From Wikipedia, the free encyclopedia
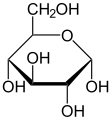
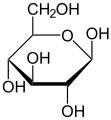
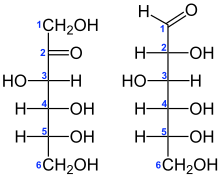
Structural formulae of fructose (left) and glucose (right)
High-fructose corn syrup (
HFCS), also known as
glucose-fructose,
isoglucose and
glucose-fructose syrup, is a
sweetener made from
corn starch. As in the production of conventional
corn syrup, the starch is broken down into glucose by enzymes. To make HFCS, the corn syrup is further
processed by
glucose isomerase to convert some of its
glucose into
fructose.
HFCS was first marketed in the early 1970s by the Clinton Corn
Processing Company, together with the Japanese Agency of Industrial
Science and Technology, where the enzyme was discovered in 1965.
As a sweetener, HFCS is often compared to
granulated sugar, but manufacturing advantages of HFCS over sugar include that it is easier to handle and more cost-effective. "HFCS 42" and "HFCS 55" refer to 42% and 55% fructose composition respectively, the rest being glucose and water. HFCS 42 is mainly used for processed foods and
breakfast cereals, whereas HFCS 55 is used mostly for production of
soft drinks.
The United States
Food and Drug Administration states that HFCS is a safe ingredient for food and beverage manufacturing. Uses and exports of HFCS from American producers have grown steadily during the early
21st century.
Food
In the U.S., HFCS is among the sweeteners that mostly replaced
sucrose (table sugar) in the food industry. Factors contributing to the rise of HFCS include production quotas of domestic sugar, import
tariffs on foreign sugar, and
subsidies of U.S. corn, raising the price of sucrose and lowering that of HFCS, making it cheapest for many sweetener applications. In spite of having a 10% greater fructose content, the relative
sweetness of HFCS 55, used most commonly in soft drinks, is comparable to that of
sucrose. HFCS (and/or standard
corn syrup) is the primary ingredient in most brands of commercial "pancake syrup", as a less expensive substitute for
maple syrup.
Because of its similar sugar profile and lower price, HFCS is often added to adulterate honey.
Assays to detect adulteration with HFCS use
differential scanning calorimetry and other advanced testing methods.
Production
Process
In the contemporary process, corn is milled to extract
corn starch
and an "acid-enzyme" process is used, in which the corn-starch solution
is acidified to begin breaking up the existing carbohydrates.
High-temperature enzymes are added to further metabolize the starch and
convert the resulting sugars to fructose.
The first enzyme added is
alpha-amylase, which breaks the long chains down into shorter sugar chains –
oligosaccharides.
Glucoamylase is mixed in and converts them to glucose. The resulting solution is filtered to remove protein, then using
activated carbon, and then demineralized using
ion-exchange resins. The purified solution is then run over immobilized
xylose isomerase,
which turns the sugars to ~50–52% glucose with some unconverted
oligosaccharides and 42% fructose (HFCS 42), and again demineralized and
again purified using activated carbon. Some is processed into HFCS 90
by liquid
chromatography, and then mixed with HFCS 42 to form HFCS 55. The enzymes used in the process are made by
microbial fermentation.
Composition and varieties
HFCS is 24% water, the rest being mainly fructose and glucose with 0–5% unprocessed
glucose oligomers.
The most common forms of HFCS used for food and beverage
manufacturing contain fructose in either 42% ("HFCS 42") or 55% ("HFCS
55") amounts, as described in the US
Code of Federal Regulations (21 CFR 184.1866).
- HFCS 42 (approx. 42% fructose if water were ignored) is used in beverages, processed foods, cereals, and baked goods.
- HFCS 55 is mostly used in soft drinks.
- HFCS 65 is used in soft drinks dispensed by Coca-Cola Freestyle machines.
- HFCS 70 is used in filling jellies
- HFCS 90 has some niche uses, but is mainly mixed with HFCS 42 to make HFCS 55.
