- The political and technical support for the program in the United States was too thin geographically. Within the United States, only in Oak Ridge, Tennessee, was the technology well understood.[6]
- The MSR program was in competition with the fast breeder program at the time, which got an early start and had copious government development funds allocated to many parts of the United States. When the MSR development program had progressed far enough to justify an expanded program leading to commercial development, the AEC could not justify the diversion of substantial funds from the LMFBR to a competing program.[6]
- Inherently safe design (safety by passive components and the strong negative temperature coefficient of reactivity of some designs). In some designs, the fuel and the coolant are the same fluid, so a loss of coolant removes the reactor's fuel. Unlike steam, fluoride salts dissolve poorly in water, and do not form burnable hydrogen. Unlike steel and solid uranium oxide, molten salts are not damaged by the core's neutron bombardment.
- A low-pressure MSR lacks a LWR's high-pressure radioactive steam and therefore do not experience leaks of radioactive steam and cooling water, and the expensive containment, steel core vessel, piping and safety equipment needed to contain radioactive steam.
- MSRs make closed nuclear fuel cycles cheaper and more practical. If fully implemented, a closed nuclear fuel cycle reduces environmental impacts: The chemical separation makes long-lived actinides back into reactor fuel. The discharged wastes are mostly fission products (nuclear ashes) with short half-lives. This reduces the needed geologic containment to 300 years rather than the tens of thousands of years needed by a light-water reactor's spent nuclear fuel. It also permits society to use more-abundant nuclear fuels.
- The fuel's liquid phase might be pyroprocessed to separate fission products (nuclear ashes) from actinide fuels. This may have advantages over conventional reprocessing, though much development is still needed.
- Fuel rods are not required.
- In new solid-fueled reactor designs, the longest-lead item is the safety testing of fuel element designs. Fuel tests usually must cover several three-year refueling cycles. However, several molten salt fuels have already been validated.
- Some designs can "burn" problematic transuranic elements from traditional solid-fuel nuclear reactors.
- An MSR can react to load changes in less than 60 seconds (unlike "traditional" solid-fuel nuclear power plants that suffer from xenon poisoning).
- Molten salt reactors can run at high temperatures, yielding high production efficiency. This reduces the size, expense and environmental impacts of a power plant.
- MSRs can offer a high "specific power," that is high power at a low mass as demonstrated by the ARE.[3] Simplified MSR power plants may be suitable for ships.
- A possibly good neutron economy makes the MSR attractive for the neutron poor thorium fuel cycle.
- LWR's (and most other solid-fuel reactors) have no fundamental "off switch", but once the initial criticality is overcome, an MSR is comparatively easy and fast to turn off by letting the freeze plug melt.
- Little development compared to most Gen IV designs .
- Required onsite chemical plant to manage core mixture and remove fission products.
- Required regulatory changes to deal with radically different design features.
- MSR designs rely on nickel-based alloys to hold the molten salt. Alloys based on nickel and iron are prone to embrittlement under high neutron flux.[65](p83)
- Corrosion risk.[70]
- As a breeder reactor, a modified MSR might be able to produce weapons-grade nuclear material.[71]
- The MSRE and aircraft nuclear reactors used enrichment levels so high that they approach the levels of nuclear weapons. These levels would be illegal in most modern regulatory regimes for power plants. Some modern designs avoid this issue.[72]
- Neutron damage to solid moderator materials can limit the core lifetime of an MSR that makes moderately fast neutrons. For example, the MSRE was designed so that its graphite moderator sticks had very low tolerances, so neutron damage could change their size without damage. "Two fluid" MSR designs are unable to use graphite piping because graphite changes size when it is bombarded with neutrons, and graphite pipes would crack and leak.
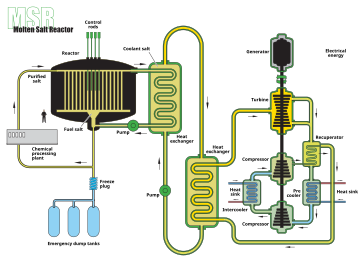
Example of a molten salt reactor scheme
A molten salt reactor (MSR) is a class of generation IV nuclear fission reactor in which the primary nuclear reactor coolant, or even the fuel itself, is a molten salt mixture. MSRs can run at higher temperatures than water-cooled reactors for a higher thermodynamic efficiency, while staying at low vapour pressure.
