From Wikipedia, the free encyclopedia
Medical laboratory scientist

|
| Occupation |
|---|
| Names |
- Medical Laboratory Scientist (MLS) / Clinical Laboratory Scientist (CLS) / Medical Technologist (MT)
- Doctor of Medical Laboratory Science (DMLS)
|
|---|
Activity sectors
| Health care, Research & Development, Allied Health, Biomedical research |
|---|
| Description |
|---|
| Competencies | Analytical skills, quality control and knowledge of laboratory medicine and technology. |
|---|
Education required
|
|
|---|
A
medical laboratory scientist (
MLS), also traditionally referred to as a
clinical laboratory scientist (
CLS), or
medical technologist (
MT), is a
healthcare professional who performs
chemical,
hematological,
immunologic,
histopathological,
cytopathological,
microscopic, and
bacteriological diagnostic analyses on
body fluids such as
blood,
urine,
sputum,
stool,
cerebrospinal fluid (CSF),
peritoneal fluid,
pericardial fluid, and
synovial fluid, as well as other specimens. Medical laboratory scientists work in
clinical laboratories at
hospitals, reference labs,
biotechnology labs and non-clinical industrial labs. Those that work in non clinical industrial labs are often referred to as
biomedical laboratory technologist (
BLT) in parts of the world.
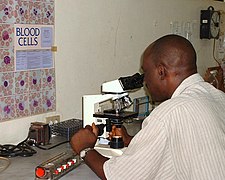
Job duties

MLS in his work environment
Medical laboratory scientists work in all areas of the clinical
laboratory, including blood banking, chemistry, hematology, immunology,
histology and microbiology . They perform a full range of laboratory
tests – from simple prenatal blood tests to more complex tests to
uncover diseases such as HIV/AIDS, diabetes, and cancer. They are also
responsible for confirming the accuracy of test results, and reporting
laboratory findings to pathologists and other physicians. The
information that a medical laboratory scientist gives to the doctor
influences the medical treatment a patient will receive. Medical
laboratory scientists operate complex electronic equipment, computers,
and precision instruments costing millions of dollars.
Medical Laboratory Scientists analyze human fluid samples using
techniques available to the clinical laboratory, such as manual white
blood cell differentials/counts, bone marrow counts, analysis via
microscopy, and advanced analytical equipment. Medical laboratory
scientists assist doctors and nurses in choosing the correct lab tests
and ensure proper collection methods.
Medical laboratory scientists receive the patient specimens, analyze
the specimens and report results. A pathologist may confirm a diagnostic
result, but often the medical laboratory scientist is responsible for
interpreting and communicating critical patient results to the
physician.
Medical laboratory scientists must recognize anomalies in their
test results and know how to correct problems with the instrumentation.
They monitor, screen, and troubleshoot analyzers featuring the latest
technology available on the market. The MLS performs equipment
validations, calibrations, quality controls, "STAT" or run-by-run
assessment, statistical control of observed data, and recording normal
operations. To maintain the integrity of the laboratory process, the
medical laboratory scientist recognizes factors that could introduce
error and rejects contaminated or sub-standard specimens, as well as
investigates discrepant results.
A typical laboratory performs hundreds of different tests with a
number of methodologies. Common tests performed by medical laboratory
scientists are
complete blood count (CBC),
comprehensive metabolic panel (CMP), electrolyte panel,
liver function tests (LFT), renal function tests (RFT), thyroid function test (TFT),
urinalysis, coagulation profile, lipid profile,
blood type, semen analysis (for fertility and post-vasectomy studies), serological studies and routine
cultures. In some facilities that have few
phlebotomists,
or none at all, (such as in rural areas) medical laboratory scientists
may perform phlebotomy on patients, as this skill is part of the
clinical training.
Because medical laboratory scientists are skilled in diverse
scientific disciplines, employment outside of the medical laboratory is
common. Many MLS are employed in government positions such as the FDA,
USDA, non-medical industrial laboratories, and manufacturing. The
practical experience required to obtain the bachelor's degree in medical
technology give the MLS a unique understanding of the
inter-relationship between microbiological and chemical testing and the
resulting clinical manifestations in clinical, scientific, and
industrial settings.