Commerce and consumption
Consumption of sugar and corn-based sweeteners in the United States from 1966 to 2013, in dry-basis pounds per capita
The global market for HFCS is expected to grow from $5.9 billion in 2019 to a projected $7.6 billion in 2024.
China
HFCS in
China makes up about 20% of sweetener demand. HFCS has gained popularity
due to rising prices of sucrose, while selling for a third the price.
Production was estimated to reach 4,150,000 tonnes in 2017. About half
of total produced HFCS is exported to the Philippines, Indonesia,
Vietnam, and India.
European Union
In the
European Union
(EU), HFCS is known as isoglucose or glucose-fructose syrup (GFS) which
has 20–30% fructose content compared to 42% (HFCS 42) and 55% (HFCS 55)
in the United States. While HFCS is produced exclusively with corn in the US, manufacturers in the EU use corn and wheat to produce GFS.
GFS was once subject to a sugar production quota, which was abolished
on 1 October 2017, removing the previous production cap of 720,000
tonnes, and allowing production and export without restriction. Use of GFS in
soft drinks
is limited in the EU because manufacturers do not have a sufficient
supply of GFS containing at least 42% fructose content. As a result,
soft drinks are primarily sweetened by sucrose which has a 50% fructose
content.
Japan
In Japan, HFCS is also referred to as isomerized sugar. HFCS production arose in Japan after government policies created a rise in the price of sugar.
Japanese HFCS is manufactured mostly from imported U.S. corn, and the
output is regulated by the government. For the period from 2007 to 2012,
HFCS had a 27–30% share of the Japanese sweetener market. Japan consumed approximately 800,000 tonnes of HFCS in 2016.
The United States Department of Agriculture states that corn from the
United States is what Japan uses to produce their HFCS. Japan imports at
a level of 3 million tonnes per year, leading 20 percent of corn
imports to be for HFCS production.
Mexico
Mexico is the largest importer of U.S. HFCS. HFCS accounts for about 27 percent of total sweetener consumption, with Mexico importing 983,069 tonnes of HFCS in 2018.
Mexico's soft drink industry is shifting from sugar to HFCS which is
expected to boost U.S. HFCS exports to Mexico according to a U.S.
Department of Agriculture Foreign Agricultural Service report.
On 1 January 2002, Mexico imposed a 20% beverage tax on soft
drinks and syrups not sweetened with cane sugar. The United States
challenged the tax, appealing to the
World Trade Organization
(WTO). On 3 March 2006, the WTO ruled in favor of the U.S. citing the
tax as discriminatory against U.S. imports of HFCS without being
justified under WTO rules.
Philippines
The
Philippines was the largest importer of Chinese HFCS. Imports of HFCS
would peak at 373,137 tonnes in 2016. Complaints from domestic sugar
producers would result in a crackdown on Chinese exports.
On 1 January 2018, the Philippine government imposed a tax of 12 pesos
($.24) on drinks sweetened with HFCS versus 6 pesos ($.12) for drinks
sweetened with other sugars.
United States
In the United States, HFCS was widely used in food manufacturing from the 1970s through the early
21st century,
primarily as a replacement for sucrose because its sweetness was
similar to sucrose, it improved manufacturing quality, was easier to
use, and was cheaper. Domestic production of HFCS increased from 2.2 million tons in 1980 to a peak of 9.5 million tons in 1999.
Although HFCS use is about the same as sucrose use in the United
States, more than 90% of sweeteners used in global manufacturing is
sucrose.
Production of HFCS in the United States was 8.3 million tons in 2017.
HFCS is easier to handle than granulated sucrose, although some sucrose
is transported as solution. Unlike sucrose, HFCS cannot be hydrolyzed,
but the free fructose in HFCS may produce
hydroxymethylfurfural when stored at high temperatures; these differences are most prominent in acidic beverages. Soft drink makers such as
Coca-Cola and
Pepsi
continue to use sugar in other nations but transitioned to HFCS for
U.S. markets in 1980 before completely switching over in 1984. Large corporations, such as
Archer Daniels Midland,
lobby for the continuation of government corn subsidies.
