From Wikipedia, the free encyclopedia
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Spectral lines of mercury (UV not seen)
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, symbol | mercury, Hg | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pronunciation | /ˈmɜrkjəri/ MER-kyə-ree |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mercury in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number | 80 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight | 200.592(3) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | transition metal | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, block | group 12, d-block | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Xe] 4f14 5d10 6s2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| per shell | 2, 8, 18, 32, 18, 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | liquid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 234.3210 K (−38.8290 °C, −37.8922 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 629.88 K (356.73 °C, 674.11 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density near r.t. | 13.534 g·cm−3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Triple point | 234.3156 K, 1.65×10−7 kPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Critical point | 1750 K, 172.00 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 2.29 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 59.11 kJ·mol−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 27.983 J·mol−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
vapor pressure
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 4, 2 (mercuric), 1 (mercurous) (a mildly basic oxide) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 2.00 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies | 1st: 1007.1 kJ·mol−1 2nd: 1810 kJ·mol−1 3rd: 3300 kJ·mol−1 |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 151 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 132±5 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 155 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellanea | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | rhombohedral
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound | liquid: 1451.4 m·s−1 (at 20 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 60.4 µm·m−1·K−1 (at 25 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 8.30 W·m−1·K−1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 961 nΩ·m (at 25 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic[1] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 7439-97-6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | Ancient Chinese and Indians (before 2000 BCE) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Most stable isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Decay modes in parentheses are predicted, but have not yet been observed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Mercury is a chemical element with symbol Hg and atomic number 80. It is commonly known as quicksilver and was formerly named hydrargyrum (/haɪˈdrɑrdʒərəm/).[2] A heavy, silvery d-block element, mercury is the only metallic element that is liquid at standard conditions for temperature and pressure; the only other element that is liquid under these conditions is bromine, though metals such as caesium, gallium, and rubidium melt just above room temperature.
Mercury occurs in deposits throughout the world mostly as cinnabar (mercuric sulfide). The red pigment vermilion, a pure form of mercuric sulfide, is mostly obtained by reaction of mercury (produced by reduction from cinnabar) with sulfur. Mercury poisoning can result from exposure to water-soluble forms of mercury (such as mercuric chloride or methylmercury), inhalation of mercury vapor, or eating seafood contaminated with mercury.
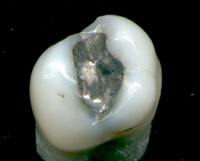
Mercury is used in thermometers, barometers, manometers, sphygmomanometers, float valves, mercury switches, mercury relays, fluorescent lamps and other devices, though concerns about the element's toxicity have led to mercury thermometers and sphygmomanometers being largely phased out in clinical environments in favour of alternatives such as alcohol- or galinstan-filled glass thermometers and thermistor- or infrared-based electronic instruments. Likewise, mechanical pressure gauges and electronic strain gauge sensors have replaced mercury sphygmomanometers. Mercury remains in use in scientific research applications and in amalgam material for dental restoration in some locales. It is used in lighting: electricity passed through mercury vapor in a fluorescent lamp produces short-wave ultraviolet light which then causes the phosphor in the tube to fluoresce, making visible light.
Properties
Physical properties

A pound coin (density ~7.6 g/cm3) floats in mercury due to the combination of the buoyant force and surface tension.
Mercury is a heavy, silvery-white metal. As compared to other metals, it is a poor conductor of heat, but a fair conductor of electricity.[3] Mercury has a freezing point of −38.83 °C and a boiling point of 356.73 °C,[4][5][6] both exceptionally low for a metal, and it is the only elemental metal known to melt at a generally cold temperature. A complete explanation of this delves deep into the realm of quantum physics, but it can be summarized as follows: mercury has a unique electron configuration where electrons fill up all the available 1s, 2s, 2p, 3s, 3p, 3d, 4s, 4p, 4d, 4f, 5s, 5p, 5d, and 6s subshells. Because this configuration strongly resists removal of an electron, mercury behaves similarly to noble gas elements, which form weak bonds and hence melt at relatively low temperatures.
The stability of the 6s shell is due to the presence of a filled 4f shell. An f shell poorly screens the nuclear charge that increases the attractive Coulomb interaction of the 6s shell and the nucleus (see lanthanide contraction). The absence of a filled inner f shell is the reason for the somewhat higher melting temperature of cadmium and zinc, although both these metals still melt easily and, in addition, have unusually low boiling points.
On the other hand, gold, which is one space to the left of mercury on the periodic table, has atoms with one fewer 6s electron than mercury. Those electrons are more easily removed and are shared between the gold atoms forming relatively strong metallic bonds.[5][4]
Chemical properties
Mercury does not react with most acids, such as dilute sulfuric acid, although oxidizing acids such as concentrated sulfuric acid and nitric acid or aqua regia dissolve it to give sulfate, nitrate, and chloride salts. Like silver, mercury reacts with atmospheric hydrogen sulfide. Mercury even reacts with solid sulfur flakes, which are used in mercury spill kits to absorb mercury vapors (spill kits also use activated carbon and powdered zinc).[7]Amalgams
Mercury dissolves many other metals such as gold and silver to form amalgams. Iron is an exception, and iron flasks have been traditionally used to trade mercury. Several other first row transition metals with the exception of manganese, copper and zinc are reluctant to form amalgams. Other elements that do not readily form amalgams with mercury include platinum and a few other metals.[8][9] Sodium amalgam is a common reducing agent in organic synthesis, and is also used in high-pressure sodium lamps.
