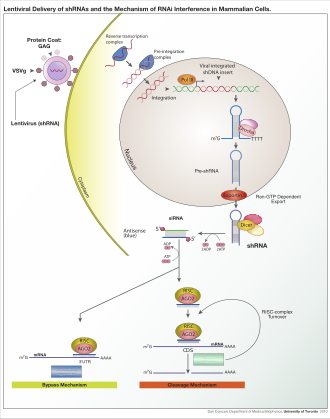
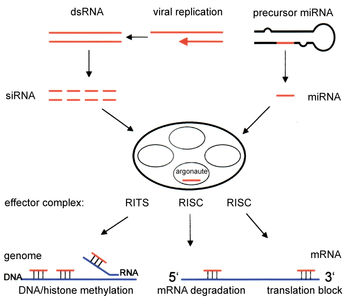
From Wikipedia, the free encyclopedia
Lentiviral delivery of designed shRNA's and the mechanism of RNA interference in mammalian cells.
RNA interference (
RNAi) is a biological process in which
RNA molecules
inhibit gene expression or translation, by neutralizing targeted
mRNA molecules. Historically, RNA interference was known by other names, including
co-suppression,
post-transcriptional gene silencing (PTGS), and
quelling.
The detailed study of each of these seemingly different processes,
elucidated that the identity of these phenomena were all actually RNAi.
Andrew Fire and
Craig C. Mello shared the 2006
Nobel Prize in Physiology or Medicine for their work on RNA interference in the
nematode worm
Caenorhabditis elegans,
which they published in 1998. Since the discovery of RNAi and its
regulatory potentials, it has become evident that RNAi has immense
potential in suppression of desired genes. RNAi is now known as precise,
efficient, stable and better than antisense technology for gene
suppression. However, antisense RNA produced intracellularly by an expression
vector may be developed and find utility as novel therapeutic agents.
Two types of small
ribonucleic acid (RNA) molecules –
microRNA (miRNA) and
small interfering RNA (
siRNA) –
are central to RNA interference. RNAs are the direct products of genes,
and these small RNAs can direct enzyme complexes to degrade
messenger RNA
(mRNA) molecules and thus decrease their activity by preventing
translation, via post-transcriptional gene silencing. Moreover,
transcription can be inhibited via the pre-transcriptional silencing
mechanism of RNA interference, through which an enzyme complex catalyzes
DNA methylation at genomic positions complementary to complexed siRNA
or miRNA. RNA interference has an important role in defending cells
against parasitic
nucleotide sequences –
viruses and
transposons. It also influences
development.
The RNAi pathway is found in many
eukaryotes, including animals, and is initiated by the enzyme
Dicer, which cleaves long
double-stranded RNA (dsRNA)
molecules into short double-stranded fragments of ~21
nucleotide siRNAs. Each
siRNA
is unwound into two single-stranded RNAs (ssRNAs), the passenger strand
and the guide strand. The passenger strand is degraded and the guide
strand is incorporated into the
RNA-induced silencing complex
(RISC). The most well-studied outcome is post-transcriptional gene
silencing, which occurs when the guide strand pairs with a complementary
sequence in a messenger RNA molecule and induces cleavage by
Argonaute 2 (Ago2), the catalytic component of the
RISC. In some organisms, this process spreads systemically, despite the initially limited molar concentrations of
siRNA.
RNAi is a valuable research tool, both in
cell culture and in
living organisms,
because synthetic dsRNA introduced into cells can selectively and
robustly induce suppression of specific genes of interest. RNAi may be
used for large-scale screens that systematically shut down each gene in
the cell, which can help to identify the components necessary for a
particular cellular process or an event such as
cell division. The pathway is also used as a practical tool in
biotechnology,
medicine and
insecticides.
[3]
Cellular mechanism
The
dicer protein from
Giardia intestinalis, which catalyzes the cleavage of dsRNA to siRNAs. The
RNase domains are colored green, the PAZ domain yellow, the platform domain red, and the connector helix blue.
[4]
RNAi is RNA-dependent
gene silencing
process that is controlled by the RNA-induced silencing complex (RISC)
and is initiated by short double-stranded RNA molecules in a cell's
cytoplasm, where they interact with the catalytic RISC component
argonaute.
[5]
When the dsRNA is exogenous (coming from infection by a virus with an
RNA genome or laboratory manipulations), the RNA is imported directly
into the
cytoplasm
and cleaved to short fragments by Dicer. The initiating dsRNA can also
be endogenous (originating in the cell), as in pre-microRNAs expressed
from
RNA-coding genes in the genome. The primary transcripts from such genes are first processed to form the characteristic
stem-loop structure of pre-miRNA in the
nucleus, then exported to the cytoplasm. Thus, the two dsRNA pathways, exogenous and endogenous, converge at the RISC.
[6]
Exogenous dsRNA initiates RNAi by activating the
ribonuclease protein Dicer,
[7]
which binds and cleaves double-stranded RNAs (dsRNAs) in plants, or
short hairpin RNAs (shRNAs) in humans, to produce double-stranded
fragments of 20–25
base pairs with a 2-nucleotide overhang at the 3' end.
[8]
Bioinformatics
studies on the genomes of multiple organisms suggest this length
maximizes target-gene specificity and minimizes non-specific effects.
[9] These short double-stranded fragments are called small interfering RNAs (
siRNAs). These
siRNAs
are then separated into single strands and integrated into an active
RISC, by RISC-Loading Complex (RLC). RLC includes Dicer-2 and R2D2, and
is crucial to unite Ago2 and RISC.
[10]
TATA-binding protein-associated factor 11 (TAF11) assembles the RLC by
facilitating Dcr-2-R2D2 tetramerization, which increases the binding
affinity to siRNA by 10-fold. Association with TAF11 would convert the
R2-D2-Initiator (RDI) complex into the RLC.
[11] R2D2 carries tandem double-stranded RNA-binding domains to recognize the thermodynamically stable terminus of
siRNA
duplexes, whereas Dicer-2 the other less stable extremity. Loading is
asymmetric: the MID domain of Ago2 recognizes the thermodynamically
stable end of the siRNA. Therefore, the "passenger" (sense) strand whose
5′ end is discarded by MID is ejected, while the saved "guide"
(antisense) strand cooperates with AGO to form the RISC.
[10]
After integration into the RISC,
siRNAs base-pair to their target mRNA and cleave it, thereby preventing it from being used as a
translation template.