Consumption of HFCS in the U.S. has declined since it peaked at
37.5 lb (17.0 kg) per person in 1999. The average American consumed
approximately 22.1 lb (10.0 kg) of HFCS in 2018, versus 40.3 lb (18.3 kg) of refined cane and beet sugar.
This decrease in domestic consumption of HFCS resulted in a push in
exporting of the product. In 2014, exports of HFCS were valued at $436
million, a decrease of 21% in one year, with Mexico receiving about 75%
of the export volume.
Vietnam
90% of Vietnam's HFCS import comes from China and South Korea. Imports would total 89,343 tonnes in 2017.
One ton of HFCS was priced at $398 in 2017, while one ton of sugar
would cost $702. HFCS has a zero cent import tax and no quota, while
sugarcane under quota has a 5% tax, and white and raw sugar not under
quota have an 85% and 80% tax. In 2018, the Vietnam Sugarcane and Sugar Association (VSSA) called for government intervention on current tax policies.
According to the VSSA, sugar companies face tighter lending policies
which cause the association's member companies with increased risk of
bankruptcy.
Health
Nutrition
Obesity and metabolic syndrome
There is no
scientific evidence that HFCS itself causes obesity or
metabolic syndrome, but rather overconsumption and excessive caloric intake of any sweetened food or beverage may contribute to these diseases.
Epidemiological research has shown that the increase in metabolic disorders, such as obesity and
non-alcoholic fatty liver disease, is linked to increased consumption of sugars and calories in general.
A 2012 review found that fructose did not appear to cause weight gain
when it replaced other carbohydrates in diets with similar calories. A 2014
systematic review found little evidence for an association between HFCS consumption and
liver diseases,
enzyme levels or fat content.
A 2018 review by the university of Colorado found that diets high in
fructose can cause the Nonalcoholic Fatty Liver Disease, due to the
conversion of fructose by fructokinase C, resulting in ATP consumption,
nucleotide turnover and uric acid generation that mediate fat
accumulation. The
American Heart Association
recommended that people limit added sugar (such as maltose, sucrose,
high fructose corn syrup, molasses or cane sugar) in their diets.
Safety and manufacturing concerns
One consumer concern about HFCS is that processing of corn is
more complex than used for “simpler” or “more natural” sugars, such as
fruit
juice concentrates or
agave nectar, but all sweetener products derived from
raw materials involve similar processing steps of
pulping,
hydrolysis,
enzyme treatment, and filtration, among other common steps of sweetener manufacturing from natural sources.
In the contemporary process to make HFCS, an "acid-enzyme" step is used
in which the corn starch solution is acidified to digest the existing
carbohydrates, then enzymes are added to further metabolize the corn
starch and convert the resulting sugars to their constituents of
fructose and glucose. Analyses published in 2014 showed that HFCS content of fructose was consistent across samples from 80 randomly selected
carbonated beverages sweetened with HFCS.
One prior concern in manufacturing was whether HFCS contains reactive
carbonyl compounds or
advanced glycation end-products evolved during processing. This concern was dismissed, however, with evidence that HFCS poses no dietary risk from these compounds.
Through the early
21st Century, some factories manufacturing HFCS had used a
chlor-alkali corn processing method which, in cases of applying
mercury cell technology for digesting corn raw material, left trace residues of
mercury in some batches of HFCS. In a 2009 release, The
Corn Refiners Association
stated that all factories in the American industry for manufacturing
HFCS had used mercury-free processing over several previous years,
making the prior report outdated. As of 2018, the
USDA, FDA and US
Centers for Disease Control list HFCS as a safe food ingredient, and do not mention mercury as a safety concern in HFCS products.
Fructose concentration and consistency
The
USFDA has recognized that studies have found differences between how
humans metabolize fructose compared to other simple sugars. The agency
does not consider HFCS-42 nor HFCS-55 to be better or worse for health
due to it providing a relatively equivalent amount of dietary fructose
as other approved sweeteners. Expressed in a 2013 review; “dietary
fructose consumption, which cannot be measured by conventional dietary
methods because the fructose content of HFCS is not disclosed, may be
much higher than...common assumptions.”