The nuclear fuel may be solid or dissolved in the coolant. In many designs the nuclear fuel dissolved in the coolant is uranium tetrafluoride (UF4). The fluid becomes critical in a graphite core that serves as the moderator. Some solid-fuel designs propose ceramic fuel dispersed in a graphite matrix, with the molten salt providing low pressure, high temperature cooling. The salts are much more efficient than compressed helium (another potential coolant in Generation IV reactor designs) at removing heat from the core, reducing the need for pumping and piping and reducing the core size.
The concept was established in the 1950s. The early Aircraft Reactor Experiment was primarily motivated by the small size that the design could provide, while the Molten-Salt Reactor Experiment was a prototype for a thorium fuel cycle breeder nuclear power plant. The increased research into Generation IV reactor designs included a renewed interest in the technology.[2]
History
Aircraft reactor experiment
Aircraft Reactor Experiment building at ORNL. It was later retrofitted for the MSRE.
Extensive research into molten salt reactors started with the U.S. aircraft reactor experiment (ARE) in support of the U.S. Aircraft Nuclear Propulsion program. The ARE was a 2.5 MWth nuclear reactor experiment designed to attain a high energy density for use as an engine in a nuclear-powered bomber.
The project included experiments, including high temperature reactor and engine tests collectively called the Heat Transfer Reactor Experiments: HTRE-1, HTRE-2 and HTRE-3 at the National Reactor Test Station (now Idaho National Laboratory) as well as an experimental high-temperature molten salt reactor at Oak Ridge National Laboratory – the ARE.
The ARE used molten fluoride salt NaF-ZrF4-UF4 (53-41-6 mol%) as fuel, moderated by beryllium oxide (BeO). Liquid sodium was a secondary coolant.
The experiment had a peak temperature of 860 °C. It produced 100 MWh over nine days in 1954. This experiment used Inconel 600 alloy for the metal structure and piping.[3]
After ARE, another reactor was operated at the Critical Experiments Facility of the Oak Ridge National Laboratory in 1957. It was part of the circulating-fuel reactor program of the Pratt & Whitney Aircraft Company (PWAC). This was called the PWAR-1, the Pratt and Whitney Aircraft Reactor-1. The experiment was run for only a few weeks and at essentially zero nuclear power, but it reached criticality. The operating temperature was held constant at approximately 675 °C (1,250 °F). The PWAR-1 used NaF-ZrF4-UF4 as the primary fuel and coolant, making it one of the three critical molten salt reactors ever built.[4]
Molten-salt reactor experiment
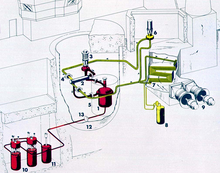
MSRE plant diagram
Oak Ridge National Laboratory (ORNL) took the lead in researching the MSR through the 1960s. Much of their work culminated with the Molten-Salt Reactor Experiment (MSRE). The MSRE was a 7.4 MWth test reactor simulating the neutronic "kernel" of a type of epithermal thorium molten salt breeder reactor called the liquid fluoride thorium reactor. The large (expensive) breeding blanket of thorium salt was omitted in favor of neutron measurements. The MSRE was located at ORNL. Its piping, core vat and structural components were made from Hastelloy-N, moderated by pyrolytic graphite. It went critical in 1965 and ran for four years. The fuel for the MSRE was LiF-BeF2-ZrF4-UF4 (65-29-5-1). The graphite core moderated it. Its secondary coolant was FLiBe (2LiF-BeF2). It reached temperatures as high as 650 °C and operated for the equivalent of about 1.5 years of full power operation.