[12] Differently from
siRNA,
a miRNA-loaded RISC complex scans cytoplasmic mRNAs for potential
complementarity. Instead of destructive cleavage (by Ago2), miRNAs
rather target the 3′ untranslated region (UTR) regions of mRNAs where
they typically bind with imperfect complementarity, thus blocking the
access of ribosomes for translation.
[13]
Exogenous dsRNA is detected and bound by an effector protein, known as RDE-4 in
C. elegans and R2D2 in
Drosophila, that stimulates dicer activity.
[14] The mechanism producing this length specificity is unknown and this protein only binds long dsRNAs.
[14]
In
C. elegans this initiation response is amplified through the synthesis of a population of 'secondary'
siRNAs during which the dicer-produced initiating or 'primary'
siRNAs are used as templates.
[15] These 'secondary'
siRNAs are structurally distinct from dicer-produced
siRNAs and appear to be produced by an
RNA-dependent RNA polymerase (RdRP).
[16][17]
MicroRNA
MicroRNAs (miRNAs) are
genomically encoded
non-coding RNAs that help regulate
gene expression, particularly during
development.
[18]
The phenomenon of RNA interference, broadly defined, includes the
endogenously induced gene silencing effects of miRNAs as well as
silencing triggered by foreign dsRNA. Mature miRNAs are structurally
similar to
siRNAs produced from exogenous dsRNA, but before reaching maturity, miRNAs must first undergo extensive
post-transcriptional modification. A miRNA is expressed from a much longer RNA-coding gene as a
primary transcript known as a
pri-miRNA which is processed, in the
cell nucleus, to a 70-nucleotide
stem-loop structure called a
pre-miRNA by the
microprocessor complex. This complex consists of an
RNase III enzyme called
Drosha and a dsRNA-binding protein
DGCR8.
The dsRNA portion of this pre-miRNA is bound and cleaved by Dicer to
produce the mature miRNA molecule that can be integrated into the RISC
complex; thus, miRNA and
siRNA share the same downstream cellular machinery.
[19] First, viral encoded miRNA was described in EBV.
[20]
Thereafter, an increasing number of microRNAs have been described in
viruses. VIRmiRNA is a comprehensive catalogue covering viral microRNA,
their targets and anti-viral miRNAs
[21] (see also VIRmiRNA resource:
http://crdd.osdd.net/servers/virmirna/).
siRNAs
derived from long dsRNA precursors differ from miRNAs in that miRNAs,
especially those in animals, typically have incomplete base pairing to a
target and inhibit the translation of many different mRNAs with similar
sequences. In contrast,
siRNAs typically base-pair perfectly and induce mRNA cleavage only in a single, specific target.
[22] In
Drosophila and
C. elegans, miRNA and
siRNA are processed by distinct argonaute proteins and dicer enzymes.
[23][24]
Three prime untranslated regions and microRNAs
Three prime untranslated regions (3'UTRs) of
messenger RNAs
(mRNAs) often contain regulatory sequences that post-transcriptionally
cause RNA interference. Such 3'-UTRs often contain both binding sites
for
microRNAs
(miRNAs) as well as for regulatory proteins. By binding to specific
sites within the 3'-UTR, miRNAs can decrease gene expression of various
mRNAs by either inhibiting translation or directly causing degradation
of the transcript. The 3'-UTR also may have silencer regions that bind
repressor proteins that inhibit the expression of a mRNA.
The 3'-UTR often contains
microRNA response elements (MREs).
MREs are sequences to which miRNAs bind. These are prevalent motifs
within 3'-UTRs. Among all regulatory motifs within the 3'-UTRs (e.g.
including silencer regions), MREs make up about half of the motifs.
As of 2014, the
miRBase web site,
[25] an archive of
miRNA sequences
and annotations, listed 28,645 entries in 233 biologic species. Of
these, 1,881 miRNAs were in annotated human miRNA loci. miRNAs were
predicted to have an average of about four hundred target
mRNAs (affecting expression of several hundred genes).
[26] Friedman et al.
[26]
estimate that &45,000 miRNA target sites within human mRNA 3'UTRs
are conserved above background levels, and & 60% of human
protein-coding genes have been under selective pressure to maintain
pairing to miRNAs.
Direct experiments show that a single miRNA can reduce the stability of hundreds of unique mRNAs.
[27]
Other experiments show that a single miRNA may repress the production
of hundreds of proteins, but that this repression often is relatively
mild (less than 2-fold).
[28][29]
The effects of miRNA dysregulation of gene expression seem to be important in cancer.
[30] For instance, in gastrointestinal cancers, nine miRNAs have been identified as
epigenetically altered and effective in down regulating DNA repair enzymes.
[31]
The effects of miRNA dysregulation of gene expression also seem
to be important in neuropsychiatric disorders, such as schizophrenia,
bipolar disorder, major depression, Parkinson's disease, Alzheimer's
disease and autism spectrum disorders.
[32][33][34]
RISC activation and catalysis
Exogenous dsRNA is detected and bound by an effector protein, known as RDE-4 in
C. elegans and R2D2 in
Drosophila, that stimulates dicer activity.
[14] This protein only binds long dsRNAs, but the mechanism producing this length specificity is unknown.
[14] This RNA-binding protein then facilitates the transfer of cleaved
siRNAs to the RISC complex.
[35]
In
C. elegans this initiation response is amplified through the synthesis of a population of 'secondary'
siRNAs during which the dicer-produced initiating or 'primary'
siRNAs are used as templates.
[15] These 'secondary'
siRNAs are structurally distinct from dicer-produced
siRNAs and appear to be produced by an
RNA-dependent RNA polymerase (RdRP).
[16][17]
small RNA
Biogenesis: primary miRNAs (pri-miRNAs) are transcribed in the nucleus
and fold back onto themselves as hairpins that are then trimmed in the
nucleus by a
microprocessor complex to form a ~60-70nt hairpin pre-RNA. This pre-miRNA is transported through the
nuclear pore complex (NPC) into the cytoplasm, where
Dicer
further trims it to a ~20nt miRNA duplex (pre-siRNAs also enter the
pathway at this step). This duplex is then loaded into Ago to form the
“pre-RISC(RNA induced silencing complex)” and the passenger strand is
released to form active
RISC.
