From Wikipedia, the free encyclopedia
Activation and response in the sensory nervous system
The sensory nervous system is a part of the nervous system responsible for processing sensory information. A sensory system consists of sensory neurons (including the sensory receptor cells), neural pathways, and parts of the brain involved in sensory perception and interoception. Commonly recognized sensory systems are those for vision, hearing, touch, taste, smell, balance and visceral sensation. Sense organs are transducers
that convert data from the outer physical world to the realm of the
mind where people interpret the information, creating their perception of the world around them.
The receptive field
is the area of the body or environment to which a receptor organ and
receptor cells respond. For instance, the part of the world an eye can
see, is its receptive field; the light that each rod or cone can see, is its receptive field. Receptive fields have been identified for the visual system, auditory system and somatosensory system.
Stimulus
- Organisms need information to solve at least three kinds of problems: (a) to maintain an appropriate environment, i.e., homeostasis; (b) to time activities (e.g., seasonal changes in behavior) or synchronize activities with those of conspecifics;
and (c) to locate and respond to resources or threats (e.g., by moving
towards resources or evading or attacking threats). Organisms also need
to transmit information in order to influence another's behavior: to
identify themselves, warn conspecifics of danger, coordinate activities,
or deceive.
Sensory systems code for four aspects of a stimulus; type (modality), intensity, location, and duration. Arrival time of a sound pulse and phase differences of continuous sound are used for sound localization. Certain receptors are sensitive to certain types of stimuli (for example, different mechanoreceptors respond best to different kinds of touch stimuli, like sharp or blunt objects). Receptors send impulses
in certain patterns to send information about the intensity of a
stimulus (for example, how loud a sound is). The location of the
receptor that is stimulated gives the brain information about the
location of the stimulus (for example, stimulating a mechanoreceptor in a
finger will send information to the brain about that finger). The
duration of the stimulus (how long it lasts) is conveyed by firing
patterns of receptors. These impulses are transmitted to the brain
through afferent neurons.
Quiescent state
Most sensory systems have a quiescent state, that is, the state that a sensory system converges to when there is no input.
This is well-defined for a linear time-invariant system,
whose input space is a vector space, and thus by definition has a point
of zero. It is also well-defined for any passive sensory system, that
is, a system that operates without needing input power. The quiescent
state is the state the system converges to when there is no input power.
It is not always well-defined for nonlinear, nonpassive sensory
organs, since they can't function without input energy. For example, a
cochlea is not a passive organ, but actively vibrates its own sensory
hairs to improve its sensitivity. This manifests as otoacoustic emissions in healthy ears, and tinnitus in pathological ears.
There is still a quiescent state for the cochlea, since there is a
well-defined mode of power input that it receives (vibratory energy on
the eardrum), which provides an unambiguous definition of "zero input
power".
Some sensory systems can have multiple quiescent states depending on its history, like flip-flops, and magnetic material with hysteresis.
It can also adapt to different quiescent states. In complete darkness,
the retinal cells become extremely sensitive, and there is noticeable "visual snow"
caused by the retinal cells firing randomly without any light input. In
brighter light, the retinal cells become a lot less sensitive, and
consequently visual noise decreases.
Quiescent state is less well-defined when the sensory organ can
be controlled by other systems, like a dog's ears that turn towards the
front or the sides as the brain commands. Some spiders can use their
nets as a large touch-organ, like weaving a skin for themselves. Even in
the absence of anything falling on the net, hungry spiders may increase
web thread tension, so as to respond promptly even to usually less
noticeable, and less profitable prey, such as small fruit flies,
creating two different "quiescent states" for the net.
Things become completely ill-defined for a system which connects
its output to its own input, thus ever-moving without any external
input. The prime example is the brain, with its default mode network.
Senses and receptors
While debate exists among neurologists as to the specific number of senses due to differing definitions of what constitutes a sense, Gautama Buddha and Aristotle classified five 'traditional' human senses which have become universally accepted: touch, taste, smell, sight, and hearing. Other senses that have been well-accepted in most mammals, including humans, include nociception, equilibrioception, kinaesthesia, and thermoception. Furthermore, some nonhuman animals have been shown to possess alternate senses, including magnetoreception and electroreception.
Receptors
The initialization of sensation stems from the response of a specific
receptor to a physical stimulus. The receptors which react to the
stimulus and initiate the process of sensation are commonly
characterized in four distinct categories: chemoreceptors, photoreceptors, mechanoreceptors, and thermoreceptors. All receptors receive distinct physical stimuli and transduce the signal into an electrical action potential. This action potential then travels along afferent neurons to specific brain regions where it is processed and interpreted.