Oak Ridge National Laboratory molten salt breeder reactor
The culmination of the Oak Ridge National Laboratory research during the 1970–1976 timeframe resulted in a proposed molten salt breeder reactor (MSBR) design which would use LiF-BeF2-ThF4-UF4 (72-16-12-0.4) as fuel. It was to be moderated by graphite with a 4-year replacement schedule. The secondary coolant was to be NaF-NaBF4. Its peak operating temperature was to be 705 °C.[5] Despite the success, the MSR program closed down in the early 1970s in favor of the liquid metal fast-breeder reactor (LMFBR),[6] after which research stagnated in the United States.[7][8] As of 2011, the ARE and the MSRE remained the only molten-salt reactors ever operated.The MSBR project received funding until 1976. Inflation-adjusted to 1991 dollars, the project received $38.9 million from 1968 to 1976.[9]
Officially, the program was cancelled because:
Oak Ridge National Laboratory denatured molten salt reactor (DMSR)
In 1980, the engineering technology division at Oak Ridge National Laboratory published a paper entitled "Conceptual Design Characteristics of a Denatured Molten-Salt Reactor with Once-Through Fueling." In it, the authors "examine the conceptual feasibility of a molten-salt power reactor fueled with denatured uranium-235 (i.e. with low-enriched uranium) and operated with a minimum of chemical processing." The main priority behind the design characteristics is proliferation resistance.[10] Lessons learned from past projects and research at ORNL were considered. Although the DMSR can theoretically be fueled partially by thorium or plutonium, fueling solely with low enriched uranium (LEU) helps maximize proliferation resistance.Another important goal of the DMSR was to minimize R&D and to maximize feasibility. The Generation IV international Forum (GIF) includes "salt processing" as a technology gap for molten salt reactors.[11] The DMSR requires minimal chemical processing because it is a burner rather than a breeder. Both reactors built at ORNL were burner designs. In addition, the choices to use graphite for neutron moderation and enhanced Hastelloy-N for piping simplify the design and reduce R&D.
United Kingdom
The UK's Atomic Energy Research Establishment (AERE) were developing an alternative MSR design across its National Laboratories at Harwell, Culham, Risley and Winfrith. AERE opted to focus on a lead-cooled 2.5 GWe Molten Salt Fast Reactor (MSFR) concept using a chloride.[12] They also researched the option of helium gas as an alternative coolant.The UK MSFR would be fuelled by plutonium, a fuel considered to be 'free' by the program's research scientists, because of the UK's plutonium stockpile.
Despite their different designs, ORNL and AERE maintained contact during this period with information exchange and expert visits. Theoretical work on the concept was conducted between 1964 and 1966, while experimental work was ongoing between 1968 and 1973. The program received annual government funding of around £100,000-£200,000 (equivalent to £2m-£3m in 2005). This funding came to an end in 1974, partly due to the success of the Prototype Fast Reactor at Dounreay which was considered a priority for funding as it went critical in the same year.[12]
AERE reports and findings from its MSR Program conducted in the 1960s and 1970s are available for public viewing at the UK National Archives in Kew, London.[12]
Soviet Union
In the USSR, a molten-salt reactor research program was started in the second half of the 1970s at the Kurchatov Institute. It included theoretical and experimental studies, particularly the investigation of mechanical, corrosion and radiation properties of the molten salt container materials. The main findings supported the conclusion that there were no physical nor technological obstacles to the practical implementation of MSRs.[15] A reduction in activity occurred after 1986 due to the Chernobyl accident, along with a general stagnation of nuclear power and the nuclear industry.[16](p381)Twenty-first century
Canada
Terrestrial Energy Inc. (TEI), a Canadian-based company, is developing a DMSR design called the Integral Molten Salt Reactor (IMSR). The IMSR is designed to be deployable as a small modular reactor (SMR). Their design currently undergoing licensing is 400MW thermal (190MW electrical). With high operating temperatures, the IMSR has applications in industrial heat markets as well as traditional power markets. The main design features include neutron moderation from graphite, fueling with low-enriched uranium and a compact and replaceable Core-unit. Decay heat is removed passively using nitrogen (with air as an emergency alternative). The latter feature permits the operational simplicity necessary for industrial deployment.[17]Terrestrial has completed the first phase of a prelicensing review by the Canadian Nuclear Safety Commission, which provides a regulatory opinion that the design features are generally safe enough to eventually obtain a license to construct the reactor.[18]