The active components of an RNA-induced silencing complex (RISC) are
endonucleases called argonaute proteins, which cleave the target mRNA strand
complementary to their bound
siRNA.
[5] As the fragments produced by dicer are double-stranded, they could each in theory produce a functional
siRNA. However, only one of the two strands, which is known as the
guide strand, binds the argonaute protein and directs gene silencing. The other
anti-guide strand or
passenger strand is degraded during RISC activation.
[36] Although it was first believed that an
ATP-dependent
helicase separated these two strands,
[37] the process proved to be ATP-independent and performed directly by the protein components of RISC.
[38][39] However, an
in vitro
kinetic analysis of RNAi in the presence and absence of ATP showed that
ATP may be required to unwind and remove the cleaved mRNA strand from
the RISC complex after catalysis.
[40] The guide strand tends to be the one whose
5' end is less stably paired to its complement,
[41] but strand selection is unaffected by the direction in which dicer cleaves the dsRNA before RISC incorporation.
[42] Instead, the R2D2 protein may serve as the differentiating factor by binding the more-stable 5' end of the passenger strand.
[43]
The structural basis for binding of RNA to the argonaute protein was examined by
X-ray crystallography of the binding
domain of an RNA-bound argonaute protein. Here, the
phosphorylated 5' end of the RNA strand enters a
conserved basic surface
pocket and makes contacts through a
divalent cation (an atom with two positive charges) such as
magnesium and by
aromatic stacking (a process that allows more than one atom to share an electron by passing it back and forth) between the 5' nucleotide in the
siRNA and a conserved
tyrosine residue. This site is thought to form a nucleation site for the binding of the
siRNA to its mRNA target.
[44]
Analysis of the inhibitory effect of mismatches in either the 5’ or 3’
end of the guide strand has demonstrated that the 5’ end of the guide
strand is likely responsible for matching and binding the target mRNA,
while the 3’ end is responsible for physically arranging target mRNA
into a cleavage-favorable RISC region.
[40]
It is not understood how the activated RISC complex locates
complementary mRNAs within the cell. Although the cleavage process has
been proposed to be linked to
translation, translation of the mRNA target is not essential for RNAi-mediated degradation.
[45] Indeed, RNAi may be more effective against mRNA targets that are not translated.
[46] Argonaute proteins are localized to specific regions in the cytoplasm called
P-bodies (also cytoplasmic bodies or GW bodies), which are regions with high rates of mRNA decay;
[47] miRNA activity is also clustered in P-bodies.
[48]
Disruption of P-bodies decreases the efficiency of RNA interference,
suggesting that they are a critical site in the RNAi process.
[49]
Transcriptional silencing
Components of the RNAi pathway are used in many eukaryotes in the maintenance of the organization and structure of their
genomes. Modification of
histones and associated induction of
heterochromatin formation serves to downregulate genes pre-
transcriptionally;
[51] this process is referred to as
RNA-induced transcriptional silencing (RITS), and is carried out by a complex of proteins called the RITS complex. In
fission yeast this complex contains argonaute, a
chromodomain protein Chp1, and a protein called Tas3 of unknown function.
[52] As a consequence, the induction and spread of heterochromatic regions requires the argonaute and RdRP proteins.
[53] Indeed, deletion of these genes in the fission yeast
S. pombe disrupts
histone methylation and
centromere formation,
[54] causing slow or stalled
anaphase during
cell division.
[55] In some cases, similar processes associated with histone modification have been observed to transcriptionally upregulate genes.
[56]
The mechanism by which the RITS complex induces heterochromatin
formation and organization is not well understood. Most studies have
focused on the
mating-type region
in fission yeast, which may not be representative of activities in
other genomic regions/organisms. In maintenance of existing
heterochromatin regions, RITS forms a complex with
siRNAs
complementary
to the local genes and stably binds local methylated histones, acting
co-transcriptionally to degrade any nascent pre-mRNA transcripts that
are initiated by
RNA polymerase.
The formation of such a heterochromatin region, though not its
maintenance, is dicer-dependent, presumably because dicer is required to
generate the initial complement of
siRNAs that target subsequent transcripts.
[57]
Heterochromatin maintenance has been suggested to function as a
self-reinforcing feedback loop, as new siRNAs are formed from the
occasional nascent transcripts by RdRP for incorporation into local RITS
complexes.
[58] The relevance of observations from fission yeast mating-type regions and centromeres to
mammals is not clear, as heterochromatin maintenance in mammalian cells may be independent of the components of the RNAi pathway.
[59]
Crosstalk with RNA editing
The type of
RNA editing that is most prevalent in higher eukaryotes converts
adenosine nucleotides into
inosine in dsRNAs via the enzyme
adenosine deaminase (ADAR).
[60] It was originally proposed in 2000 that the RNAi and A→I RNA editing pathways might compete for a common dsRNA substrate.
[61] Some pre-miRNAs do undergo A→I RNA editing
[62][63] and this mechanism may regulate the processing and expression of mature miRNAs.
[63] Furthermore, at least one mammalian ADAR can sequester
siRNAs from RNAi pathway components.
[64] Further support for this model comes from studies on ADAR-null
C. elegans strains indicating that A→I RNA editing may counteract RNAi silencing of endogenous genes and transgenes.
[65]
Illustration of the major differences between plant and animal gene silencing. Natively expressed
microRNA or exogenous
small interfering RNA is processed by
dicer and integrated into the
RISC complex, which mediates gene silencing.
[66]
Variation among organisms
Organisms
vary in their ability to take up foreign dsRNA and use it in the RNAi
pathway. The effects of RNA interference can be both systemic and
heritable in plants and
C. elegans, although not in
Drosophila or mammals. In plants, RNAi is thought to propagate by the transfer of
siRNAs between cells through
plasmodesmata (channels in the cell walls that enable communication and transport).
[37] Heritability comes from
methylation of promoters targeted by RNAi; the new methylation pattern is copied in each new generation of the cell.
[67]
A broad general distinction between plants and animals lies in the
targeting of endogenously produced miRNAs; in plants, miRNAs are usually
perfectly or nearly perfectly complementary to their target genes and
induce direct mRNA cleavage by RISC, while animals' miRNAs tend to be
more divergent in sequence and induce translational repression.
[66] This translational effect may be produced by inhibiting the interactions of translation
initiation factors with the messenger RNA's
polyadenine tail.