Mercury readily combines with aluminium to form a mercury-aluminium amalgam when the two pure metals come into contact. Since the amalgam destroys the aluminium oxide layer which protects metallic aluminium from oxidizing in-depth (as in iron rusting), even small amounts of mercury can seriously corrode aluminium. For this reason, mercury is not allowed aboard an aircraft under most circumstances because of the risk of it forming an amalgam with exposed aluminium parts in the aircraft.[10]
Mercury embrittlement is the most common type of liquid metal embrittlement.
Isotopes
There are seven stable isotopes of mercury with 202Hg being the most abundant (29.86%). The longest-lived radioisotopes are 194Hg with a half-life of 444 years, and 203Hg with a half-life of 46.612 days. Most of the remaining radioisotopes have half-lives that are less than a day. 199Hg and 201Hg are the most often studied NMR-active nuclei, having spins of 1⁄2 and 3⁄2 respectively.[3]Etymology and history
Mercury was found in Egyptian tombs that date from 1500 BC.[11]
In China and Tibet, mercury use was thought to prolong life, heal fractures, and maintain generally good health, although it is now known that exposure to mercury vapor leads to serious adverse health effects.[12] The first emperor of China, Qín Shǐ Huáng Dì—allegedly buried in a tomb that contained rivers of flowing mercury on a model of the land he ruled, representative of the rivers of China—was killed by drinking a mercury and powdered jade mixture formulated by Qin alchemists (causing liver failure, mercury poisoning, and brain death) who intended to give him eternal life.[13][14]
The ancient Greeks used mercury in ointments; the ancient Egyptians and the Romans used it in cosmetics which sometimes deformed the face[citation needed]. In Lamanai, once a major city of the Maya civilization, a pool of mercury was found under a marker in a Mesoamerican ballcourt.[15][16] By 500 BC mercury was used to make amalgams (Medieval Latin amalgama, "alloy of mercury") with other metals.[17]
Alchemists thought of mercury as the First Matter from which all metals were formed. They believed that different metals could be produced by varying the quality and quantity of sulfur contained within the mercury. The purest of these was gold, and mercury was called for in attempts at the transmutation of base (or impure) metals into gold, which was the goal of many alchemists.[18]
Hg is the modern chemical symbol for mercury. It comes from hydrargyrum, a Latinized form of the Greek word ὑδράργυρος (hydrargyros), which is a compound word meaning "water-silver" (from ὑδρ- hydr-, the root of ὕδωρ, "water," and ἄργυρος argyros "silver") – since it is liquid like water and shiny like silver. The element was named after the Roman god Mercury, known for his speed and mobility. It is associated with the planet Mercury; the astrological symbol for the planet is also one of the alchemical symbols for the metal; the Sanskrit word for alchemy is Rasavātam which means "the way of mercury".[19] Mercury is the only metal for which the alchemical planetary name became the common name.[18]
The mines in Almadén (Spain), Monte Amiata (Italy), and Idrija (now Slovenia) dominated mercury production from the opening of the mine in Almadén 2500 years ago, until new deposits were found at the end of the 19th century.[20]
Occurrence

Mercury is an extremely rare element in Earth's crust, having an average crustal abundance by mass of only 0.08 parts per million (ppm).[21] However, because it does not blend geochemically with those elements that constitute the majority of the crustal mass, mercury ores can be extraordinarily concentrated considering the element's abundance in ordinary rock. The richest mercury ores contain up to 2.5% mercury by mass, and even the leanest concentrated deposits are at least 0.1% mercury (12,000 times average crustal abundance). It is found either as a native metal (rare) or in cinnabar, corderoite, livingstonite and other minerals, with cinnabar (HgS) being the most common ore.[22] Mercury ores usually occur in very young orogenic belts where rocks of high density are forced to the crust of Earth, often in hot springs or other volcanic regions.[23]
Beginning in 1558, with the invention of the patio process to extract silver from ore using mercury, mercury became an essential resource in the economy of Spain and its American colonies. Mercury was used to extract silver from the lucrative mines in New Spain and Peru. Initially, the Spanish Crown's mines in Almadén in Southern Spain supplied all the mercury for the colonies.[24] Mercury deposits were discovered in the New World, and more than 100,000 tons of mercury were mined from the region of Huancavelica, Peru, over the course of three centuries following the discovery of deposits there in 1563. The patio process and later pan amalgamation process continued to create great demand for mercury to treat silver ores until the late 19th century.[25]

Native mercury with cinnabar, Socrates mine, Sonoma County, California. Cinnabar sometimes alters to native mercury in the oxidized zone of mercury deposits.
Former mines in Italy, the United States and Mexico, which once produced a large proportion of the world supply, have now been completely mined out or, in the case of Slovenia (Idrija) and Spain (Almadén), shut down due to the fall of the price of mercury. Nevada's McDermitt Mine, the last mercury mine in the United States, closed in 1992. The price of mercury has been highly volatile over the years and in 2006 was $650 per 76-pound (34.46 kg) flask.[26]
Mercury is extracted by heating cinnabar in a current of air and condensing the vapor. The equation for this extraction is
- HgS + O2 → Hg + SO2
Because of the high toxicity of mercury, both the mining of cinnabar and refining for mercury are hazardous and historic causes of mercury poisoning.[28] In China, prison labor was used by a private mining company as recently as the 1950s to create new cinnabar mercury mines. Thousands of prisoners were used by the Luo Xi mining company to establish new tunnels.[29] Worker health in functioning mines is at high risk.