In the United Kingdom and the United States, senior laboratory
scientists, who are typically post-doctoral scientists, take on
significantly greater clinical responsibilities in the laboratory. In
the United States these scientists may function in the role of clinical
laboratory directors, while in the United Kingdom they are known as
consultant clinical scientists.
Though clinical scientists have existed in the UK National Health
Service for ~60 years, the introduction of formally trained and
accredited consultant level clinical scientists is relatively new, and
was introduced as part of the new Modernising Scientific Careers
framework.
Consultant clinical scientists are expected to provide expert
scientific and clinical leadership alongside and, at the same level as,
medical consultant colleagues. While specialists in healthcare science
will follow protocols, procedures and clinical guidelines, consultant
clinical scientists will help shape future guidelines and the
implementation of new and emerging technologies to help advance patient
care.
Role in the healthcare process
A
Medical Laboratory Scientist's role is to provide accurate laboratory
results in a timely manner. An estimated 70 percent of all decisions
regarding a patient's diagnosis and treatment, hospital admission and
discharge are based on laboratory test results.
in the United Kingdom, Healthcare Scientists including Clinical
Scientists may intervene throughout entire care pathways from diagnostic
tests to therapeutic treatments and rehabilitation. Although this
workforce comprises approximately 5% of the healthcare workforce in the
UK, their work underpins 80% of all diagnoses and clinical decisions
made.
Specialty areas
Many Medical Laboratory Scientists are
generalists,
skilled in most areas of the clinical laboratory. However some are
specialists, qualified by unique undergraduate education or additional
training to perform more complex analyses than usual within a specific
field. Specialties include
clinical biochemistry,
hematology,
coagulation,
microbiology,
bacteriology,
toxicology,
virology,
parasitology,
mycology,
immunology,
immunohematology (
blood bank),
histopathology,
histocompatibility,
cytopathology,
genetics,
cytogenetics,
electron microscopy, and
IVF
labs. Medical Technologists specialty may use additional credentials,
such as "SBB" (Specialist in Blood Banking) from the American
Association of Blood Banks, "SM" (Specialist in Microbiology) from the
American Society for Microbiology, "SC" (Specialist in Chemistry) from
the American Association for Clinical Chemistry, or "SH" (Specialist in
Hematology) from the American Society for Clinical Pathology (ASCP).
These additional notations may be appended to the base credential, for
example, "MLS(ASCP)SBB". Additional information can be found in the ASCP Procedures for Examination & Certification.
Andrology Laboratory Scientist, Embryology Laboratory Scientist,
and Molecular Diagnostics Technologist certifications are provided by
the American Association of Bioanalysts; those with the certifications
are classified as ALS(AAB), ELS(AAB), and MDxT(AAB) respectively.
Certified Histocompatibility Associate, Certified Histocompatibility
Technologist, Certified Histocompatibility Specialist, and Diplomate of
the ABHI are titles granted by the American Board of Hisocompatibility
and Immunogenetics after meeting education and experience requirements
and passing the required examination; those individuals would hold the
credentials CHA(ABHI), CHT(ABHI), CHS(AHBI), and D(ABHI) upon passing
the corresponding examination.
In the United States, Medical Laboratory Scientists can be certified and employed in
infection control. These professionals monitor and report infectious disease findings to help limit
iatrogenic and
nosocomial infections. They may also educate other healthcare workers about such problems and ways to minimize them.
In the United Kingdom the number of Clinical Scientists in a
pathology discipline are typically greater, where less medically
qualified pathologists train as consultants. Clinical Biochemistry,
Clinical Immunology and Genomic Medicine are specialities with an
abundance of UK Clinical Scientists, and where the role is well
established. Infection services in the United Kingdom are generally
undertaken by medically qualified Microbiologists, who may have overall
responsibility for laboratory services in addition to Infection
Prevention and Control responsibilities, and may be required to
contribute to ward rounds and patient clinics. Therefore, the Royal
College of Pathologists and Royal College of Physicians have developed
Combined Infection Training[10], that medical trainees gain a much more
patient focused experience, and undertake Physician examinations in
addition to Pathology training. The end result of this is that several
regional medical deaneries no longer permit Medical Doctors to train in
Microbiology or Virology as single disciplines, and instead advocate
dual-specialisation as Infectious Disease/Microbiology or Infectious
Disease/Virology [11]. Simultaneously the expansion of higher specialist
scientist trainees in microbiology mean that many of the laboratory and
scientific responsibilities of medical doctors may be taken on my
Clinical Scientists, and medical doctors will instead be expected to
perform a much more patient facing role. The exception in Microbiology
is the sub-discipline of Virology, which is well suited to the expertise
of clinical scientists due to reliance on cutting edge scientific
methods, increasing use of specialised genetic technologies, and a
technical understanding of virus biology, with a reduced emphasis on
patient management compared with Microbiology as a whole.
