antimicrobial consumption in livestock globally, it was
predicted that in the 228 countries studied, there would be a total 67%
increase in consumption of antibiotics by livestock by 2030. In some
countries such as Brazil, Russia, India, China, and South Africa it is
predicted that a 99% increase will occur.
Several countries have restricted the use of antibiotics in livestock,
including Canada, China, Japan, and the US. These restrictions are
sometimes associated with a reduction of the prevalence of antimicrobial resistance in humans.
Pesticides
Most pesticides
protect crops against insects and plants, but in some cases
antimicrobial pesticides are used to protect against various
microorganisms such as bacteria, viruses, fungi, algae, and protozoa.
The overuse of many pesticides in an effort to have a higher yield of
crops has resulted in many of these microbes evolving a tolerance
against these antimicrobial agents. Currently there are over 4000
antimicrobial pesticides registered with the EPA and sold to market, showing the widespread use of these agents.
It is estimated that for every single meal a person consumes, 0.3 g of
pesticides is used, as 90% of all pesticide use is used on agriculture.
A majority of these products are used to help defend against the spread
of infectious diseases, and hopefully protect public health. But out of
the large amount of pesticides used, it is also estimated that less
than 0.1% of those antimicrobial agents, actually reach their targets.
That leaves over 99% of all pesticides used available to contaminate
other resources.
In soil, air, and water these antimicrobial agents are able to spread,
coming in contact with more microorganisms and leading to these microbes
evolving mechanisms to tolerate and further resist pesticides.
Prevention
Mission Critical: Preventing Antibiotic Resistance (CDC report, 2014)
There have been increasing public calls for global collective action
to address the threat, including a proposal for international treaty on
antimicrobial resistance. Further detail and attention is still needed
in order to recognize and measure trends in resistance on the
international level; the idea of a global tracking system has been
suggested but implementation has yet to occur. A system of this nature
would provide insight to areas of high resistance as well as information
necessary for evaluating programs and other changes made to fight or
reverse antibiotic resistance.
Duration of antibiotics
Antibiotic treatment duration should be based on the infection and other health problems a person may have. For many infections once a person has improved there is little evidence that stopping treatment causes more resistance. Some therefore feel that stopping early may be reasonable in some cases. Other infections, however, do require long courses regardless of whether a person feels better.
Monitoring and mapping
There are multiple national and international monitoring programs for drug-resistant threats, including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant S. aureus (VRSA), extended spectrum beta-lactamase (ESBL), vancomycin-resistant Enterococcus (VRE), and multidrug-resistant Acinetobacter baumannii (MRAB).
ResistanceOpen is an online global map of antimicrobial resistance developed by HealthMap which displays aggregated data on antimicrobial resistance from publicly available and user submitted data. The website can display data for a 25-mile radius from a location. Users may submit data from antibiograms
for individual hospitals or laboratories. European data is from the
EARS-Net (European Antimicrobial Resistance Surveillance Network), part
of the ECDC.
ResistanceMap is a website by the Center for Disease Dynamics, Economics & Policy and provides data on antimicrobial resistance on a global level.
Limiting antibiotic use
Antibiotic stewardship programmes appear useful in reducing rates of antibiotic resistance.
The antibiotic stewardship program will also provide pharmacists with
the knowledge to educate patients that antibiotics will not work for a
virus.
Excessive antibiotic use has become one of the top contributors
to the evolution of antibiotic resistance. Since the beginning of the
antibiotic era, antibiotics have been used to treat a wide range of
disease.
Overuse of antibiotics has become the primary cause of rising levels of
antibiotic resistance. The main problem is that doctors are willing to
prescribe antibiotics to ill-informed individuals who believe that
antibiotics can cure nearly all illnesses, including viral infections
like the common cold. In an analysis of drug prescriptions, 36% of
individuals with a cold or an upper respiratory infection (both viral in
origin) were given prescriptions for antibiotics. These prescriptions accomplished nothing other than increasing the risk of further evolution of antibiotic resistant bacteria.
Using antibiotics without prescription is another driving force leading
to the overuse of antibiotics to self-treat diseases like the common
cold, cough, fever, and dysentery resulting in a pandemic of antibiotic
resistance in countries like Bangladesh, risking its spread around the
globe. Introducing strict antibiotic stewardship in the outpatient setting may reduce the emerging bacterial resistance.
At the hospital level
Antimicrobial stewardship teams in hospitals are encouraging optimal use of antimicrobials.
The goals of antimicrobial stewardship are to help practitioners pick
the right drug at the right dose and duration of therapy while
preventing misuse and minimizing the development of resistance.