The European Union directive calling for compact fluorescent bulbs to be made mandatory by 2012 has encouraged China to re-open deadly cinnabar mines to obtain the mercury required for CFL bulb manufacture. As a result, environmental dangers have been a concern, particularly in the southern cities of Foshan and Guangzhou, and in Guizhou province in the southwest.[29]
Abandoned mercury mine processing sites often contain very hazardous waste piles of roasted cinnabar calcines. Water run-off from such sites is a recognized source of ecological damage. Former mercury mines may be suited for constructive re-use. For example, in 1976 Santa Clara County, California purchased the historic Almaden Quicksilver Mine and created a county park on the site, after conducting extensive safety and environmental analysis of the property.[30]
Chemistry
Mercury exists in two main oxidation states, I and II. Higher oxidation states are rare (e.g., mercury(IV) fluoride, HgF4), but have been detected under extraordinary conditions.[31]Compounds of mercury(I)
Different from its lighter neighbors, cadmium and zinc, mercury usually forms simple stable compounds with metal-metal bonds. Most mercury(I) compounds are diamagnetic and feature the dimeric cation, Hg2+2. Stable derivatives include the chloride and nitrate. Treatment of Hg(I) compounds complexation with strong ligands such as sulfide, cyanide, etc. induces disproportionation to Hg2+ and elemental mercury.[32] Mercury(I) chloride, a colorless solid also known as calomel, is really the compound with the formula Hg2Cl2, with the connectivity Cl-Hg-Hg-Cl. It is a standard in electrochemistry. It reacts with chlorine to give mercuric chloride, which resists further oxidation. Mercury(I) hydride, a colorless gas, is the compound with the formula HgH, containing no Hg-Hg bond.
Indicative of its tendency to bond to itself, mercury forms mercury polycations, which consist of linear chains of mercury centers, capped with a positive charge. One example is Hg2+
3(AsF−
6)
2.[33]
Compounds of mercury(II)
Mercury(II) is the most common oxidation state and is the main one in nature as well. All four mercuric halides are known. They form tetrahedral complexes with other ligands but the halides adopt linear coordination geometry, somewhat like Ag+ does. Best known is mercury(II) chloride, an easily sublimating white solid. HgCl2 forms coordination complexes that are typically tetrahedral, e.g. HgCl2−4.
Mercury(II) oxide, the main oxide of mercury, arises when the metal is exposed to air for long periods at elevated temperatures. It reverts to the elements upon heating near 400 °C, as was demonstrated by Joseph Priestley in an early synthesis of pure oxygen.[7] Hydroxides of mercury are poorly characterized, as they are for its neighbors gold and silver.
Being a soft metal, mercury forms very stable derivatives with the heavier chalcogens. Preeminent is mercury(II) sulfide, HgS, which occurs in nature as the ore cinnabar and is the brilliant pigment vermillion. Like ZnS, HgS crystallizes in two forms, the reddish cubic form and the black zinc blende form.[3] Mercury(II) selenide (HgSe) and mercury(II) telluride (HgTe) are also known, these as well as various derivatives, e.g. mercury cadmium telluride and mercury zinc telluride being semiconductors useful as infrared detector materials.[34]
Mercury(II) salts form a variety of complex derivatives with ammonia. These include Millon's base (Hg2N+), the one-dimensional polymer (salts of HgNH+
2)
n), and "fusible white precipitate" or [Hg(NH3)2]Cl2. Known as Nessler's reagent, potassium tetraiodomercurate(II) (HgI2−
4) is still occasionally used to test for ammonia owing to its tendency to form the deeply colored iodide salt of Millon's base.
Mercury fulminate is a detonator widely used in explosives.[3]
Higher oxidation states
Oxidation states above +2 in a non-charged species are extremely rare, although a cyclic mercurinium(IV) cation, with three substituents, may be an intermediate in oxymercuration reactions.[35][36] In 2007, a report of synthesis of a mercury(IV) compound, mercury(IV) fluoride, was published.[37] In the 1970s, there was a claim on synthesis of a mercury(III) compound, but it is now thought to be false.[38]Organomercury compounds
Organic mercury compounds are historically important but are of little industrial value in the western world. Mercury(II) salts are a rare example of simple metal complexes that react directly with aromatic rings. Organomercury compounds are always divalent and usually two-coordinate and linear geometry. Unlike organocadmium and organozinc compounds, organomercury compounds do not react with water. They usually have the formula HgR2, which are often volatile, or HgRX, which are often solids, where R is aryl or alkyl and X is usually halide or acetate. Methylmercury, a generic term for compounds with the formula CH3HgX, is a dangerous family of compounds that are often found in polluted water.[39] They arise by a process known as biomethylation.Applications
Mercury is used primarily for the manufacture of industrial chemicals or for electrical and electronic applications. It is used in some thermometers, especially ones which are used to measure high temperatures. A still increasing amount is used as gaseous mercury in fluorescent lamps, while most of the other applications are slowly phased out due to health and safety regulations and is in some applications replaced with less toxic but considerably more expensive Galinstan alloy.[40]
Medicine
Mercury and its compounds have been used in medicine, although they are much less common today than they once were, now that the toxic effects of mercury and its compounds are more widely understood. The first edition of the Merck's Manual featured many mercuric compounds[41] such as:- Mercauro
- Mercuro-iodo-hemol.