Chemoreceptors
Chemoreceptors, or chemosensors, detect certain chemical stimuli and
transduce that signal into an electrical action potential. The two
primary types of chemoreceptors are:
Photoreceptors
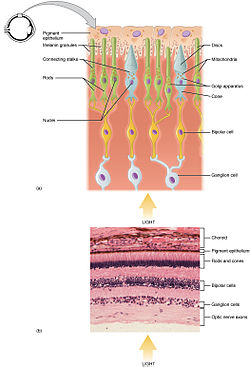
Photoreceptors are capable of phototransduction, a process which converts light (electromagnetic radiation) into, among other types of energy, a membrane potential. The three primary types of photoreceptors are:
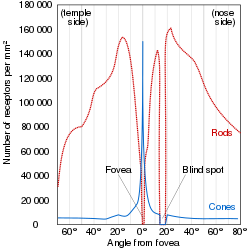
Cones are photoreceptors which respond significantly to color.
In humans the three different types of cones correspond with a primary
response to short wavelength (blue), medium wavelength (green), and
long wavelength (yellow/red).
Rods
are photoreceptors which are very sensitive to the intensity of light,
allowing for vision in dim lighting. The concentrations and ratio of
rods to cones is strongly correlated with whether an animal is diurnal or nocturnal. In humans rods outnumber cones by approximately 20:1, while in nocturnal animals, such as the tawny owl, the ratio is closer to 1000:1.
Ganglion Cells reside in the adrenal medulla and retina where they are involved in the sympathetic response. Of the ~1.3 million ganglion cells present in the retina, 1-2% are believed to be photosensitive ganglia. These photosensitive ganglia play a role in conscious vision for some animals, and are believed to do the same in humans.
Mechanoreceptors
Mechanoreceptors are sensory receptors which respond to mechanical forces, such as pressure or distortion. While mechanoreceptors are present in hair cells and play an integral role in the vestibular and auditory systems, the majority of mechanoreceptors are cutaneous and are grouped into four categories:
- Slowly adapting type 1 receptors have small receptive fields and respond to static stimulation. These receptors are primarily used in the sensations of form and roughness.
- Slowly adapting type 2 receptors have large receptive fields
and respond to stretch. Similarly to type 1, they produce sustained
responses to a continued stimuli.
- Rapidly adapting receptors have small receptive fields and underlie the perception of slip.
- Pacinian receptors have large receptive fields and are the predominant receptors for high-frequency vibration.
Thermoreceptors
Thermoreceptors are sensory receptors which respond to varying temperatures. While the mechanisms through which these receptors operate is unclear, recent discoveries have shown that mammals have at least two distinct types of thermoreceptors:
TRPV1 is a heat-activated channel that acts as a small heat detecting
thermometer in the membrane which begins the polarization of the neural
fiber when exposed to changes in temperature. Ultimately, this allows
us to detect ambient temperature in the warm/hot range. Similarly, the
molecular cousin to TRPV1, TRPM8, is a cold-activated ion channel that
responds to cold. Both cold and hot receptors are segregated by distinct
subpopulations of sensory nerve fibers, which shows us that the
information coming into the spinal cord is originally separate. Each
sensory receptor has its own "labeled line" to convey a simple sensation
experienced by the recipient. Ultimately, TRP channels act as
thermosensors, channels that help us to detect changes in ambient
temperatures.
Nociceptors
Nociceptors respond to potentially damaging stimuli by sending signals to the spinal cord and brain. This process, called nociception, usually causes the perception of pain.
They are found in internal organs, as well as on the surface of the
body. Nociceptors detect different kinds of damaging stimuli or actual
damage. Those that only respond when tissues are damaged are known as
"sleeping" or "silent" nociceptors.
- Thermal nociceptors are activated by noxious heat or cold at various temperatures.
- Mechanical nociceptors respond to excess pressure or mechanical deformation.
- Chemical nociceptors respond to a wide variety of chemicals, some of
which are signs of tissue damage. They are involved in the detection of
some spices in food.
Sensory cortex
All stimuli received by the receptors listed above are transduced to an action potential, which is carried along one or more afferent neurons towards a specific area of the brain. While the term sensory cortex is often used informally to refer to the somatosensory cortex, the term more accurately refers to the multiple areas of the brain at which senses are received to be processed. For the five traditional senses in humans, this includes the primary and secondary cortices of the different senses: the somatosensory cortex, the visual cortex, the auditory cortex, the primary olfactory cortex, and the gustatory cortex. Other modalities have corresponding sensory cortex areas as well, including the vestibular cortex for the sense of balance.