Stewardship may reduce the length of stay by an average of slightly over
1 day while not increasing the risk of death.
At the farming level
It
is established that the use of antibiotics in animal husbandry can give
rise to AMR resistances in bacteria found in food animals to the
antibiotics being administered (through injections or medicated feeds). For this reason only antimicrobials that are deemed "not-clinically relevant" are used in these practices.
Recent studies have shown that the prophylactic use of
"non-priority" or "non-clinically relevant" antimicrobials in feeds can
potentially, under certain conditions, lead to co-selection of
environmental AMR bacteria with resistance to medically important
antibiotics.
The possibility for co-selection of AMR resistances in the food chain
pipeline may have far-reaching implications for human health.
At the level of GP
Given
the volume of care provided in primary care (General Practice), recent
strategies have focused on reducing unnecessary antibiotic prescribing
in this setting. Simple interventions, such as written information
explaining the futility of antibiotics for common infections such as
upper respiratory tract infections, have been shown to reduce antibiotic
prescribing.
The prescriber should closely adhere to the five rights of drug
administration: the right patient, the right drug, the right dose, the
right route, and the right time.
Cultures should be taken before treatment when indicated and treatment potentially changed based on the susceptibility report.
About a third of antibiotic prescriptions written in outpatient settings
in the United States were not appropriate in 2010 and 2011. Doctors in
the U.S. wrote 506 annual antibiotic scripts for every 1,000 people,
with 353 being medically necessary.
Health workers and pharmacists can help tackle resistance by:
enhancing infection prevention and control; only prescribing and
dispensing antibiotics when they are truly needed; prescribing and
dispensing the right antibiotic(s) to treat the illness.
At the individual level
People
can help tackle resistance by using antibiotics only when prescribed by
a doctor; completing the full prescription, even if they feel better;
never sharing antibiotics with others or using leftover prescriptions.
Country examples
- The Netherlands has the lowest rate of antibiotic prescribing in the OECD, at a rate of 11.4 defined daily doses (DDD) per 1,000 people per day in 2011.
- Germany and Sweden also have lower prescribing rates, with Sweden's rate having been declining since 2007.
- Greece, France and Belgium have high prescribing rates of more than 28 DDD.
Water, sanitation, hygiene
Infectious disease control through improved water, sanitation and hygiene (WASH)
infrastructure needs to be included in the antimicrobial resistance
(AMR) agenda. The "Interagency Coordination Group on Antimicrobial
Resistance" stated in 2018 that "the spread of pathogens through unsafe
water results in a high burden of gastrointestinal disease, increasing
even further the need for antibiotic treatment." This is particularly a problem in developing countries where the spread of infectious diseases caused by inadequate WASH standards is a major driver of antibiotic demand.
Growing usage of antibiotics together with persistent infectious
disease levels have led to a dangerous cycle in which reliance on
antimicrobials increases while the efficacy of drugs diminishes.
The proper use of infrastructure for water, sanitation and hygiene
(WASH) can result in a 47–72 percent decrease of diarrhea cases treated
with antibiotics depending on the type of intervention and its
effectiveness.
A reduction of the diarrhea disease burden through improved
infrastructure would result in large decreases in the number of diarrhea
cases treated with antibiotics. This was estimated as ranging from 5
million in Brazil to up to 590 million in India by the year 2030.
The strong link between increased consumption and resistance indicates
that this will directly mitigate the accelerating spread of AMR. Sanitation and water for all by 2030 is Goal Number 6 of the Sustainable Development Goals.
An increase in hand washing compliance by hospital staff results in decreased rates of resistant organisms.
Water supply and sanitation infrastructure in health facilities
offer significant co-benefits for combatting AMR, and investment should
be increased.
There is much room for improvement: WHO and UNICEF estimated in 2015
that globally 38% of health facilities did not have a source of water,
nearly 19% had no toilets and 35% had no water and soap or alcohol-based
hand rub for handwashing.
Industrial wastewater treatment
Manufacturers of antimicrobials need to improve the treatment of their wastewater (by using industrial wastewater treatment processes) to reduce the release of residues into the environment.
Management in animal use
Europe
In 1997, European Union health ministers voted to ban avoparcin and four additional antibiotics used to promote animal growth in 1999.
In 2006 a ban on the use of antibiotics in European feed, with the
exception of two antibiotics in poultry feeds, became effective.
In Scandinavia, there is evidence that the ban has led to a lower
prevalence of antibiotic resistance in (nonhazardous) animal bacterial
populations.