- Mercury-ammonium chloride
- Mercury Benzoate
- Mercuric
- Mercury Bichloride (Corrosive Mercuric Chloride, U.S.P.)
- Mercury Chloride
- Mild Mercury Cyanide
- Mercury Succinimide
- Mercury Iodide
- Red Mercury Biniodide
- Mercury Iodide
- Yellow Mercury Proto-iodide
- Black (Hahnemann), Soluble Mercury Oxide
- Red Mercury Oxide
- Yellow Mercury Oxide
- Mercury Salicylate
- Mercury Succinimide
- Mercury Imido-succinate
- Mercury Sulphate
- Basic Mercury Subsulphate; Turpeth Mineral
- Mercury Tannate
- Mercury-Ammonium Chloride
Another mercury compound, merbromin (Mercurochrome), is a topical antiseptic used for minor cuts and scrapes is still in use in some countries.
Mercury in the form of one of its common ores, cinnabar, is used in various traditional medicines, especially in traditional Chinese medicine. Review of its safety has found that cinnabar can lead to significant mercury intoxication when heated, consumed in overdose, or taken long term, and can have adverse effects at therapeutic doses, though effects from therapeutic doses are typically reversible. Although this form of mercury appears to be less toxic than other forms, its use in traditional Chinese medicine has not yet been justified, as the therapeutic basis for the use of cinnabar is not clear.[45]
Today, the use of mercury in medicine has greatly declined in all respects, especially in developed countries. Thermometers and sphygmomanometers containing mercury were invented in the early 18th and late 19th centuries, respectively. In the early 21st century, their use is declining and has been banned in some countries, states and medical institutions. In 2002, the U.S. Senate passed legislation to phase out the sale of non-prescription mercury thermometers. In 2003, Washington and Maine became the first states to ban mercury blood pressure devices.[46] Mercury compounds are found in some over-the-counter drugs, including topical antiseptics, stimulant laxatives, diaper-rash ointment, eye drops, and nasal sprays. The FDA has "inadequate data to establish general recognition of the safety and effectiveness" of the mercury ingredients in these products.[47] Mercury is still used in some diuretics although substitutes now exist for most therapeutic uses.
Production of chlorine and caustic soda
Chlorine is produced from sodium chloride (common salt, NaCl) using electrolysis to separate the metallic sodium from the chlorine gas. Usually the salt is dissolved in water to produce a brine. By-products of any such chloralkali process are hydrogen (H2) and sodium hydroxide (NaOH), which is commonly called caustic soda or lye. By far the largest use of mercury[48][49] in the late 20th century was in the mercury cell process (also called the Castner-Kellner process) where metallic sodium is formed as an amalgam at a cathode made from mercury; this sodium is then reacted with water to produce sodium hydroxide.[50] Many of the industrial mercury releases of the 20th century came from this process, although modern plants claimed to be safe in this regard.[49] After about 1985, all new chloralkali production facilities that were built in the United States used either membrane cell or diaphragm cell technologies to produce chlorine.Laboratory uses
Some medical thermometers, especially those for high temperatures, are filled with mercury; however, they are gradually disappearing. In the United States, non-prescription sale of mercury fever thermometers has been banned since 2003.[51]Mercury is also found in liquid mirror telescopes.
Some transit telescopes use a basin of mercury to form a flat and absolutely horizontal mirror, useful in determining an absolute vertical or perpendicular reference. Concave horizontal parabolic mirrors may be formed by rotating liquid mercury on a disk, the parabolic form of the liquid thus formed reflecting and focusing incident light. Such telescopes are cheaper than conventional large mirror telescopes by up to a factor of 100, but the mirror cannot be tilted and always points straight up.[52][53][54]
Liquid mercury is a part of popular secondary reference electrode (called the calomel electrode) in electrochemistry as an alternative to the standard hydrogen electrode. The calomel electrode is used to work out the electrode potential of half cells.[55] Last, but not least, the triple point of mercury, −38.8344 °C, is a fixed point used as a temperature standard for the International Temperature Scale (ITS-90).[3]
In polarography both the dropping mercury electrode [56]and the hanging mercury drop electrode [57] use elemental mercury. This use allows a new uncontaminated electrode to be available for each measurement or each new experiment.
Niche uses
Gaseous mercury is used in mercury-vapor lamps and some "neon sign" type advertising signs and fluorescent lamps. Those low-pressure lamps emit very spectrally narrow lines, which are traditionally used in optical spectroscopy for calibration of spectral position. Commercial calibration lamps are sold for this purpose; however simply reflecting some of the fluorescent-lamp ceiling light into a spectrometer is a common calibration practice.[58] Gaseous mercury is also found in some electron tubes, including ignitrons, thyratrons, and mercury arc rectifiers.[59] It is also used in specialist medical care lamps for skin tanning and disinfection (see pictures).[60] Gaseous mercury is added to cold cathode argon-filled lamps to increase the ionization and electrical conductivity. An argon filled lamp without mercury will have dull spots and will fail to light correctly. Lighting containing mercury can be bombarded/oven pumped only once. When added to neon filled tubes the light produced will be inconsistent red/blue spots until the initial burning-in process is completed; eventually it will light a consistent dull off-blue color.[61]-
The deep violet glow of a mercury vapor discharge in a germicidal lamp, whose spectrum is rich in invisible ultraviolet radiation.