It is therefore likely that many patients in UK hospitals may
come into contact with Clinical Scientists working in a patient facing
speciality, who may be confused with medical doctors due to the complex
nature of their role.
Educational requirements
Educational and licensing requirements vary by country due to differing scopes of practice and legislative differences.
Australia
In
Australia, medical laboratory scientists complete a four-year
undergraduate degree program in medical laboratory science or Master of
Medical Laboratory science . These programs should be accredited by the
Australian Institute of Medical Scientists (AIMS).
Canada
In
Canada, three-year college or technical school programs are offered that
include seven semesters, two of them comprising an unpaid internship.
The student graduates before taking a standard examination (such as the
Canadian Society for Medical Laboratory Science, or CSMLS, exam) to be
qualified as a medical laboratory technologist.
Many MLTs go on to receive a bachelor of science degree after they are
certified, but a few university programs affiliated with a college MLT
program to allow students to graduate with both MLT certification and a
degree such as the
University of New Brunswick's Bachelor of Medical Laboratory Sciences program.
Canada is currently experiencing an increasing problem with staffing shortages in medical laboratories.
New Zealand
In
New Zealand, a medical laboratory scientist must complete a bachelor's
degree in medical laboratory science or biological or chemical science
recognized by the Medical Sciences Council of New Zealand. As part of
this degree they must complete clinical placement.
Once they graduate they must have worked at least six months under
supervision, be registered with the Medical Sciences Counsel of New
Zealand, and hold a current Annual Practicing Certificate.
Ghana
In Ghana, a doctor of medical laboratory scientist (MLS.D) is a professional with a six (6) years professional
doctorate degree in medical laboratory science, the medical laboratory scientist (MLS) has four (4) years
bachelor's degree in medical laboratory science and the medical laboratory technicians (MLT) has three (3) years
diploma in medical laboratory science.
The curriculum for the programme include clinical rotations,
where the students get hands-on experiences in each discipline of the
laboratory and performs diagnostic testing in a functioning laboratory
under supervision.
Pakistan
In
Pakistan National Institute of Health (NIH) Islamabad is the pioneer in
Laboratory Sciences, College of Medical Lab Technology, (CMLT), NIH,
Islamabad offers 2 years F.Sc in Medical Lab Technology (MLT),
Previously 2 Years B.Sc (MLT) that was discontinued and replaced by 4
years Bachelor Program in Medical Lab Sciences. University of Health
Sciences, Lahore also offering 4 year Bachelor program in Medical Lab
Sciences through approved colleges. University of Lahore, University of
Faisalabad, University of Sargodha and Superior University Lahore
offering 5-years Doctor of Medical Lab Sciences (DMLS) Program;
Eligibility criteria for 4 years BS Medical Lab Sciences and 5 years
Doctor of Medical Lab Sciences (DMLS) is F.Sc Pre-Medical.
United States
In
the United States, a medical laboratory scientist (MLS), medical
technologist (MT), or a clinical laboratory scientist (CLS) typically
earns a
bachelor's degree
in medical laboratory science, clinical laboratory science, or medical
technology. Other routes include attaining a degree in biomedical
science or in a life / biological science (biology, biochemistry,
microbiology, etc.). Both routes typically requires the MLS/MT/CLS to
obtain certification from a national certifying board (AAB, AMT, or
ASCP) as most laboratories exceed the federal minimum requirements
established by the Clinical Laboratory Improvement Amendments (CLIA).
Common comprehensive Medical laboratory scientist degree programs are set up in a few different ways.
- In 3+1 programs, the student attends classroom courses for three
years and complete a clinical rotation their final year of study.
- In 2+2 programs, students have already completed their lower
division coursework and return to complete their last two years of study
in a CLS program.