As of 2004, several European countries established a decline of
antimicrobial resistance in humans through limiting the use of
antimicrobials in agriculture and food industries without jeopardizing
animal health or economic cost.
United States
The United States Department of Agriculture (USDA) and the Food and Drug Administration (FDA) collect data on antibiotic use in humans and in a more limited fashion in animals.
The FDA first determined in 1977 that there is evidence of emergence of
antibiotic-resistant bacterial strains in livestock. The
long-established practice of permitting OTC sales of antibiotics
(including penicillin and other drugs) to lay animal owners for
administration to their own animals nonetheless continued in all states.
In 2000, the FDA announced their intention to revoke approval of fluoroquinolone use in poultry production because of substantial evidence linking it to the emergence of fluoroquinolone-resistant Campylobacter
infections in humans. Legal challenges from the food animal and
pharmaceutical industries delayed the final decision to do so until
2006.
Fluroquinolones have been banned from extra-label use in food animals
in the USA since 2007. However, they remain widely used in companion and
exotic animals.
Global action plans and awareness
The
increasing interconnectedness of the world and the fact that new
classes of antibiotics have not been developed and approved for more
than 25 years highlight the extent to which antimicrobial resistance is a
global health challenge.
A global action plan to tackle the growing problem of resistance to
antibiotics and other antimicrobial medicines was endorsed at the
Sixty-eighth World Health Assembly in May 2015.
One of the key objectives of the plan is to improve awareness and
understanding of antimicrobial resistance through effective
communication, education and training. This global action plan developed
by the World Health Organization was created to combat the issue of
antimicrobial resistance and was guided by the advice of countries and
key stakeholders. The WHO's global action plan is composed of five key
objectives that can be targeted through different means, and represents
countries coming together to solve a major problem that can have future
health consequences. These objectives are as follows:
- improve awareness and understanding of antimicrobial resistance through effective communication, education and training.
- strengthen the knowledge and evidence base through surveillance and research.
- reduce the incidence of infection through effective sanitation, hygiene and infection prevention measures.
- optimize the use of antimicrobial medicines in human and animal health.
- develop the economic case for sustainable investment that takes
account of the needs of all countries and to increase investment in new
medicines, diagnostic tools, vaccines and other interventions.
Steps towards progress
- React based in Sweden has produced informative material on AMR for the general public.
- Videos are being produced for the general public to generate interest and awareness.
- The Irish Department of Health published a National Action Plan on Antimicrobial Resistance in October 2017.
The Strategy for the Control of Antimicrobial Resistance in Ireland
(SARI), Iaunched in 2001 developed Guidelines for Antimicrobial
Stewardship in Hospitals in Ireland
in conjunction with the Health Protection Surveillance Centre, these
were published in 2009. Following their publication a public information
campaign 'Action on Antibiotics'
was launched to highlight the need for a change in antibiotic
prescribing. Despite this, antibiotic prescribing remains high with
variance in adherence to guidelines.
Antibiotic Awareness Week
The
World Health Organization has promoted the first World Antibiotic
Awareness Week running from 16 to 22 November 2015. The aim of the week
is to increase global awareness of antibiotic resistance. It also wants
to promote the correct usage of antibiotics across all fields in order
to prevent further instances of antibiotic resistance.
World Antibiotic Awareness Week has been held every November
since 2015. For 2017, the Food and Agriculture Organization of the
United Nations (FAO), the World Health Organization (WHO) and the World Organisation for Animal Health
(OIE) are together calling for responsible use of antibiotics in humans
and animals to reduce the emergence of antibiotic resistance.
United Nations
In 2016 the Secretary-General of the United Nations convened the Interagency Coordination Group (IACG) on Antimicrobial Resistance.
The IACG worked with international organizations and experts in human,
animal, and plant health to create a plan to fight antimicrobial
resistance.
Their report released in April 2019 highlights the seriousness of
antimicrobial resistance and the threat it poses to world health. It
suggests five recommendations for member states to follow in order to
tackle this increasing threat. The IACG recommendations are as follows:
- Accelerate progress in countries
- Innovate to secure the future
- Collaborate for more effective action
- Invest for a sustainable response
- Strengthen accountability and global governance
Mechanisms and organisms
Bacteria
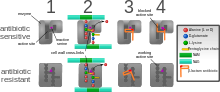
Diagram
depicting antibiotic resistance through alteration of the antibiotic's
target site, modeled after MRSA's resistance to penicillin. Beta-lactam
antibiotics permanently inactivate
PBP enzymes, which are essential for bacterial life, by permanently binding to their active sites.