-
Skin tanner containing a low-pressure mercury vapor lamp and two infrared lamps, which act both as light source and electrical ballast
Cosmetics
Mercury, as thiomersal, is widely used in the manufacture of mascara. In 2008, Minnesota became the first state in the United States to ban intentionally added mercury in cosmetics, giving it a tougher standard than the federal government.[62]A study in geometric mean urine mercury concentration identified a previously unrecognized source of exposure (skin care products) to inorganic mercury among New York City residents. Population-based biomonitoring also showed that mercury concentration levels are higher in consumers of seafood and fish meals.[63]
Historic uses
Many historic applications made use of the peculiar physical properties of mercury, especially as a dense liquid and a liquid metal:
- Quantities of liquid mercury ranging from 90 to 600 grams (3.2 to 21.2 oz) have been recovered from elite Maya tombs or ritual caches at six sites. This mercury may have been used in bowls as mirrors for divinatory purposes. Five of these date to the Classic Period of Maya civilization (c. 250–900) but one example predated this.[64]
- In Islamic Spain, it was used for filling decorative pools. Later, the American artist Alexander Calder built a mercury fountain for the Spanish Pavilion at the 1937 World Exhibition in Paris. The fountain is now on display at the Fundació Joan Miró in Barcelona.[65]
- Mercury was used inside wobbler lures. Its heavy, liquid form made it useful since the lures made an attractive irregular movement when the mercury moved inside the plug. Such use was stopped due to environmental concerns, but illegal preparation of modern fishing plugs has occurred.
- The Fresnel lenses of old lighthouses used to float and rotate in a bath of mercury which acted like a bearing.[66]
- Mercury sphygmomanometers (blood pressure meter), barometers, diffusion pumps, coulometers, and many other laboratory instruments. As an opaque liquid with a high density and a nearly linear thermal expansion, it is ideal for this role.[67]
- As an electrically conductive liquid, it was used in mercury switches (including home mercury light switches installed prior to 1970), tilt switches used in old fire detectors, and tilt switches in some home thermostats.[68]
- Owing to its acoustic properties, mercury was used as the propagation medium in delay line memory devices used in early digital computers of the mid-20th century.
- Experimental mercury vapor turbines were installed to increase the efficiency of fossil-fuel electrical power plants.[69] The South Meadow power plant in Hartford, CT employed mercury as its working fluid, in a binary configuration with a secondary water circuit, for a number of years starting in the late 1920s in a drive to improve plant efficiency. Several other plants were built, including the Schiller Station in Portsmouth, NH, which went online in 1950. The idea did not catch on industry-wide due to the weight and toxicity of mercury, as well as the advent of supercritical steam plants in later years.[70][71]
- Similarly, liquid mercury was used as a coolant for some nuclear reactors; however, sodium is proposed for reactors cooled with liquid metal, because the high density of mercury requires much more energy to circulate as coolant.[72]
- Mercury was a propellant for early ion engines in electric space propulsion systems. Advantages were mercury's high molecular weight, low ionization energy, low dual-ionization energy, high liquid density and liquid storability at room temperature. Disadvantages were concerns regarding environmental impact associated with ground testing and concerns about eventual cooling and condensation of some of the propellant on the spacecraft in long-duration operations. The first spaceflight to use electric propulsion was a mercury-fueled ion thruster developed by NASA Lewis and flown on the Space Electric Rocket Test "SERT-1" spacecraft launched by NASA at its Wallops Flight Facility in 1964. The SERT-1 flight was followed up by the SERT-2 flight in 1970. Mercury and caesium were preferred propellants for ion engines until Hughes Research Laboratory performed studies finding xenon gas to be a suitable replacement. Xenon is now the preferred propellant for ion engines as it has a high molecular weight, little or no reactivity due to its noble gas nature, and has a high liquid density under mild cryogenic storage.[73][74]
- The mercury battery is a non-rechargeable electrochemical battery, a primary cell, that was common throughout the middle of the 20th century. It was used in a wide variety of applications and was available in various sizes, particularly button sizes. Its constant voltage output and long shelf life gave it a niche use for camera light meters and hearing aids. The mercury cell was effectively banned in most countries in the 1990s due to concerns about the mercury contaminating landfills.[75]
- Mercury was used for preserving wood, developing daguerreotypes, silvering mirrors, anti-fouling paints (discontinued in 1990), herbicides (discontinued in 1995), handheld maze games, cleaning, and road leveling devices in cars. Mercury compounds have been used in antiseptics, laxatives, antidepressants, and in antisyphilitics.
- It was allegedly used by allied spies to sabotage Luftwaffe planes: a mercury paste was applied to bare aluminium, causing the metal to rapidly corrode; this would cause structural failures.[76]
- Chloralkali process: The largest industrial use of mercury during the 20th century was in electrolysis for separating chlorine and sodium from brine; mercury being the anode of the Castner-Kellner process. The chlorine was used for bleaching paper (hence the location of many of these plants near paper mills) while the sodium was used to make sodium hydroxide for soaps and other cleaning products. This usage has largely been discontinued, replaced with other technologies that utilize membrane cells.[77]
- As electrodes in some types of electrolysis, batteries (mercury cells), sodium hydroxide and chlorine production, handheld games, catalysts, insecticides.