- In 4+1 program, students who have already completed an undergraduate
program return to complete a year of medical laboratory training. The
training is typically completed at a clinical site rather than a
college.
The core curriculum in medical technology generally comprises 20
credits in clinical chemistry, 20 credits in hematology, and 20 credits
in clinical microbiology.
During clinical rotations, the student experiences hands-on
learning in each discipline of the laboratory and performs diagnostic
testing in a functioning laboratory under supervision. With limited or
no compensation, a student in the clinical phase of training usually
works 40 hours per week for 20 to 52 weeks. Some programs in the United
States have had the time students spend completing their clinical
rotation reduced due to staffing shortages. For example, in 2015, the
MLS program at the University of Minnesota reduced the clinical rotation
portion of the program from 22 weeks to 12 weeks.
In the United States, a two-year academic program (
associate's degree)
qualifies the graduate to work as a medical laboratory technician
(MLT). MLTs receive training more exclusively in laboratory sciences
without the basic science coursework often required by MLS programs;
however, there are many MLT training programs that require substantial
basic didactic science course work prior to entry into a clinical
practicum.
Although the didactic coursework may be less for the MLT, the clinical
practicum, in many cases, is similar to that of the MLS student's. This
equates to MLTs who are well equipped to enter the work force with
relevant and knowledge based practical application. The shorter
training time may be attractive to many students, but there are
disadvantages to this route. MTs, MLSs and CLSs usually earn higher
salaries and have more responsibilities than MLTs. In 2018, medical
laboratory technicians earned an average salary of $51,219, while
medical laboratory scientists earned a salary of $67,888.
An added disadvantage for MLTs is that some institutions will only
employ MLSs, although that practice is starting to change due to recent
efforts in cost reduction, and due to staffing shortages.
In practice, the term medical laboratory technician may
apply to persons who are trained to operate equipment and perform tests,
usually under the supervision of the certified medical technologist or
laboratory scientist. Depending on the state where employment is
granted, the job duties between MLSs and MLTs may or may not be similar.
For example, in Florida, a MLT may only perform highly complex testing
while under the direct supervision of a clinical laboratory
technologist, a clinical laboratory supervisor, or a clinical laboratory
director.
This may make it impractical for a MLT to lawfully work in a Florida
blood bank. California has similar restrictions on MLTs. To accommodate
California's restrictions, the American Association of Bioanalysts (AAB)
developed a separate certification examination for California
licensure. However, this exam does not include material covering the
areas of immunohematology or microscopy.
Although the typical entry-level academic requirement for most MLTs is
an associate degree, a 60 credit certificate program exists through
military training programs; such as the U.S. Army's 68K military
occupational specialty.
As in other countries, staffing shortages have become a major
issue in many clinical laboratories in the United States. Due to
several factors, including boomer retirement, and inadequate recruitment
and retention efforts, the medical laboratory workforce is shrinking.
For the decade 2010–2020, workforce needs are expected to grow by 13%.
This translates into about 11,300 positions per year that will need to
be filled, with only about 5000 new graduates per year coming out of
various programs. By 2025, it is estimated that the shortage of medical
laboratory professionals will reach 98,700 in the U.S.
United Kingdom
In
the United Kingdom (UK) there are two varieties of registered
healthcare scientist in hospitals - Clinical Scientists and Biomedical
Scientists (BMS). There is a strict and formal post graduate training
programme for both careers followed by statutory registration for each
with the Health & Care Professions Council UK (HCPC):[1], for the
safety and assurance of the customers - the patients. They are two
similar but distinct careers with parallel but different training paths
and different entry requirements.
The role of Clinical Scientists is to improve the health and
well-being of patients and the public by practising alongside doctors,
nurses, and other health and social care professionals in the delivery
of healthcare. Their aim is to provide expert scientific and clinical
advice to clinician colleagues, to aid in the diagnosis, treatment and
management of patient care.
Examples of the type of work they undertake include:
- Advising, diagnosing, interpreting, and treating patients.
- Advising health and social care professionals in the diagnosis and treatment of patients.
- Researching the science, technology, and practise used in healthcare to innovate and improve services.
- Designing, building, and operating technology for diagnosing and treating patients.
- Ensuring the safety and reliability of tests and equipment used in healthcare.