MRSA, however, expresses a PBP that does not allow the antibiotic into its active site.
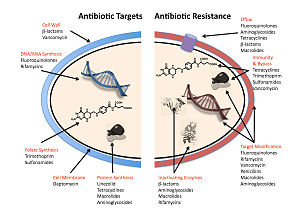
The five main mechanisms by which bacteria exhibit resistance to antibiotics are:
- Drug inactivation or modification: for example, enzymatic deactivation of penicillin G in some penicillin-resistant bacteria through the production of β-lactamases. Drugs may also be chemically modified through the addition of functional groups by transferase enzymes; for example, acetylation, phosphorylation, or adenylation are common resistance mechanisms to aminoglycosides. Acetylation is the most widely used mechanism and can affect a number of drug classes.
- Alteration of target- or binding site: for example, alteration of PBP—the binding target site of penicillins—in MRSA
and other penicillin-resistant bacteria. Another protective mechanism
found among bacterial species is ribosomal protection proteins. These
proteins protect the bacterial cell from antibiotics that target the
cell's ribosomes to inhibit protein synthesis. The mechanism involves
the binding of the ribosomal protection proteins to the ribosomes of the
bacterial cell, which in turn changes its conformational shape. This
allows the ribosomes to continue synthesizing proteins essential to the
cell while preventing antibiotics from binding to the ribosome to
inhibit protein synthesis.
- Alteration of metabolic pathway: for example, some sulfonamide-resistant bacteria do not require para-aminobenzoic acid (PABA), an important precursor for the synthesis of folic acid and nucleic acids in bacteria inhibited by sulfonamides, instead, like mammalian cells, they turn to using preformed folic acid.
- Reduced drug accumulation: by decreasing drug permeability or increasing active efflux (pumping out) of the drugs across the cell surface
These pumps within the cellular membrane of certain bacterial species
are used to pump antibiotics out of the cell before they are able to do
any damage. They are often activated by a specific substrate associated
with an antibiotic, as in fluoroquinolone resistance.
- Ribosome splitting and recycling: for example, drug-mediated stalling of the ribosome by lincomycin and erythromycin unstalled by a heat shock protein found in Listeria monocytogenes,
which is a homologue of HflX from other bacteria. Liberation of the
ribosome from the drug allows further translation and consequent
resistance to the drug.
A number of mechanisms used by common antibiotics to deal with bacteria and ways by which bacteria become resistant to them.
There are several different types of germs that have developed a resistance over time. For example, Penicillinase-producing Neisseria gonorrhoeae developed a resistance to penicillin in 1976. Another example is Azithromycin-resistant Neisseria gonorrhoeae, which developed a resistance to azithromycin in 2011.
In gram-negative bacteria, plasmid-mediated resistance genes produce proteins that can bind to DNA gyrase, protecting it from the action of quinolones. Finally, mutations at key sites in DNA gyrase or topoisomerase IV can decrease their binding affinity to quinolones, decreasing the drug's effectiveness.
Some bacteria are naturally resistant to certain antibiotics; for example, gram-negative bacteria are resistant to most β-lactam antibiotics due to the presence of β-lactamase. Antibiotic resistance can also be acquired as a result of either genetic mutation or horizontal gene transfer. Although mutations are rare, with spontaneous mutations in the pathogen genome occurring at a rate of about 1 in 105 to 1 in 108 per chromosomal replication,
the fact that bacteria reproduce at a high rate allows for the effect
to be significant. Given that lifespans and production of new
generations can be on a timescale of mere hours, a new (de novo)
mutation in a parent cell can quickly become an inherited mutation of widespread prevalence, resulting in the microevolution
of a fully resistant colony. However, chromosomal mutations also confer
a cost of fitness. For example, a ribosomal mutation may protect a
bacterial cell by changing the binding site of an antibiotic but may
result in slower growth rate. Moreover, some adaptive mutations can propagate not only through inheritance but also through horizontal gene transfer. The most common mechanism of horizontal gene transfer is the transferring of plasmids carrying antibiotic resistance genes between bacteria of the same or different species via conjugation. However, bacteria can also acquire resistance through transformation, as in Streptococcus pneumoniae uptaking of naked fragments of extracellular DNA that contain antibiotic resistance genes to streptomycin, through transduction, as in the bacteriophage-mediated transfer of tetracycline resistance genes between strains of S. pyogenes, or through gene transfer agents, which are particles produced by the host cell that resemble bacteriophage structures and are capable of transferring DNA.