- Mercury was once used as a gun barrel bore cleaner.[78][79]
- From the mid-18th to the mid-19th centuries, a process called "carroting" was used in the making of felt hats. Animal skins were rinsed in an orange solution (the term "carroting" arose from this color) of the mercury compound mercuric nitrate, Hg(NO3)2·2H2O.[80] This process separated the fur from the pelt and matted it together. This solution and the vapors it produced were highly toxic. The United States Public Health Service banned the use of mercury in the felt industry in December 1941. The psychological symptoms associated with mercury poisoning inspired the phrase "mad as a hatter". Lewis Carroll's "Mad Hatter" in his book Alice's Adventures in Wonderland was a play on words based on the older phrase, but the character himself does not exhibit symptoms of mercury poisoning.[81]
- Gold and silver mining. Historically, mercury was used extensively in hydraulic gold mining in order to help the gold to sink through the flowing water-gravel mixture. Thin gold particles may form mercury-gold amalgam and therefore increase the gold recovery rates.[3] Large-scale use of mercury stopped in the 1960s. However, mercury is still used in small scale, often clandestine, gold prospecting. It is estimated that 45,000 metric tons of mercury used in California for placer mining have not been recovered.[82] Mercury was also used in silver mining.[83]
Historic medicinal uses
Mercury(I) chloride (also known as calomel or mercurous chloride) has been used in traditional medicine as a diuretic, topical disinfectant, and laxative. Mercury(II) chloride (also known as mercuric chloride or corrosive sublimate) was once used to treat syphilis (along with other mercury compounds), although it is so toxic that sometimes the symptoms of its toxicity were confused with those of the syphilis it was believed to treat.[84] It is also used as a disinfectant. Blue mass, a pill or syrup in which mercury is the main ingredient, was prescribed throughout the 19th century for numerous conditions including constipation, depression, child-bearing and toothaches.[85] In the early 20th century, mercury was administered to children yearly as a laxative and dewormer, and it was used in teething powders for infants. The mercury-containing organohalide merbromin (sometimes sold as Mercurochrome) is still widely used but has been banned in some countries such as the U.S.[86]Toxicity and safety
Mercury and most of its compounds are extremely toxic and must be handled with care; in cases of spills involving mercury (such as from certain thermometers or fluorescent light bulbs), specific cleaning procedures are used to avoid exposure and contain the spill.[87] Protocols call for physically merging smaller droplets on hard surfaces, combining them into a single larger pool for easier removal with an eyedropper, or for gently pushing the spill into a disposable container. Vacuum cleaners and brooms cause greater dispersal of the mercury and should not be used. Afterwards, fine sulfur, zinc, or some other powder that readily forms an amalgam (alloy) with mercury at ordinary temperatures is sprinkled over the area before itself being collected and properly disposed of.
Cleaning porous surfaces and clothing is not effective at removing all traces of mercury and it is therefore advised to discard these kinds of items should they be exposed to a mercury spill.Mercury can be absorbed through the skin and mucous membranes and mercury vapors can be inhaled, so containers of mercury are securely sealed to avoid spills and evaporation. Heating of mercury, or of compounds of mercury that may decompose when heated, is always carried out with adequate ventilation in order to avoid exposure to mercury vapor. The most toxic forms of mercury are its organic compounds, such as dimethylmercury and methylmercury. Mercury can cause both chronic and acute poisoning.
Preindustrial deposition rates of mercury from the atmosphere may be about 4 ng /(1 L of ice deposit). Although that can be considered a natural level of exposure, regional or global sources have significant effects. Volcanic eruptions can increase the atmospheric source by 4–6 times.[88]
Natural sources, such as volcanoes, are responsible for approximately half of atmospheric mercury emissions. The human-generated half can be divided into the following estimated percentages:[89][90][91]
Recent atmospheric mercury contamination in outdoor urban air was measured at 0.01–0.02 µg/m3. A 2001 study measured mercury levels in 12 indoor sites chosen to represent a cross-section of building types, locations and ages in the New York area. This study found mercury concentrations significantly elevated over outdoor concentrations, at a range of 0.0065 – 0.523 μg/m3. The average was 0.069 μg/m3.[93]
Mercury also enters into the environment through the improper disposal (e.g., land filling, incineration) of certain products. Products containing mercury include: auto parts, batteries, fluorescent bulbs, medical products, thermometers, and thermostats.[94] Due to health concerns (see below), toxics use reduction efforts are cutting back or eliminating mercury in such products. For example, the amount of mercury sold in thermostats in the United States decreased from 14.5 tons in 2004 to 3.9 tons in 2007.[95]
Most thermometers now use pigmented alcohol instead of mercury, and galinstan alloy thermometers are also an option. Mercury thermometers are still occasionally used in the medical field because they are more accurate than alcohol thermometers, though both are commonly being replaced by electronic thermometers and less commonly by galinstan thermometers. Mercury thermometers are still widely used for certain scientific applications because of their greater accuracy and working range.