Trainee Clinical Scientist posts are advertised nationally, usually
between November and February on the Clinical Scientists Recruitment
webpages where application forms may be obtained and electronic
submission of applications can be made. These posts are for the approved
Pre-registration Training Programme, designed to prepare entrants for
higher professional qualifications, further clinical training and
eventual Consultant responsibility.
Clinical Scientist training involves enrolment of graduates (1st
or 2nd class honours degree or better is essential due to the high
competition for limited training places) into an intensive 3-year
training scheme leading to certification and eventual registration
before starting the higher career structure. The basic qualification for
becoming a Clinical Biochemist, Clinical Immunologist or Clinical
Microbiologist is a good Honours degree in an appropriate subject: for
Clinical Biochemistry, that subject might be Biochemistry or Chemistry
(or another life science subject which contains a substantial
Biochemistry component); for Clinical Immunology, that subject might be
any life science degree with an immunology component; for Clinical
Microbiology that subject might be any life science degree with a
microbiology component.
Although not essential, some candidates will apply with higher
degrees in an attempt to improve their chances of selection for training
and several universities currently offer MSc courses in Clinical
Biochemistry, Immunology and Microbiology which have been approved by
the ACB or the AHCS. Full-time and 'sandwich' courses are available, and
further information may be obtained from individual programmes,
although the level of financial support provided varies, and should be
clarified at interview. Some entrants to the profession will already
have obtained a PhD, and the training and research experience that this
provides is invaluable to the work of the Clinical Scientist. In larger
Departments, there may be opportunities to study for a research degree
after entering the profession and acquiring registration, but since this
has to be fitted in with other responsibilities, it may take some years
to complete. It should be clearly understood that the major role of the
profession is patient care and that research, management and all the
other aspects will come as side issues and not be the predominating
factor in the career path. The work of Biomedical Scientists and
Clinical Scientists have impact on the diagnosis and treatment of almost
every patient admitted to hospitals in the United Kingdom.
The United Kingdom is facing a shortage of qualified Clinical and
Biomedical Scientists. The Royal College of Pathologists and the Royal
College of Physicians have pointed out the need for increased
government funding for medical training programs to prevent diagnostic
facilities and medical infrastructure from being overwhelmed.
Nigeria
In
Nigeria, Medical Laboratory Science is a high skilled profession charged
by Act. 2004 Cap 114 Laws of the Federation of Nigeria. The initial
qualification awarded graduates of the programme, like some other
medical programmes, was Associate of the Institute of Medical Laboratory
Technology/Science (AIMLT/AIMLS)
The Medical Laboratory Science Council of Nigeria, which was established
by Act. 2004 Cap 114 Laws of the Federation of Nigeria, regulates the
practice of Medical Laboratory Science in Nigeria. In Nigeria, the
Medical Laboratory Science programme is Bachelor of Medical Laboratory
Science (BMLS), regulated by National Universities Commission (NUC) and
by the Medical Laboratory Science Council of Nigeria (MLSCN). Students
at their first year (100 level) are trained under the Faculty of Science
in Basic Sciences and Faculty of Arts, Management and Social science in
General studies and Entrepreneurship. At the 200 level, students are
taught basic medical sciences and are introduced to Medical Laboratory
Science. The third year of the programme marks the beginning of the
professional training as students are engaged in the classroom for
lectures as well as in the Hospital laboratory for the professional or
practical training. At the fourth year students are taught the basics in
all the special areas of Medical Laboratory Science. At the end of 400
level programme, successful students are presented for the First
professional examination, to be moderated by the Medical Laboratory
Science Council of Nigeria At the fifth year, students break into 4
core or specialized areas of Medical Laboratory Science, namely: medical
microbiology/parasitology, chemical pathology/immunology,
haematology/blood transfusion science and histopathology/cytopathology.
At the end of the fifth year, suitable students are presented for final
professional examination by the Medical Laboratory Science Council of
Nigeria.
Certification and licensing
United States
There
are currently three major certification agencies in the United States
of America for clinical laboratory scientists. They are the American
Association of Bioanalysts (AAB), the American Medical Technologists
(AMT), and the American Society for Clinical Pathology (ASCP). All three
national accrediting agencies will certify scientists in the clinical
laboratory as generalist (chemistry, hematology, immunology,
immunohematology/blood bank, and microbiology).