Antibiotic resistance can be introduced artificially into a microorganism through laboratory protocols, sometimes used as a selectable marker
to examine the mechanisms of gene transfer or to identify individuals
that absorbed a piece of DNA that included the resistance gene and
another gene of interest.
Recent findings show no necessity of large populations of
bacteria for the appearance of antibiotic resistance. Small populations
of Escherichia coli
in an antibiotic gradient can become resistant. Any heterogeneous
environment with respect to nutrient and antibiotic gradients may
facilitate antibiotic resistance in small bacterial populations.
Researchers hypothesize that the mechanism of resistance evolution is
based on four SNP mutations in the genome of E. coli produced by the gradient of antibiotic.
In one study, which has implications for space microbiology, a non-pathogenic strain E. coli MG1655 was exposed to trace levels of the broad spectrum antibiotic chloramphenicol,
under simulated microgravity (LSMMG, or, Low Shear Modeled
Microgravity) over 1000 generations. The adapted strain acquired
resistance to not only chloramphenicol, but also cross-resistance to
other antibiotics;
this was in contrast to the observation on the same strain, which was
adapted to over 1000 generations under LSMMG, but without any antibiotic
exposure; the strain in this case did not acquire any such resistance.
Thus, irrespective of where they are used, the use of an antibiotic
would likely result in persistent resistance to that antibiotic, as well
as cross-resistance to other antimicrobials.
In recent years, the emergence and spread of β-lactamases called carbapenemases has become a major health crisis. One such carbapenemase is New Delhi metallo-beta-lactamase 1 (NDM-1), an enzyme that makes bacteria resistant to a broad range of beta-lactam antibiotics. The most common bacteria that make this enzyme are gram-negative such as E. coli and Klebsiella pneumoniae, but the gene for NDM-1 can spread from one strain of bacteria to another by horizontal gene transfer.
Viruses
Specific antiviral drugs
are used to treat some viral infections. These drugs prevent viruses
from reproducing by inhibiting essential stages of the virus's
replication cycle in infected cells. Antivirals are used to treat HIV, hepatitis B, hepatitis C, influenza, herpes viruses including varicella zoster virus, cytomegalovirus and Epstein-Barr virus. With each virus, some strains have become resistant to the administered drugs.
Antiviral drugs typically target key components of viral reproduction; for example, oseltamivir targets influenza neuraminidase,
while guanosine analogs inhibit viral DNA polymerase. Resistance to
antivirals is thus acquired through mutations in the genes that encode
the protein targets of the drugs.
Resistance to HIV antivirals is problematic, and even multi-drug resistant strains have evolved. One source of resistance is that many current HIV drugs, including NRTIs and NNRTIs, target reverse transcriptase; however, HIV-1 reverse transcriptase is highly error prone and thus mutations conferring resistance arise rapidly. Resistant strains of the HIV virus emerge rapidly if only one antiviral drug is used. Using three or more drugs together, termed combination therapy,
has helped to control this problem, but new drugs are needed because of
the continuing emergence of drug-resistant HIV strains.
Fungi
Infections by fungi are a cause of high morbidity and mortality in immunocompromised persons, such as those with HIV/AIDS, tuberculosis or receiving chemotherapy. The fungi candida, Cryptococcus neoformans and Aspergillus fumigatus cause most of these infections and antifungal resistance occurs in all of them.
Multidrug resistance in fungi is increasing because of the widespread
use of antifungal drugs to treat infections in immunocompromised
individuals.
Of particular note, Fluconazole-resistant Candida species have been highlighted as a growing problem by the CDC. More than 20 species of Candida can cause Candidiasis infection, the most common of which is Candida albicans.
Candida yeasts normally inhabit the skin and mucous membranes without
causing infection. However, overgrowth of Candida can lead to
Candidiasis. Some Candida strains are becoming resistant to first-line
and second-line antifungal agents such as azoles and echinocandins.
Parasites
The protozoan parasites that cause the diseases malaria, trypanosomiasis, toxoplasmosis, cryptosporidiosis and leishmaniasis are important human pathogens.
Malarial parasites that are resistant to the drugs that are
currently available to infections are common and this has led to
increased efforts to develop new drugs. Resistance to recently developed drugs such as artemisinin has also been reported. The problem of drug resistance in malaria has driven efforts to develop vaccines.
Trypanosomes are parasitic protozoa that cause African trypanosomiasis and Chagas disease (American trypanosomiasis). There are no vaccines to prevent these infections so drugs such as pentamidine and suramin, benznidazole and nifurtimox are used to treat infections. These drugs are effective but infections caused by resistant parasites have been reported.