[68]
Some eukaryotic
protozoa such as
Leishmania major and
Trypanosoma cruzi lack the RNAi pathway entirely.
[69][70] Most or all of the components are also missing in some
fungi, most notably the
model organism Saccharomyces cerevisiae.
[71] The presence of RNAi in other budding yeast species such as
Saccharomyces castellii and
Candida albicans, further demonstrates that inducing two RNAi-related proteins from
S. castellii facilitates RNAi in
S. cerevisiae.
[72] That certain
ascomycetes and
basidiomycetes
are missing RNA interference pathways indicates that proteins required
for RNA silencing have been lost independently from many fungal
lineages, possibly due to the evolution of a novel pathway with similar function, or to the lack of selective advantage in certain
niches.
[73]
Related prokaryotic systems
Gene
expression in prokaryotes is influenced by an RNA-based system similar
in some respects to RNAi. Here, RNA-encoding genes control mRNA
abundance or translation by producing a complementary RNA that anneals
to an mRNA. However these regulatory RNAs are not generally considered
to be analogous to miRNAs because the dicer enzyme is not involved.
[74] It has been suggested that
CRISPR interference systems in prokaryotes are analogous to eukaryotic RNA interference systems, although none of the protein components are
orthologous.
[75]
Biological functions
Immunity
RNA interference is a vital part of the
immune response to viruses and other foreign
genetic material, especially in plants where it may also prevent the self-propagation of transposons.
[76] Plants such as
Arabidopsis thaliana express multiple dicer
homologs that are specialized to react differently when the plant is exposed to different viruses.
[77]
Even before the RNAi pathway was fully understood, it was known that
induced gene silencing in plants could spread throughout the plant in a
systemic effect and could be transferred from stock to
scion plants via
grafting.
[78]
This phenomenon has since been recognized as a feature of the plant
adaptive immune system and allows the entire plant to respond to a virus
after an initial localized encounter.
[79] In response, many plant viruses have evolved elaborate mechanisms to suppress the RNAi response.
[80]
These include viral proteins that bind short double-stranded RNA
fragments with single-stranded overhang ends, such as those produced by
dicer.
[81] Some plant genomes also express endogenous
siRNAs in response to infection by specific types of
bacteria.
[82]
These effects may be part of a generalized response to pathogens that
downregulates any metabolic process in the host that aids the infection
process.
[83]
Although animals generally express fewer variants of the dicer
enzyme than plants, RNAi in some animals produces an antiviral response.
In both juvenile and adult
Drosophila, RNA interference is important in antiviral
innate immunity and is active against pathogens such as
Drosophila X virus.
[84][85] A similar role in immunity may operate in
C. elegans,
as argonaute proteins are upregulated in response to viruses and worms
that overexpress components of the RNAi pathway are resistant to viral
infection.
[86][87]
The role of RNA interference in mammalian innate immunity is
poorly understood, and relatively little data is available. However, the
existence of viruses that encode genes able to suppress the RNAi
response in mammalian cells may be evidence in favour of an
RNAi-dependent mammalian immune response,
[88][89] although this hypothesis has been challenged as poorly substantiated.
[90]
Maillard et al.
[91] and Li et al.
[92]
provide evidence for the existence of a functional antiviral RNAi
pathway in mammalian cells. Other functions for RNAi in mammalian
viruses also exist, such as miRNAs expressed by the
herpes virus that may act as
heterochromatin organization triggers to mediate viral latency.
[93]
Downregulation of genes
Endogenously expressed miRNAs, including both
intronic and
intergenic miRNAs, are most important in translational repression
[66] and in the regulation of development, especially on the timing of
morphogenesis and the maintenance of
undifferentiated or incompletely differentiated cell types such as
stem cells.
[94] The role of endogenously expressed miRNA in downregulating
gene expression was first described in
C. elegans in 1993.
[95] In plants this function was discovered when the "JAW microRNA" of
Arabidopsis was shown to be involved in the regulation of several genes that control plant shape.
[96] In plants, the majority of genes regulated by miRNAs are
transcription factors;
[97] thus miRNA activity is particularly wide-ranging and regulates entire
gene networks during development by modulating the expression of key regulatory genes, including transcription factors as well as
F-box proteins.
[98] In many organisms, including humans, miRNAs are linked to the formation of
tumors and dysregulation of the
cell cycle. Here, miRNAs can function as both
oncogenes and
tumor suppressors.
[99]
Upregulation of genes
RNA sequences (
siRNA and miRNA) that are complementary to parts of a promoter can increase gene transcription, a phenomenon dubbed
RNA activation.
Part of the mechanism for how these RNA upregulate genes is known:
dicer and argonaute are involved, possibly via histone demethylation.
[100] miRNAs have been proposed to upregulate their target genes upon cell cycle arrest, via unknown mechanisms.
[101]
Evolution
Based on
parsimony-based phylogenetic analysis, the
most recent common ancestor of all
eukaryotes
most likely already possessed an early RNA interference pathway; the
absence of the pathway in certain eukaryotes is thought to be a derived
characteristic.
[102] This ancestral RNAi system probably contained at least one dicer-like protein, one argonaute, one
PIWI protein, and an
RNA-dependent RNA polymerase that may also have played other cellular roles. A large-scale
comparative genomics study likewise indicates that the eukaryotic
crown group
already possessed these components, which may then have had closer
functional associations with generalized RNA degradation systems such as
the
exosome.
[103]
This study also suggests that the RNA-binding argonaute protein family,
which is shared among eukaryotes, most archaea, and at least some
bacteria (such as
Aquifex aeolicus), is homologous to and originally evolved from components of the
translation initiation system.
The ancestral function of the RNAi system is generally agreed to
have been immune defense against exogenous genetic elements such as
transposons and viral genomes.
[102][104]
Related functions such as histone modification may have already been
present in the ancestor of modern eukaryotes, although other functions
such as regulation of development by miRNA are thought to have evolved
later.
[102]
RNA interference genes, as components of the antiviral innate immune system in many eukaryotes, are involved in an
evolutionary arms race
with viral genes. Some viruses have evolved mechanisms for suppressing
the RNAi response in their host cells, particularly for plant viruses.
[80] Studies of evolutionary rates in
Drosophila have shown that genes in the RNAi pathway are subject to strong
directional selection and are among the fastest-
evolving genes in the
Drosophila genome.