The American Association of Bioanalysts and the American Medical
Technologists certifications continue to use the traditional designation
Medical Technologist (MT), while the American Society for Clinical
Pathology has adopted the designation of Medical Laboratory Scientist
(MLS). Regardless of terminology, these highly qualified individuals
serve as scientists in the clinical laboratory.
There are two other organizations that have previously provided
proficiency examinations to clinical laboratory scientist. The first, is
the US Department of Health and Human Services.
The second, is the National Credentialing Agency for Laboratory
Personnel (NCA). The NCA was absorbed by the American Society for
Clinical Pathology in 2009 and promptly dissolved.
In the
United States, the
Clinical Laboratory Improvement Amendments (CLIA '88) define the level of qualification required to perform tests of various complexity.
Clinical Laboratory Scientists, Medical Technologists and Medical
Laboratory Scientists are near the highest level of qualification among
general testing personnel and are usually qualified to perform the most
complex clinical testing including
HLA testing (also known as tissue typing) and
blood type reference
testing. Provider Performed Microscopy, or PPM (doctorate or master's
level health provider) and Cytology have additional requirements.
In addition to the national certification, 12 states (California,
Florida, Georgia, Hawaii, Louisiana, Montana, Nevada, North Dakota,
Rhode Island, Tennessee, West Virginia and New York) and Puerto Rico
also require a state license. Puerto Rico, in order to provide the state
license, requires either a local board certification with a state
examination, or any of both the ASCP and the NCA.
Minnesota, Texas, Illinois, Massachusetts, Michigan, Vermont,
Washington, New Jersey, Iowa, Utah, Ohio, South Carolina, Wyoming,
Pennsylvania, Virginia, South Dakota, Delaware, Missouri, and Alaska are
currently attempting to obtain licensure. All states require
documentation from a professional certification agency before issuing a
state certification. A person applying for state certification may also
be expected to submit fingerprints, education and training records, and
competency certification. Some states also require completion of a
specified number of continuing education contact hours prior to issuing
or renewing a license. Licensing is somewhat controversial as it adds a
bureaucratic layer in a field that is severely understaffed. Simply
requiring testing personnel to obtain and maintain their national
certification would help ensure competent testing personnel without
increasing costs to testing personnel.
Some states recognize another state's license if it is equal or
more stringent, but currently California does not recognize any other
state license.
United Kingdom
In
the United Kingdom all clinical scientists and biomedical scientists
have had to be registered with the Health & Care Professions Council
(HCPC) in order to work unsupervised, to develop through the careers
grades of their profession and to use the protected titles of "Clinical
Scientist" or "Biomedical Scientist". The HCPC registers nearly 200,000
healthcare professionals[3] and while success in an approved degree
course from an accredited University is sufficient for all other
professions, both clinical scientists and biomedical scientists have
post graduate training and no approved degree courses. Autonomous
assessment of applicants in these two professions with subsequent
certification for successful ones, is the only approved UK route to
registration for them.
"Clinical Scientist", just as "Biomedical Scientist", is a
protected title under the law (there is a £5000 fine for transgressors
who fraudulently use the title without being registered by the state).
The HCPC can strike people off the register for malpractice in just the
same way as for doctors with the General Medical Council (GMC).
Those who are working in "Trainee" positions in the profession
are permitted to use the title with an appropriate caveat, for example –
"Pre-registration Clinical Scientist", Trainee Clinical Scientist, etc.
Alternatively some may use titles specific to the discipline they train
in, such as Trainee Clinical Biochemist", "Clinical Immunologist in
Training" or “ Pre-Registrant Clinical Microbiologist” which is also
perfectly acceptable since it is not implying the protected "Clinical
Scientist" title of fully qualified and registered practitioners. It is
against the law to formally work with the title of “Clinical Scientist”
without professional registration.
Nigeria
In Nigeria successful student at the end of the training in both
academic and professional assessments with respect to the graduation
requirements is certified by the respective University, inducted and
licensed by the Medical Laboratory Science Council of Nigeria after a
successful internship training. http://mlscn.gov.ng
Further education
As
in many healthcare professions, a Medical Laboratory Scientist may
pursue higher education to advance or further specialize in their
career.
- Master of Science, Master of Business Administration, Master of Health Administration, Doctor of medical laboratory science for specialization, education and management roles.