Leishmaniasis
is caused by protozoa and is an important public health problem
worldwide, especially in sub-tropical and tropical countries. Drug
resistance has "become a major concern".
History
The
1950s to 1970s represented the golden age of antibiotic discovery, where
countless new classes of antibiotics were discovered to treat
previously incurable diseases such as tuberculosis and syphilis.
However, since that time the discovery of new classes of antibiotics
has been almost nonexistent, and represents a situation that is
especially problematic considering the resiliency of bacteria shown over time and the continued misuse and overuse of antibiotics in treatment.
The phenomenon of antimicrobial resistance caused by overuse of antibiotics was predicted as early as 1945 by Alexander Fleming
who said "The time may come when penicillin can be bought by anyone in
the shops. Then there is the danger that the ignorant man may easily
under-dose himself and by exposing his microbes to nonlethal quantities
of the drug make them resistant."
Without the creation of new and stronger antibiotics an era where
common infections and minor injuries can kill, and where complex
procedures such as surgery and chemotherapy become too risky, is a very
real possibility.
Antimicrobial resistance threatens the world as we know it, and can
lead to epidemics of enormous proportions if preventive actions are not
taken. In this day and age current antimicrobial resistance leads to
longer hospital stays, higher medical costs, and increased mortality.
Society and culture
Since
the mid-1980s pharmaceutical companies have invested in medications for
cancer or chronic disease that have greater potential to make money and
have "de-emphasized or dropped development of antibiotics". On 20 January 2016 at the World Economic Forum in Davos, Switzerland,
more than "80 pharmaceutical and diagnostic companies" from around the
world called for "transformational commercial models" at a global level
to spur research and development on antibiotics and on the "enhanced use
of diagnostic tests that can rapidly identify the infecting organism".
Legal frameworks
Some
global health scholars have argued that a global, legal framework is
needed to prevent and control antimicrobial resistance.
For instance, binding global policies could be used to create
antimicrobial use standards, regulate antibiotic marketing, and
strengthen global surveillance systems. Ensuring compliance of involved parties is a challenge.
Global antimicrobial resistance policies could take lessons from the
environmental sector by adopting strategies that have made international
environmental agreements successful in the past such as: sanctions for
non-compliance, assistance for implementation, majority vote
decision-making rules, an independent scientific panel, and specific
commitments.
United States
For the United States 2016 budget, U.S. president Barack Obama
proposed to nearly double the amount of federal funding to "combat and
prevent" antibiotic resistance to more than $1.2 billion. Many international funding agencies like USAID, DFID, SIDA and Bill & Melinda Gates Foundation have pledged money for developing strategies to counter antimicrobial resistance.
On 27 March 2015, the White House
released a comprehensive plan to address the increasing need for
agencies to combat the rise of antibiotic-resistant bacteria. The Task
Force for Combating Antibiotic-Resistant Bacteria developed The National Action Plan for Combating Antibiotic-Resistant Bacteria
with the intent of providing a roadmap to guide the US in the
antibiotic resistance challenge and with hopes of saving many lives.
This plan outlines steps taken by the Federal government over the next
five years needed in order to prevent and contain outbreaks of
antibiotic-resistant infections; maintain the efficacy of antibiotics
already on the market; and to help to develop future diagnostics,
antibiotics, and vaccines.
The Action Plan was developed around five goals with focuses on
strengthening health care, public health veterinary medicine,
agriculture, food safety and research, and manufacturing. These goals,
as listed by the White House, are as follows:
- Slow the Emergence of Resistant Bacteria and Prevent the Spread of Resistant Infections
- Strengthen National One-Health Surveillance Efforts to Combat Resistance
- Advance Development and use of Rapid and Innovative Diagnostic Tests
for Identification and Characterization of Resistant Bacteria
- Accelerate Basic and Applied Research and Development for New Antibiotics, Other Therapeutics, and Vaccines
- Improve International Collaboration and Capacities for Antibiotic
Resistance Prevention, Surveillance, Control and Antibiotic Research and
Development
The following are goals set to meet by 2020:
- Establishment of antimicrobial programs within acute care hospital settings
- Reduction of inappropriate antibiotic prescription and use by at least 50% in outpatient settings and 20% inpatient settings
- Establishment of State Antibiotic Resistance (AR) Prevention Programs in all 50 states
- Elimination of the use of medically important antibiotics for growth promotion in food-producing animals.