Somatosensory cortex
Located in the parietal lobe, the primary somatosensory cortex is the primary receptive area for the sense of touch and proprioception in the somatosensory system. This cortex is further divided into Brodmann areas 1, 2, and 3. Brodmann area 3 is considered the primary processing center of the somatosensory cortex as it receives significantly more input from the thalamus, has neurons highly responsive to somatosensory stimuli, and can evoke somatic sensations through electrical stimulation. Areas 1 and 2 receive most of their input from area 3. There are also pathways for proprioception (via the cerebellum), and motor control (via Brodmann area 4). See also: S2 Secondary somatosensory cortex.
Visual cortex
The visual cortex refers to the primary visual cortex, labeled V1 or Brodmann area 17, as well as the extrastriate visual cortical areas V2-V5. Located in the occipital lobe, V1 acts as the primary relay station for visual input, transmitting information to two primary pathways labeled the dorsal and ventral streams.
The dorsal stream includes areas V2 and V5, and is used in interpreting
visual 'where' and 'how.' The ventral stream includes areas V2 and V4,
and is used in interpreting 'what.' Increases in Task-negative activity are observed in the ventral attention network, after abrupt changes in sensory stimuli, at the onset and offset of task blocks, and at the end of a completed trial.
Auditory cortex
Located in the temporal lobe,
the auditory cortex is the primary receptive area for sound
information. The auditory cortex is composed of Brodmann areas 41 and
42, also known as the anterior transverse temporal area 41 and the posterior transverse temporal area 42, respectively. Both areas act similarly and are integral in receiving and processing the signals transmitted from auditory receptors.
Primary olfactory cortex
Located in the temporal lobe, the primary olfactory cortex is the primary receptive area for olfaction, or smell. Unique to the olfactory and gustatory systems, at least in mammals, is the implementation of both peripheral and central mechanisms of action. The peripheral mechanisms involve olfactory receptor neurons which transduce a chemical signal along the olfactory nerve, which terminates in the olfactory bulb. The chemoreceptors in the receptor neurons that start the signal cascade are G protein-coupled receptors. The central mechanisms include the convergence of olfactory nerve axons into glomeruli in the olfactory bulb, where the signal is then transmitted to the anterior olfactory nucleus, the piriform cortex, the medial amygdala, and the entorhinal cortex, all of which make up the primary olfactory cortex.
In contrast to vision and hearing, the olfactory bulbs are not cross-hemispheric; the right bulb connects to the right hemisphere and the left bulb connects to the left hemisphere.
Gustatory cortex
The gustatory cortex is the primary receptive area for taste. The word taste
is used in a technical sense to refer specifically to sensations coming
from taste buds on the tongue. The five qualities of taste detected by
the tongue include sourness, bitterness, sweetness, saltiness, and the
protein taste quality, called umami. In contrast, the term flavor
refers to the experience generated through integration of taste with
smell and tactile information. The gustatory cortex consists of two
primary structures: the anterior insula, located on the insular lobe, and the frontal operculum, located on the frontal lobe. Similarly to the olfactory cortex, the gustatory pathway operates through both peripheral and central mechanisms. Peripheral taste receptors, located on the tongue, soft palate, pharynx, and esophagus, transmit the received signal to primary sensory axons, where the signal is projected to the nucleus of the solitary tract in the medulla, or the gustatory nucleus of the solitary tract complex. The signal is then transmitted to the thalamus, which in turn projects the signal to several regions of the neocortex, including the gustatory cortex.
The neural processing of taste is affected at nearly every stage
of processing by concurrent somatosensory information from the tongue,
that is, mouthfeel.
Scent, in contrast, is not combined with taste to create flavor until
higher cortical processing regions, such as the insula and orbitofrontal
cortex.
Human sensory system
The human sensory system consists of the following subsystems:
Diseases
Disability-adjusted life year for sense organ diseases per 100,000 inhabitants in 2002.
no data
less than 200
200-400
400-600
600-800
800-1000
1000-1200
1200-1400
1400-1600
1600-1800
1800-2000
2000-2300
more than 2300







![Anatomy of a Rod Cell[4]](https://upload.wikimedia.org/wikipedia/commons/thumb/b/bb/Rod%26Cone.jpg/179px-Rod%26Cone.jpg)