[105]
Applications
Gene knockdown
The RNA interference pathway is often exploited in
experimental biology to study the function of genes in
cell culture and
in vivo in
model organisms.
[5]
Double-stranded RNA is synthesized with a sequence complementary to a
gene of interest and introduced into a cell or organism, where it is
recognized as exogenous genetic material and activates the RNAi pathway.
Using this mechanism, researchers can cause a drastic decrease in the
expression of a targeted gene. Studying the effects of this decrease can
show the physiological role of the gene product. Since RNAi may not
totally abolish expression of the gene, this technique is sometimes
referred as a "
knockdown", to distinguish it from "
knockout" procedures in which expression of a gene is entirely eliminated.
[106]
In a recent study validation of RNAi silencing efficiency using gene
array data showed 18.5% failure rate across 429 independent experiments.
[107]
Extensive efforts in
computational biology
have been directed toward the design of successful dsRNA reagents that
maximize gene knockdown but minimize "off-target" effects. Off-target
effects arise when an introduced RNA has a base sequence that can pair
with and thus reduce the expression of multiple genes. Such problems
occur more frequently when the dsRNA contains repetitive sequences. It
has been estimated from studying the genomes of humans,
C. elegans and
S. pombe that about 10% of possible
siRNAs have substantial off-target effects.
[9] A multitude of software tools have been developed implementing
algorithms for the design of general
[108][109] mammal-specific,
[110] and virus-specific
[111] siRNAs that are automatically checked for possible cross-reactivity.
Depending on the organism and experimental system, the exogenous
RNA may be a long strand designed to be cleaved by dicer, or short RNAs
designed to serve as
siRNA substrates. In most mammalian cells, shorter RNAs are used because long double-stranded RNA molecules induce the mammalian
interferon response, a form of
innate immunity that reacts nonspecifically to foreign genetic material.
[112] Mouse
oocytes and cells from early mouse
embryos lack this reaction to exogenous dsRNA and are therefore a common model system for studying mammalian gene-knockdown effects.
[113]
Specialized laboratory techniques have also been developed to improve
the utility of RNAi in mammalian systems by avoiding the direct
introduction of
siRNA, for example, by stable
transfection with a
plasmid encoding the appropriate sequence from which
siRNAs can be transcribed,
[114] or by more elaborate
lentiviral vector systems allowing the inducible activation or deactivation of transcription, known as
conditional RNAi.
[115][116]
Functional genomics
A normal adult
Drosophila fly, a common model organism used in RNAi experiments.
Most
functional genomics applications of RNAi in animals have used
C. elegans[117] and
Drosophila,
[118] as these are the common
model organisms in which RNAi is most effective.
C. elegans
is particularly useful for RNAi research for two reasons: firstly, the
effects of gene silencing are generally heritable, and secondly because
delivery of the dsRNA is extremely simple. Through a mechanism whose
details are poorly understood, bacteria such as
E. coli
that carry the desired dsRNA can be fed to the worms and will transfer
their RNA payload to the worm via the intestinal tract. This "delivery
by feeding" is just as effective at inducing gene silencing as more
costly and time-consuming delivery methods, such as soaking the worms in
dsRNA solution and injecting dsRNA into the gonads.
[119]
Although delivery is more difficult in most other organisms, efforts
are also underway to undertake large-scale genomic screening
applications in cell culture with mammalian cells.
[120]
Approaches to the design of genome-wide RNAi libraries can require more sophistication than the design of a single
siRNA for a defined set of experimental conditions.
Artificial neural networks are frequently used to design
siRNA libraries
[121] and to predict their likely efficiency at gene knockdown.
[122] Mass genomic screening is widely seen as a promising method for
genome annotation and has triggered the development of high-throughput screening methods based on
microarrays.
[123][124]
However, the utility of these screens and the ability of techniques
developed on model organisms to generalize to even closely related
species has been questioned, for example from
C. elegans to related parasitic nematodes.
[125][126]
Functional genomics using RNAi is a particularly attractive
technique for genomic mapping and annotation in plants because many
plants are
polyploid,
which presents substantial challenges for more traditional genetic
engineering methods. For example, RNAi has been successfully used for
functional genomics studies in
bread wheat (which is hexaploid)
[127] as well as more common plant model systems
Arabidopsis and
maize.
[128]
Medicine
History of RNAi use in medicine
Timeline of the use of RNAi in medicine between 1996 and 2017
The first instance of
RNA silencing in animals was documented in 1996, when Guo and Kemphues observed that, by introducing
sense and
antisense RNA to par-1 mRNA in
Caenorhabditis elegans caused degradation of the par-1 message.
[129]
It was thought that this degradation was triggered by single stranded
RNA (ssRNA), but two years later, in 1998, Fire and Mello discovered
that this ability to silence the par-1 gene expression was actually
triggered by double-stranded RNA (dsRNA).
[129] They would eventually share the
Nobel Prize in Physiology or Medicine for this discovery.
[130] Just after Fire and Mello's ground-breaking discovery, Elbashir et al. discovered, by using synthetically made
small interfering RNA (siRNA), it was possible to target the silencing of specific sequences in a gene, rather than silencing the entire gene.
[131]
Only a year later, McCaffrey and colleagues demonstrated that this
sequence specific silencing had therapeutic applications by targeting a
sequence from the
Hepatitis C virus in
transgenic mice.
[132]
Since then, multiple researchers have been attempting to expand the
therapeutic applications of RNAi, specifically looking to target genes
that cause various types of
cancer.
[133][134] Finally, in 2004, this new gene silencing technology entered a
Phase I clinical trial in humans for wet age-related
macular degeneration.
[131] Six years later the first-in-human Phase I clinical trial was started, using a
nanoparticle delivery system to target
solid tumors.
[135]
Although most research is currently looking into the applications of
RNAi in cancer treatment, the list of possible applications is
extensive. RNAi could potentially be used to treat
viruses,
[136] bacterial diseases,
[137] parasites,
[138] maladaptive genetic mutations,
[139] control drug consumption,
[140] provide pain relief,
[141] and even
modulate sleep.
[142]
Therapeutic applications
Viral infection
Antiviral
treatment is one of the earliest proposed RNAi-based medical
applications, and two different types have been developed. The first
type is to target viral RNAs. Many studies have shown that targeting
viral RNAs can suppress the replication of numerous viruses, including
HIV,
[143] HPV,
[144] hepatitis A,
[145] hepatitis B,
[146] Influenza virus,
[147] and
Measles virus.