United Kingdom
Public Health England
reported that the total number of antibiotic resistant infections in
England rose by 9% from 55,812 in 2017 to 60,788 in 2018, but antibiotic
consumption had fallen by 9% from 20.0 to 18.2 defined daily doses per
1,000 inhabitants per day between 2014 and 2018.
Policies
According to World Health Organization,
policymakers can help tackle resistance by strengthening
resistance-tracking and laboratory capacity and by regulating and
promoting the appropriate use of medicines.
Policymakers and industry can help tackle resistance by: fostering
innovation and research and development of new tools; and promoting
cooperation and information sharing among all stakeholders.
Further research
Rapid viral testing
Clinical
investigation to rule out bacterial infections are often done for
patients with pediatric acute respiratory infections. Currently it is
unclear if rapid viral testing affects antibiotic use in children.
Vaccines
Microorganisms usually do not develop resistance to vaccines
because vaccines reduce the spread of the infection and target the
pathogen in multiple ways in the same host and possibly in different
ways between different hosts. Furthermore, if the use of vaccines
increases, there is evidence that antibiotic resistant strains of
pathogens will decrease; the need for antibiotics will naturally
decrease as vaccines prevent infection before it occurs.
However, there are well documented cases of vaccine resistance,
although these are usually much less of a problem than antimicrobial
resistance.
While theoretically promising, antistaphylococcal vaccines have
shown limited efficacy, because of immunological variation between Staphylococcus
species, and the limited duration of effectiveness of the antibodies
produced. Development and testing of more effective vaccines is
underway.
Two registrational trials have evaluated vaccine candidates in active immunization strategies against S. aureus
infection. In a phase II trial, a bivalent vaccine of capsulat protein 5
& 8 was tested in 1804 hemodialysis patients with a primary fistula
or synthetic graft vascular access. After 40 weeks following
vaccination a protective effect was seen against S. aureus bacteremia,
but not at 54 weeks following vaccination. Based on these results, a second trial was conducted which failed to show efficacy.
Merck tested V710, a vaccine targeting IsdB, in a blinded
randomized trial in patients undergoing median sternotomy. The trial was
terminated after a higher rate of multiorgan system failure–related
deaths was found in the V710 recipients. Vaccine recipients who
developed S. aureus infection were 5 times more likely to die than control recipients who developed S. aureus infection.
Numerous investigators have suggested that a multiple-antigen
vaccine would be more effective, but a lack of biomarkers defining human
protective immunity keep these proposals in the logical, but strictly
hypothetical arena.
Alternating therapy
Alternating
therapy is a proposed method in which two or three antibiotics are
taken in a rotation versus taking just one antibiotic such that bacteria
resistant to one antibiotic are killed when the next antibiotic is
taken. Studies have found that this method reduces the rate at which
antibiotic resistant bacteria emerge in vitro relative to a single drug
for the entire duration.
Studies have found that bacteria that evolve antibiotic
resistance towards one group of antibiotic may become more sensitive to
others. This phenomenon can be used to select against resistant bacteria using an approach termed collateral sensitivity cycling, which has recently been found to be relevant in developing treatment strategies for chronic infections caused by Pseudomonas aeruginosa.
Development of new drugs
Since the discovery of antibiotics, research and development
(R&D) efforts have provided new drugs in time to treat bacteria
that became resistant to older antibiotics, but in the 2000s there has
been concern that development has slowed enough that seriously ill
people may run out of treatment options.
Another concern is that doctors may become reluctant to perform routine
surgeries because of the increased risk of harmful infection. Backup treatments can have serious side-effects; for example, treatment of multi-drug-resistant tuberculosis can cause deafness or psychological disability. The potential crisis at hand is the result of a marked decrease in industry R&D. Poor financial investment in antibiotic research has exacerbated the situation.
The pharmaceutical industry has little incentive to invest in
antibiotics because of the high risk and because the potential financial
returns are less likely to cover the cost of development than for other pharmaceuticals. In 2011, Pfizer,
one of the last major pharmaceutical companies developing new
antibiotics, shut down its primary research effort, citing poor
shareholder returns relative to drugs for chronic illnesses.
However, small and medium-sized pharmaceutical companies are still
active in antibiotic drug research. In particular, apart from classical
synthetic chemistry methodologies, researchers have developed a
combinatorial synthetic biology platform on single cell level in a high-throughput screening manner to diversify novel lanthipeptides.
In the United States, drug companies and the administration of President Barack Obama had been proposing changing the standards by which the FDA approves antibiotics targeted at resistant organisms.