Historically, one of the largest releases was from the Colex plant, a lithium-isotope separation plant at Oak Ridge, Tennessee. The plant operated in the 1950s and 1960s. Records are incomplete and unclear, but government commissions have estimated that some two million pounds of mercury are unaccounted for.[96]
A serious industrial disaster was the dumping of mercury compounds into Minamata Bay, Japan. It is estimated that over 3,000 people suffered various deformities, severe mercury poisoning symptoms or death from what became known as Minamata disease.[97][98]
Case control studies have shown effects such as tremors, impaired cognitive skills, and sleep disturbance in workers with chronic exposure to mercury vapor even at low concentrations in the range 0.7–42 μg/m3.[99][100] A study has shown that acute exposure (4 – 8 hours) to calculated elemental mercury levels of 1.1 to 44 mg/m3 resulted in chest pain, dyspnea, cough, hemoptysis, impairment of pulmonary function, and evidence of interstitial pneumonitis.[101] Acute exposure to mercury vapor has been shown to result in profound central nervous system effects, including psychotic reactions characterized by delirium, hallucinations, and suicidal tendency. Occupational exposure has resulted in broad-ranging functional disturbance, including erethism, irritability, excitability, excessive shyness, and insomnia. With continuing exposure, a fine tremor develops and may escalate to violent muscular spasms. Tremor initially involves the hands and later spreads to the eyelids, lips, and tongue. Long-term, low-level exposure has been associated with more subtle symptoms of erethism, including fatigue, irritability, loss of memory, vivid dreams and depression.[102][103]
The United States Clean Air Act, passed in 1990, put mercury on a list of toxic pollutants that need to be controlled to the greatest possible extent. Thus, industries that release high concentrations of mercury into the environment agreed to install maximum achievable control technologies (MACT). In March 2005, the EPA promulgated a regulation[111] that added power plants to the list of sources that should be controlled and instituted a national cap and trade system. States were given until November 2006 to impose stricter controls, but after a legal challenge from several states, the regulations were struck down by a federal appeals court on 8 February 2008. The rule was deemed not sufficient to protect the health of persons living near coal-fired power plants, given the negative effects documented in the EPA Study Report to Congress of 1998.[112]
The EPA announced new rules for coal-fired power plants on 22 December 2011.[113] Cement kilns that burn hazardous waste are held to a looser standard than are standard hazardous waste incinerators in the United States, and as a result are a disproportionate source of mercury pollution.[114]
There are restrictions for mercury concentration in packaging (the limit is 100 ppm for sum of mercury, lead, hexavalent chromium and cadmium) and batteries (the limit is 5 ppm).[116] In July 2007, the European Union also banned mercury in non-electrical measuring devices, such as thermometers and barometers. The ban applies to new devices only, and contains exemptions for the health care sector and a two-year grace period for manufacturers of barometers. [117]
Releases in the environment
Preindustrial deposition rates of mercury from the atmosphere may be about 4 ng /(1 L of ice deposit). Although that can be considered a natural level of exposure, regional or global sources have significant effects. Volcanic eruptions can increase the atmospheric source by 4–6 times.[88]
Natural sources, such as volcanoes, are responsible for approximately half of atmospheric mercury emissions. The human-generated half can be divided into the following estimated percentages:[89][90][91]
- 65% from stationary combustion, of which coal-fired power plants are the largest aggregate source (40% of U.S. mercury emissions in 1999). This includes power plants fueled with gas where the mercury has not been removed. Emissions from coal combustion are between one and two orders of magnitude higher than emissions from oil combustion, depending on the country.[89]
- 11% from gold production. The three largest point sources for mercury emissions in the U.S. are the three largest gold mines. Hydrogeochemical release of mercury from gold-mine tailings has been accounted as a significant source of atmospheric mercury in eastern Canada.[92]
- 6.8% from non-ferrous metal production, typically smelters.
- 6.4% from cement production.
- 3.0% from waste disposal, including municipal and hazardous waste, crematoria, and sewage sludge incineration.
- 3.0% from caustic soda production.
- 1.4% from pig iron and steel production.
- 1.1% from mercury production, mainly for batteries.
- 2.0% from other sources.
Recent atmospheric mercury contamination in outdoor urban air was measured at 0.01–0.02 µg/m3. A 2001 study measured mercury levels in 12 indoor sites chosen to represent a cross-section of building types, locations and ages in the New York area. This study found mercury concentrations significantly elevated over outdoor concentrations, at a range of 0.0065 – 0.523 μg/m3. The average was 0.069 μg/m3.[93]
Mercury also enters into the environment through the improper disposal (e.g., land filling, incineration) of certain products. Products containing mercury include: auto parts, batteries, fluorescent bulbs, medical products, thermometers, and thermostats.[94] Due to health concerns (see below), toxics use reduction efforts are cutting back or eliminating mercury in such products. For example, the amount of mercury sold in thermostats in the United States decreased from 14.5 tons in 2004 to 3.9 tons in 2007.[95]
Most thermometers now use pigmented alcohol instead of mercury, and galinstan alloy thermometers are also an option. Mercury thermometers are still occasionally used in the medical field because they are more accurate than alcohol thermometers, though both are commonly being replaced by electronic thermometers and less commonly by galinstan thermometers. Mercury thermometers are still widely used for certain scientific applications because of their greater accuracy and working range.