[148]
The other strategy is to block the initial viral entries by targeting
the host cell genes. For example, suppression of chemokine receptors (
CXCR4 and
CCR5)on host cells can prevent HIV viral entry.
[149]
Cancer
While traditional
chemotherapy
can effectively kill cancer cells, lack of specificity for
discriminating normal cells and cancer cells in these treatments usually
cause severe side effects. Numerous studies have demonstrated that RNAi
can provide a more specific approach to inhibit tumor growth by
targeting cancer-related genes (i.e.,
oncogene).
[150] It has also been proposed that RNAi can enhance the sensitivity of cancer cells to
chemotherapeutic agents, providing a combinatorial therapeutic approach with chemotherapy.
[151] Another potential RNAi-based treatment is to inhibit cell invasion and
migration.
[152]
Neurological diseases
RNAi strategies also show potential for treating
neurodegenerative diseases. Studies in cells and in mouse have shown that specifically targeting
Amyloid beta-producing
genes (e.g. BACE1 and APP) by RNAi can significantly reduced the amount
of Aβ peptide which is correlated with the cause of
Alzheimer's disease.
[153][154][155] In addition, this silencing-based approaches also provide promising results in treatment of
Parkinson's disease and
Polyglutamine disease.
[156][157][158]
Difficulties in Therapeutic Application
To
achieve the clinical potential of RNAi, siRNA must be efficiently
transportated to the cells of target tissues. However, there are various
barriers that must be fixed before it can be used clinically.
For example, "Naked" siRNA is susceptible to several obstacles that reduce its therapeutic efficacy.
[159]
Additionally, once siRNA has entered the bloodstream, naked RNA can be
degraded by serum nucleases and can stimulate the innate immune system.
[160]
Due to its size and highly polyanionic (containing negative charges at
several sites) nature, unmodified siRNA molecules cannot readily enter
the cells through the cell membrane. Therefore, artificial or
nanoparticle encapsulated siRNA
must be used. However, transporting siRNA across the cell membrane
still has its own unique challenges. If siRNA is transferred across the
cell membrane, unintended toxicities can occur if therapeutic doses are
not optimized, and siRNAs can exhibit off-target effects (e.g.
unintended downregulation of genes with
partial sequence complementarity).
[161] Even after entering the cells, repeated dosing is required since their effects are diluted at each cell division.
Safety and Uses in Cancer treatment
Compared with chemotherapy or other anti-cancer drugs, there are a lot of advantages of siRNA drug.
[162] SiRNA acts on the post-translational stage of gene expression, so it doesn’t modify or change DNA in a deleterious effect.
[162]
SiRNA can also be used to produced a specific response in a certain
type of way, such as by downgrading suppression of gene expression.
[162] In a single cancer cell, siRNA can cause dramatic suppression of gene expression with just several copies.
[162] This happens by silencing cancer-promoting genes with RNAi, as well as targeting an mRNA sequence.
[162]
RNAi drugs treat cancer by silencing certain cancer promoting genes.
[162]
This is done by complementing the cancer genes with the RNAi, such as
keeping the mRNA sequences in accordance with the RNAi drug.
[162] Ideally, RNAi is should be injected and/or chemically modified so the RNAi can reach cancer cells more efficiently.
[162] RNAi uptake and regulation is monitored by the kidneys.
[162]
Stimulation of immune response
The human immune system is divided into two separate branches: the innate immune system and the adaptive immune system.
[163] The innate immune system is the first defense against infection and responds to pathogens in a generic fashion.
[163]
On the other hand, the adaptive immune system, a system that was
evolved later than the innate, is composed mainly of highly specialized B
and T cells that are trained to react to specific portions of
pathogenic molecules.
[163]
The challenge between old pathogens and new has helped create a
system of guarded cells and particles that are called safe framework.
[163]
This framework has given humans an army systems that search out and
destroy invader particles, such as pathogens, microscopic organisms,
parasites, and infections.
[163]
The mammalian safe framework has developed to incorporate siRNA as a
tool to indicate viral contamination, which has allowed siRNA is create
an intense innate immune response.
[163]
siRNA is controlled by the innate immune system, which can be
divided into the acute inflammatory responses and antiviral responses.
[163] The inflammatory response is created with signals from small signaling molecules, or cytokines.
[163] These include interleukin-1 (IL-1), interleukin-6 (IL-6), interleukin-12 (IL-12) and tumor necrosis factor α (TNF-α).
[163]
The innate immune system generates inflammation and antiviral
responses, which cause the release pattern recognition receptors
(PRRs).
[163] These receptors help in labeling which pathogens are viruses, fungi, or bacteria.
[163] Moreover, the importance of siRNA and the innate immune system is to
include more PRRs to help recognize different RNA structures.
[163] This makes it more likely for the siRNA to cause an immunostimulant response in the event of the pathogen.
[163]
Prospects as a Therapeutic Technique
Clinical Phase I and II studies of siRNA therapies conducted between 2015 and 2017 have demonstrated potent and durable
gene knockdown in the
liver, with some signs of clinical improvement and without unacceptable toxicity.
[164] Two Phase III studies are in progress to treat familial neurodegenerative and cardiac syndromes caused by mutations in
transthyretin (TTR).
[165] Numerous publications have shown that in vivo delivery systems are very
promising and are diverse in characteristics, allowing numerous
applications. The nanoparticle delivery system shows the most promise
yet this method presents additional challenges in the
scale-up
of the manufacturing process, such as the need for tightly controlled
mixing processes to achieve consistent quality of the drug product.
[166] The table below shows different drugs using RNA interference and what their phases and status is in clinical trials.