On 18 September 2014 Obama signed an executive order to implement the recommendations proposed in a report by the President's Council of Advisors on Science and Technology
(PCAST) which outlines strategies to stream-line clinical trials and
speed up the R&D of new antibiotics. Among the proposals:
- Create a 'robust, standing national clinical trials network for
antibiotic testing' which will promptly enroll patients once identified
to be suffering from dangerous bacterial infections. The network will
allow testing multiple new agents from different companies
simultaneously for their safety and efficacy.
- Establish a 'Special Medical Use (SMU)' pathway for FDA to approve
new antimicrobial agents for use in limited patient populations, shorten
the approval timeline for new drug so patients with severe infections
could benefit as quickly as possible.
- Provide economic incentives, especially for development of new
classes of antibiotics, to offset the steep R&D costs which drive
away the industry to develop antibiotics.
Biomaterials
Using
antibiotic-free alternatives in bone infection treatment may help
decrease the use of antibiotics and thus antimicrobial resistance. The bone regeneration material bioactive glass S53P4 has shown to
effectively inhibit the bacterial growth of up to 50 clinically relevant
bacteria including MRSA and MRSE.
Nanomaterials
During the last decades, copper and silver nanomaterials have demonstrated appealing features for the development of a new family of antimicrobial agents.
Rediscovery of ancient treatments
Similar to the situation in malaria therapy, where successful treatments based on ancient recipes have been found,
there has already been some success in finding and testing ancient
drugs and other treatments that are effective against AMR bacteria.
Rapid diagnostics
Distinguishing infections requiring antibiotics from self-limiting
ones is clinically challenging. In order to guide appropriate use of
antibiotics and prevent the evolution and spread of antimicrobial
resistance, diagnostic tests that provide clinicians with timely,
actionable results are needed.
Acute febrile illness is a common reason for seeking medical care
worldwide and a major cause of morbidity and mortality. In areas with
decreasing malaria incidence, many febrile patients are inappropriately
treated for malaria, and in the absence of a simple diagnostic test to
identify alternative causes of fever, clinicians presume that a
non-malarial febrile illness is most likely a bacterial infection,
leading to inappropriate use of antibiotics. Multiple studies have shown
that the use of malaria rapid diagnostic tests without reliable tools
to distinguish other fever causes has resulted in increased antibiotic
use.
Antimicrobial susceptibility testing (AST) can help practitioners avoid prescribing unnecessary antibiotics in the style of precision medicine, and help them prescribe effective antibiotics, but with the traditional approach it could take 12 to 48 hours. Rapid testing, possible from molecular diagnostics innovations, is defined as "being feasible within an 8-h working shift". Progress has been slow due to a range of reasons including cost and regulation.
Optical techniques such as phase contrast microscopy in
combination with single-cell analysis are another powerful method to
monitor bacterial growth. In 2017, scientists from Sweden published a
method that applies principles of microfluidics
and cell tracking, to monitor bacterial response to antibiotics in less
than 30 minutes overall manipulation time. Recently, this platform has
been advanced by coupling microfluidic chip with optical tweezing in order to isolate bacteria with altered phenotype directly from the analytical matrix.
Phage therapy
Phage therapy is the therapeutic use of bacteriophages to treat pathogenic bacterial infections. Phage therapy has many potential applications in human medicine as well as dentistry, veterinary science, and agriculture.
Phage therapy relies on the use of naturally-occurring
bacteriophages to infect and lyse bacteria at the site of infection in a
host. Due to current advances in genetics and biotechnology these
bacteriophages can possibly be manufactured to treat specific
infections.
Phages can be bioengineered to target multidrug-resistant bacterial
infections, and their use involves the added benefit of preventing the
elimination of beneficial bacteria in the human body.
Phages destroy bacterial cell walls and membrane through the use of
lytic proteins which kill bacteria by making many holes from the inside
out.
Bacteriophages can even possess the ability to digest the biofilm that
many bacteria develop that protect them from antibiotics in order to
effectively infect and kill bacteria. Bioengineering can play a role in
creating successful bacteriophages.
Understanding the mutual interactions and evolutions of bacterial
and phage populations in the environment of a human or animal body is
essential for rational phage therapy.
Bacteriophagics are used against antibiotic resistant bacteria in Georgia (George Eliava Institute) and in one institute in Wrocław, Poland. Bacteriophage cocktails are common drugs sold over the counter in pharmacies in eastern countries.
In Belgium, four patients with severe musculoskeletal infections
received bacteriophage therapy with concomitant antibiotics. After a
single course of phage therapy, no recurrence of infection occurred and
no severe side-effects related to the therapy were detected.