Other
Taste difference
Most countries, including Mexico, use
sucrose,
or table sugar, in soft drinks. In the U.S., soft drinks, such as
Coca-Cola, are typically made with HFCS 55. HFCS has a sweeter taste
than glucose. Some Americans seek out drinks such as
Mexican Coca-Cola in ethnic groceries because they prefer the taste over that of HFCS-sweetened Coca-Cola.
Kosher Coca-Cola, sold in the U.S. around the
Jewish holiday of
Passover, also uses sucrose rather than HFCS and is highly sought after by people who prefer the original taste.
While these are simply opinions, a 2011 study further backed up the
idea that people enjoy sucrose (table sugar) more than HFCS. The study,
conducted by Michigan State University, included a 99-member panel that
evaluated yogurt sweetened with sucrose (table sugar), HFCS, and
different varieties of honey for likeness. The results showed that,
overall, the panel enjoyed the yogurt with sucrose (table sugar) added
more than those that contained HFCS or honey.
Beekeeping
In
apiculture in the United States, HFCS is a honey substitute for some managed
honey bee colonies during times when nectar is in low supply. However, when HFCS is heated to about 45 °C (113 °F),
hydroxymethylfurfural, which is toxic to bees, can form from the breakdown of fructose. Although some researchers cite honey substitution with HFCS as one factor among many for
colony collapse disorder, there is no evidence that HFCS is the only cause. Compared to hive honey, both HFCS and sucrose caused signs of malnutrition in bees fed with them, apparent in the
expression of genes involved in
protein metabolism and other processes affecting honey bee health.
Public relations
There are various public relations concerns with HFCS, including how
HFCS products are advertised and labeled as "natural". As a consequence,
several companies reverted to manufacturing with sucrose (table sugar)
from products that had previously been made with HFCS. In 2010, the
Corn Refiners Association (CRA) applied to allow HFCS to be renamed "corn sugar", but that petition was rejected by the United States
Food and Drug Administration in 2012.
In August 2016 in a move to please consumers with health concerns,
McDonald's announced they would be replacing all HFCS in their
buns with sucrose (table sugar) and would cut out preservatives and other artificial additives from their menu items.
Marion Gross, senior vice president of McDonald's stated, "We know that
they [consumers] don't feel good about high-fructose corn syrup so
we're giving them what they're looking for instead." Over the early 21st century, other companies such as
Yoplait,
Gatorade, and
Hershey's also phased out HFCS, replacing it with conventional sugar because consumers perceived sugar to be healthier. Companies such as
PepsiCo and Heinz have also released products that use sugar in lieu of HFCS, although they still sell HFCS-sweetened products.
History
Commercial production of corn syrup began in 1964.
In the late 1950s, scientists at Clinton Corn Processing Company of
Clinton, Iowa, tried to turn glucose from corn starch into fructose, but the process was not scalable. In 1965–1970 Yoshiyuki Takasaki, at the Japanese
National Institute of Advanced Industrial Science and Technology (AIST) developed a heat-stable
xylose isomerase enzyme from yeast. In 1967, the Clinton Corn Processing Company obtained an exclusive license to manufacture glucose
isomerase derived from
Streptomyces bacteria and began shipping an early version of HFCS in February 1967.
In 1983, the FDA approved HFCS as
Generally Recognized as Safe (GRAS), and that decision was reaffirmed in 1996.
Prior to the development of the worldwide sugar industry, dietary
fructose was limited to only a few items. Milk, meats, and most
vegetables, the staples of many early diets, have no fructose, and only
5–10% fructose by weight is found in fruits such as grapes, apples, and
blueberries. Most
traditional dried fruits, however, contain about 50% fructose. From 1970 to 2000, there was a 25% increase in "added sugars" in the U.S. When recognized as a cheaper, more versatile sweetener, HFCS replaced
sucrose as the main sweetener of
soft drinks in the United States.
Since 1789, the U.S. sugar industry has had trade protection against tariffs imposed by foreign-produced sugar, while subsidies to corn growers cheapen the primary ingredient in HFCS,
corn. Industrial users looking for cheaper replacements rapidly adopted HFCS in the 1970s.