[159]
| Drug
|
Target
|
Delivery System
|
Disease
|
Phase
|
Status
|
Company
|
Identifier
|
| ALN–VSP02
|
KSP and VEGF
|
LNP
|
Solid tumours
|
I
|
Completed
|
Alnylam Pharmaceuticals
|
NCT01158079
|
| siRNA–EphA2–DOPC
|
EphA2
|
LNP
|
Advanced cancers
|
I
|
Recruiting
|
MD Anderson Cancer Center
|
NCT01591356
|
| Atu027
|
PKN3
|
LNP
|
Solid tumours
|
I
|
Completed
|
Silence Therapeutics
|
NCT00938574
|
| TKM–080301
|
PLK1
|
LNP
|
Cancer
|
I
|
Recruiting
|
Tekmira Pharmaceutical
|
NCT01262235
|
| TKM–100201
|
VP24, VP35, Zaire Ebola L-polymerase
|
LNP
|
Ebola-virus infection
|
I
|
Recruiting
|
Tekmira Pharmaceutical
|
NCT01518881
|
| ALN–RSV01
|
RSV nucleocapsid
|
Naked siRNA
|
Respiratory syncytial virus infections
|
II
|
Completed
|
Alnylam Pharmaceuticals
|
NCT00658086
|
| PRO-040201
|
ApoB
|
LNP
|
Hypercholesterolaemia
|
I
|
Terminated
|
Tekmira Pharmaceutical
|
NCT00927459
|
| ALN–PCS02
|
PCSK9
|
LNP
|
Hypercholesterolaemia
|
I
|
Completed
|
Alnylam Pharmaceuticals
|
NCT01437059
|
| ALN–TTR02
|
TTR
|
LNP
|
Transthyretin-mediated amyloidosis
|
II
|
Recruiting
|
Alnylam Pharmaceuticals
|
NCT01617967
|
| CALAA-01
|
RRM2
|
Cyclodextrin NP
|
Solid tumours
|
I
|
Active
|
Calando Pharmaceuticals
|
NCT00689065
|
| TD101
|
K6a (N171K mutation)
|
Naked siRNA
|
Pachyonychia congenita
|
I
|
Completed
|
Pachyonychia Congenita Project
|
NCT00716014
|
| AGN211745
|
VEGFR1
|
Naked siRNA
|
Age-related macular degeneration, choroidal neovascularization
|
II
|
Terminated
|
Allergan
|
NCT00395057
|
| QPI-1007
|
CASP2
|
Naked siRNA
|
Optic atrophy, non-arteritic anterior ischaemic optic neuropathy
|
I
|
Completed
|
Quark Pharmaceuticals
|
NCT01064505
|
| I5NP
|
p53
|
Naked siRNA
|
Kidney injury, acute renal failure
|
I
|
Completed
|
Quark Pharmaceuticals
|
NCT00554359
|
|
|
|
Delayed graft function, complications of kidney transplant
|
I, II
|
Recruiting
|
Quark Pharmaceuticals
|
NCT00802347
|
| PF-655 (PF-04523655)
|
RTP801 (Proprietary target)
|
Naked siRNA
|
Choroidal neovascularization, diabetic retinopathy, diabetic macular oedema
|
II
|
Active
|
Quark Pharmaceuticals
|
NCT01445899
|
| siG12D LODER
|
KRAS
|
LODER polymer
|
Pancreatic cancer
|
II
|
Recruiting
|
Silenseed
|
NCT01676259
|
| Bevasiranib
|
VEGF
|
Naked siRNA
|
Diabetic macular oedema, macular degeneration
|
II
|
Completed
|
Opko Health
|
NCT00306904
|
| SYL1001
|
TRPV1
|
Naked siRNA
|
Ocular pain, dry-eye syndrome
|
I, II
|
Recruiting
|
Sylentis
|
NCT01776658
|
| SYL040012
|
ADRB2
|
Naked siRNA
|
Ocular hypertension, open-angle glaucoma
|
II
|
Recruiting
|
Sylentis
|
NCT01739244
|
| CEQ508
|
CTNNB1
|
Escherichia coli-carrying shRNA
|
Familial adenomatous polyposis
|
I, II
|
Recruiting
|
Marina Biotech
|
Unknown
|
| RXi-109
|
CTGF
|
Self-delivering RNAi compound
|
Cicatrix scar prevention
|
I
|
Recruiting
|
RXi Pharmaceuticals
|
NCT01780077
|
| ALN–TTRsc
|
TTR
|
siRNA–GalNAc conjugate
|
Transthyretin-mediated amyloidosis
|
I
|
Recruiting
|
Alnylam Pharmaceuticals
|
NCT01814839
|
| ARC-520
|
Conserved regions of HBV
|
DPC
|
HBV
|
I
|
Recruiting
|
Arrowhead Research
|
NCT01872065
|
Biotechnology
RNA interference has been used for applications in
biotechnology
and is nearing commercialization in others. RNAi has developed many
novel crops such as nicotine free tobacco, decaffeinated coffee, nutrient
fortified and hypoallergenic crops. The genetically engineered Arctic
apples received FDA approval in 2015.
[167]
The apples were produced by RNAi suppression of PPO (polyphenol
oxidase) gene making apple varieties that will not undergo browning
after being sliced. PPO-silenced apples are unable to convert
chlorogenic acid into quinone product.
[1]
There are several opportunities for the applications of RNAi in
crop science for its improvement such as stress tolerance and enhanced
nutritional level. RNAi will prove its potential for inhibition of
photorespiration to enhance the productivity of C3 plants. This
knockdown technology may be useful in inducing early flowering, delayed
ripening, delayed senescence, breaking dormancy, stress-free plants,
overcoming self-sterility, etc.
[1]
Foods
RNAi has
been used to genetically engineer plants to produce lower levels of
natural plant toxins. Such techniques take advantage of the stable and
heritable RNAi phenotype in plant stocks.
Cotton seeds are rich in
dietary protein but naturally contain the toxic
terpenoid product
gossypol,
making them unsuitable for human consumption. RNAi has been used to
produce cotton stocks whose seeds contain reduced levels of
delta-cadinene synthase,
a key enzyme in gossypol production, without affecting the enzyme's
production in other parts of the plant, where gossypol is itself
important in preventing damage from plant pests.
[168] Similar efforts have been directed toward the reduction of the
cyanogenic natural product
linamarin in
cassava plants.
[169]
No plant products that use RNAi-based
genetic engineering have yet exited the experimental stage. Development efforts have successfully reduced the levels of
allergens in
tomato plants
[170] and fortification of plants such as tomatoes with dietary
antioxidants.
[171] Previous commercial products, including the
Flavr Savr tomato and two
cultivars of
ringspot-resistant
papaya, were originally developed using
antisense technology but likely exploited the RNAi pathway.
[172][173]
Other crops
Another effort decreased the precursors of likely
carcinogens in
tobacco plants.
[174] Other plant traits that have been engineered in the laboratory include the production of non-
narcotic natural products by the
opium poppy[175] and resistance to common plant viruses.