Historically, one of the largest releases was from the Colex plant, a lithium-isotope separation plant at Oak Ridge, Tennessee. The plant operated in the 1950s and 1960s. Records are incomplete and unclear, but government commissions have estimated that some two million pounds of mercury are unaccounted for.[96]
A serious industrial disaster was the dumping of mercury compounds into Minamata Bay, Japan. It is estimated that over 3,000 people suffered various deformities, severe mercury poisoning symptoms or death from what became known as Minamata disease.[97][98]
Occupational exposure
Due to the health effects of mercury exposure, industrial and commercial uses are regulated in many countries. The World Health Organization, OSHA, and NIOSH all treat mercury as an occupational hazard, and have established specific occupational exposure limits. Environmental releases and disposal of mercury are regulated in the U.S. primarily by the United States Environmental Protection Agency.Case control studies have shown effects such as tremors, impaired cognitive skills, and sleep disturbance in workers with chronic exposure to mercury vapor even at low concentrations in the range 0.7–42 μg/m3.[99][100] A study has shown that acute exposure (4 – 8 hours) to calculated elemental mercury levels of 1.1 to 44 mg/m3 resulted in chest pain, dyspnea, cough, hemoptysis, impairment of pulmonary function, and evidence of interstitial pneumonitis.[101] Acute exposure to mercury vapor has been shown to result in profound central nervous system effects, including psychotic reactions characterized by delirium, hallucinations, and suicidal tendency. Occupational exposure has resulted in broad-ranging functional disturbance, including erethism, irritability, excitability, excessive shyness, and insomnia. With continuing exposure, a fine tremor develops and may escalate to violent muscular spasms. Tremor initially involves the hands and later spreads to the eyelids, lips, and tongue. Long-term, low-level exposure has been associated with more subtle symptoms of erethism, including fatigue, irritability, loss of memory, vivid dreams and depression.[102][103]
Treatment
Research on the treatment of mercury poisoning is limited. Currently available drugs for acute mercurial poisoning include chelators N-acetyl-D, L-penicillamine (NAP), British Anti-Lewisite (BAL), 2,3-dimercapto-1-propanesulfonic acid (DMPS), and dimercaptosuccinic acid (DMSA). In one small study including 11 construction workers exposed to elemental mercury, patients were treated with DMSA and NAP.[104] Chelation therapy with both drugs resulted in the mobilization of a small fraction of the total estimated body mercury. DMSA was able to increase the excretion of mercury to a greater extent than NAP.[105]Fish
Fish and shellfish have a natural tendency to concentrate mercury in their bodies, often in the form of methylmercury, a highly toxic organic compound of mercury. Species of fish that are high on the food chain, such as shark, swordfish, king mackerel, bluefin tuna, albacore tuna, and tilefish contain higher concentrations of mercury than others. As mercury and methylmercury are fat soluble, they primarily accumulate in the viscera, although they are also found throughout the muscle tissue.[106] When this fish is consumed by a predator, the mercury level is accumulated. Since fish are less efficient at depurating than accumulating methylmercury, fish-tissue concentrations increase over time. Thus species that are high on the food chain amass body burdens of mercury that can be ten times higher than the species they consume. This process is called biomagnification. Mercury poisoning happened this way in Minamata, Japan, now called Minamata disease.Regulations
International
140 countries agreed in the Minamata Convention on Mercury by the United Nations Environment Program (UNEP) to prevent emissions. [107] Convention is expected to be open for signature in October 2013.[108]United States
In the United States, the Environmental Protection Agency is charged with regulating and managing mercury contamination. Several laws give the EPA this authority, including the Clean Air Act, the Clean Water Act, the Resource Conservation and Recovery Act, and the Safe Drinking Water Act. Additionally, the Mercury-Containing and Rechargeable Battery Management Act, passed in 1996, phases out the use of mercury in batteries, and provides for the efficient and cost-effective disposal of many types of used batteries.[109] North America contributed approximately 11% of the total global anthropogenic mercury emissions in 1995.[110]The United States Clean Air Act, passed in 1990, put mercury on a list of toxic pollutants that need to be controlled to the greatest possible extent. Thus, industries that release high concentrations of mercury into the environment agreed to install maximum achievable control technologies (MACT). In March 2005, the EPA promulgated a regulation[111] that added power plants to the list of sources that should be controlled and instituted a national cap and trade system. States were given until November 2006 to impose stricter controls, but after a legal challenge from several states, the regulations were struck down by a federal appeals court on 8 February 2008. The rule was deemed not sufficient to protect the health of persons living near coal-fired power plants, given the negative effects documented in the EPA Study Report to Congress of 1998.[112]
The EPA announced new rules for coal-fired power plants on 22 December 2011.[113] Cement kilns that burn hazardous waste are held to a looser standard than are standard hazardous waste incinerators in the United States, and as a result are a disproportionate source of mercury pollution.[114]
European Union
In the European Union, the directive on the Restriction of the Use of Certain Hazardous Substances in Electrical and Electronic Equipment (see RoHS) bans mercury from certain electrical and electronic products, and limits the amount of mercury in other products to less than 1000 ppm.[115]There are restrictions for mercury concentration in packaging (the limit is 100 ppm for sum of mercury, lead, hexavalent chromium and cadmium) and batteries (the limit is 5 ppm).[116] In July 2007, the European Union also banned mercury in non-electrical measuring devices, such as thermometers and barometers. The ban applies to new devices only, and contains exemptions for the health care sector and a two-year grace period for manufacturers of barometers. [117]