- Doctor of Philosophy
for management and directorship roles in the clinical laboratory as
well as for academic research and professorship. Doctors of Philosophy
holding a degree in a biological science, and who are board certified by
a CLIA-approved entity, are qualified as a medical laboratory director.
- Doctor of Medicine or Doctor of clinical laboratory Science - this
is the position that qualifies an individual to oversee or direct almost
all types of clinical laboratories. Under U.S. CLIA laws, a requirement
of at least year of clinical laboratory experience (any MD) or
pathology residency must be met.
In the United Kingdom The Modernising Scientific Careers (MSC)
programme sets out for the first time a comprehensive training and
career framework for the whole healthcare science workforce inclusive of
the more than 50 different scientific professional specialisms. In its
conception it aimed to provide a coherent framework that was accessible,
affordable and designed specifically to both capture scientific and
technological advances and to provide improved outcomes for patients,
the service and professionals. A key aspect of the framework from the
start was the formalisation of training to develop talented clinical
scientists to undertake quality assured Higher Specialist Scientist
Training (HSST) programmes to prepare them for roles as Consultant
Clinical Scientists. It is envisaged that Consultant Clinical Scientists
will work synergistically and in partnership with their medical
colleagues and within multiprofessional clinical teams to support
clinical scientific practice aimed at quality improvement, innovation
and world-class outcomes for patients. This scientific expertise and
leadership will provide important benefits and added value to patients
and to the service as it moves forward through the 21st century. This
will bring to fruition the vision of science and realise the potential
of scientific and technological advances for both translational and
personalised medicine.
Training through the Higher Specialist Scientist Training pathway
is discipline specific. For life science disciplines (Immunology,
Microbiology, Virology, Haematology, Biochemistry) the training
curriculum and formal examinations are administered by the Royal College
of Pathologists. The life science training pathway for Clinical
Scientists follows a similar pathway to that undertaken by medically
qualified specialist registrars in pathology. Clinical Scientists are
therefore the only discipline of non-medical healthcare professionals
examined by a Medical Royal College. Clinical Scientists who attain both
part 1 examination certification and part 2 certification are awarded
Fellowship of the Royal College of Pathologists (FRCPath) and are deemed
to have the knowledge and expertise expected of a consultant level
scientist. Consultant Clinical Scientist posts generally require
candidates to have completed FRCPath qualification to be eligible.
All Clinical Scientists regardless of seniority or specialisation
may have other responsibilities including academic appointments,
responsibilities as clinical lead for a pathology service, or may have
wider hospital responsibilities such as Directorship of Infection
Prevention and Control, or responsibility for the hospital's Research
and Development strategy. Junior clinical scientists may become involved
in academic research, working towards award of a Ph.D. or DClinSci.
Job title
Russian MLS prepares the analyses in ELISA laboratory
The informal abbreviations of job titles may be a source of
confusion. In the United States Medical Laboratory Scientist (ASCP) and
Medical Technologists (AMT) or (AAB) are often called "med techs" (based
on the era in which they were known as "medical technologists"), but
this shorthand term is shared by other healthcare employees, including
pharmacy techs,
radiographers (also known as radiologic technologists), and
respiratory therapists.
In the United States there is a formal distinction between an MLT
and a MT/MLS. Often, MT/MLS have at least a bachelor's degree, while
MLT have an associate degree. However, due to grandfathering rules and
certification requirements between the boards of registry, some MT/MLS
may only have an associate degree.
Scientists and technologists generally earn a higher income than
technicians, have more responsibilities, and have more opportunities for
advancement.
In the United Kingdom, there are defined training pathways
leading to professional registration as either a Clinical Scientist, or
as a Biomedical Scientist. The role descriptions for these healthcare
scientists are very different, where clinical scientists generally
undertake non-routine research and development, as well as improving and
providing clinical service using scientific expertise. Biomedical
Scientists in the United Kingdom are similar to the role of MLT and
MT/CLS described above, and have similar regulatory requirements for
professional regulation. Clinical Scientists in the United Kingdom may
struggle with a lack of professional recognition. This is in part due to
the myriad job titles used to describe them including Clinical
Physiologists, Medical Physicists, and Clinical Biochemists, which
generally mean the public and other healthcare workers assume Clinical
Scientists to be medically qualified doctors, due to the sometimes
complex nature of the role.