[176]
Insecticide
RNAi is under development as an
insecticide, employing multiple approaches, including genetic engineering and topical application.
[3] Cells in the midgut of some insects take up the dsRNA molecules in the process referred to as environmental RNAi.
[177] In some insects the effect is systemic as the signal spreads throughout the insect's body (referred to as systemic RNAi).
[178]
RNAi technology is shown to be safe for consumption by mammals, including humans.
[179]
RNAi has varying effects in different species of
Lepidoptera (butterflies and moths).
[180] Possibly because their
saliva and gut juice is better at breaking down RNA, the
cotton bollworm, the
beet armyworm and the
Asiatic rice borer have so far not been proven susceptible to RNAi by feeding.
[3]
To develop resistance to RNAi, the western corn rootworm would
have to change the genetic sequence of its Snf7 gene at multiple sites.
Combining multiple strategies, such as engineering the protein Cry,
derived from a bacterium called
Bacillus thuringiensis (Bt), and RNAi in one plant delay the onset of resistance.
[3][181]
Transgenic plants
Transgenic crops
have been made to express dsRNA, carefully chosen to silence crucial
genes in target pests. These dsRNAs are designed to affect only insects
that express specific gene sequences. As a
proof of principle, in 2009 a study showed RNAs that could kill any one of four fruit fly species while not harming the other three.
[3]
In 2012
Syngenta bought Belgian RNAi firm Devgen for $522 million and
Monsanto paid $29.2 million for the exclusive rights to
intellectual property from
Alnylam Pharmaceuticals. The
International Potato Center in
Lima, Peru is looking for genes to target in the sweet potato weevil, a beetle whose larvae ravage
sweet potatoes
globally. Other researchers are trying to silence genes in ants,
caterpillars and pollen beetles. Monsanto will likely be first to
market, with a transgenic corn seed that expresses dsRNA based on gene
Snf7 from the
western corn rootworm, a
beetle whose
larvae
annually cause one billion dollars in damage in the United States
alone. A 2012 paper showed that silencing Snf7 stunts larval growth,
killing them within days. In 2013 the same team showed that the RNA
affects very few other species.
[3]
Topical
Alternatively dsRNA can be supplied without genetic engineering. One approach is to add them to
irrigation water. The molecules are absorbed into the plants'
vascular
system and poison insects feeding on them. Another approach involves
spraying dsRNA like a conventional pesticide. This would allow faster
adaptation to resistance. Such approaches would require low cost sources
of dsRNAs that do not currently exist.
[3]
Genome-scale screening
Genome-scale RNAi research relies on
high-throughput screening
(HTS) technology. RNAi HTS technology allows genome-wide
loss-of-function screening and is broadly used in the identification of
genes associated with specific phenotypes. This technology has been
hailed as the second genomics wave, following the first genomics wave of
gene expression microarray and
single nucleotide polymorphism discovery platforms.
[182]
One major advantage of genome-scale RNAi screening is its ability to
simultaneously interrogate thousands of genes. With the ability to
generate a large amount of data per experiment, genome-scale RNAi
screening has led to an explosion of data generation rates. Exploiting
such large data sets is a fundamental challenge, requiring suitable
statistics/bioinformatics methods. The basic process of cell-based RNAi
screening includes the choice of an RNAi library, robust and stable cell
types, transfection with RNAi agents, treatment/incubation, signal
detection, analysis and identification of important genes or
therapeutical targets.
[183]
History
Example
petunia plants in which genes for pigmentation are silenced by RNAi. The left plant is
wild-type; the right plants contain
transgenes
that induce suppression of both transgene and endogenous gene
expression, giving rise to the unpigmented white areas of the flower.
[184]
The process of RNAi was referred to as "co-suppression" and
"quelling" when observed prior to the knowledge of an RNA-related
mechanism. The discovery of RNAi was preceded first by observations of
transcriptional inhibition by
antisense RNA expressed in
transgenic plants,
[185] and more directly by reports of unexpected outcomes in experiments performed by plant scientists in the
United States and the
Netherlands in the early 1990s.
[186] In an attempt to alter
flower colors in
petunias, researchers introduced additional copies of a gene encoding
chalcone synthase, a key enzyme for flower
pigmentation
into petunia plants of normally pink or violet flower color. The
overexpressed gene was expected to result in darker flowers, but instead
caused some flowers to have less visible purple pigment, sometimes in
variegated patterns, indicating that the activity of chalcone synthase
had been substantially decreased or became suppressed in a
context-specific manner. This would later be explained as the result of
the transgene being inserted adjacent to promoters in the opposite
direction in various positions throughout the genomes of some
transformants, thus leading to expression of antisense transcripts and
gene silencing when these promoters are active. Another early
observation of RNAi was came from a study of the
fungus Neurospora crassa,
[187]
although it was not immediately recognized as related. Further
investigation of the phenomenon in plants indicated that the
downregulation was due to post-transcriptional inhibition of gene
expression via an increased rate of mRNA degradation.
[188] This phenomenon was called
co-suppression of gene expression, but the molecular mechanism remained unknown.
[189]
Not long after, plant
virologists
working on improving plant resistance to viral diseases observed a
similar unexpected phenomenon. While it was known that plants expressing
virus-specific proteins showed enhanced tolerance or resistance to
viral infection, it was not expected that plants carrying only short,
non-coding regions of viral RNA sequences would show similar levels of
protection. Researchers believed that viral RNA produced by transgenes
could also inhibit viral replication.
[190] The reverse experiment, in which short sequences of plant genes were
introduced into viruses, showed that the targeted gene was suppressed in
an infected plant.
[191] This phenomenon was labeled "virus-induced gene silencing" (VIGS), and the set of such phenomena were collectively called
post transcriptional gene silencing.[192]
After these initial observations in plants, laboratories searched for this phenomenon in other organisms.
[193][194] Craig C. Mello and
Andrew Fire's 1998
Nature paper reported a potent gene silencing effect after injecting double stranded RNA into
C. elegans.
[195] In investigating the regulation of muscle protein production, they observed that neither mRNA nor
antisense RNA
injections had an effect on protein production, but double-stranded RNA
successfully silenced the targeted gene. As a result of this work, they
coined the term
RNAi. This discovery represented the first
identification of the causative agent for the phenomenon. Fire and Mello
were awarded the 2006
Nobel Prize in Physiology or Medicine.