China
Under Jiang Mianheng's direction, China initiated a thorium molten-salt reactor research project. It was formally announced at the Chinese Academy of Sciences (CAS) annual conference in January 2011.[19] A 100-MW demonstrator of the solid fuel version (TMSR-SF), based on pebble bed technology, was to be ready by 2024. Initially, a 10-MW pilot and a larger demonstrator of the liquid fuel (TMSR-LF) variant were targeted for 2024 and 2035 respectively.[20][21] However, China has accelerated its program to build two 12 MW reactors underground at Wuwei research facilities in Gansu Province by 2020.[22] Heat from the reaction will be used to produce electricity, hydrogen, industrial chemicals, drinking water through desalination, and minerals.[22] The project also seeks to test new corrosion resistant materials.[22]In 2017, ANSTO/Shanghai Institute Of Applied Physics announced the creation of a NiMo-SiC alloy for use in molten salt reactors.[23][24]
Denmark
Seaborg Technologies, a company based in Denmark, is developing the core for a Molten Salt Waste-burner (MSW). The MSW is a high temperature, single salt, thermal MSR designed to go critical on a combination of thorium and nuclear waste from conventional nuclear reactors. The MSW design is modular. The reactor core is estimated to be replaced every 6–10 years. However, the fuel will not be replaced and will burn for the entire power plant lifetime. The first version of the Seaborg core is planned to produce 50 MWth power and could consume approximately 1 ton (not considering natural decays) of transuranic waste over its 60 years power plant lifetime. After 60 years the 233U concentration in the fuel salt is high enough to initiate a closed thorium fuel cycle in the next generation power plant.[25]France
The CNRS project EVOL (Evaluation and viability of liquid fuel fast reactor system) project, with the objective of proposing a design of the MSFR (Molten Salt Fast Reactor),[26] released its final report in 2014.[27] The various molten salt reactor projects like FHR, MOSART, MSFR, and TMSR have common themes in basic R&D areas, according to a 2014 paper giving an overview of the MSR in a GenV context.[28] Another paper gives an overview of the MSFR.[29] More resources are available in the MSFR bibliography.[30]The EVOL project will be continued by the EU-funded SAMOFAR (Safety Assessment of the Molten Salt Fast Reactor) project, in which several European research institutes and universities collaborate[31].
India
Ratan Kumar Sinha, Chairman of Atomic Energy Commission of India, stated in 2013: "India is also investigating Molten Salt Reactor (MSR) technology. We have molten salt loops operational at BARC."[32]Japan
The Fuji Molten Salt Reactor is a 100 to 200 MWe LFTR, using technology similar to the Oak Ridge project. A consortium including members from Japan, the U.S. and Russia are developing the project. The project would likely take 20 years to develop a full size reactor,[33] but the project seems to lack funding.[34]United Kingdom
The Alvin Weinberg Foundation is a British non-profit organization founded in 2011, dedicated to raising awareness about the potential of thorium energy and LFTR. It was formally launched at the House of Lords on 8 September 2011.[35][36][37] It is named after American nuclear physicist Alvin M. Weinberg, who pioneered thorium molten salt reactor research.A study on MSRs completed in July 2015 by Energy Process Developments, funded by Innovate UK, summarizes MSR activity internationally. It looks at the feasibility of developing a pilot scale demonstration MSR in the UK. A review of potential UK sites is given along with an insight into the UK regulatory process for innovative reactor technology. The technical review of six MSR designs led to the selection of the Stable Salt Reactor, designed by Moltex Energy, as most suitable for UK implementation.[38] However, Moltex "failed to get engagement from the UK government quickly enough" and so will target Canada instead.[39]
United States
Idaho National Laboratory designed a molten-salt-cooled, molten-salt-fuelled reactor with a prospective output of 1000 MWe.[40]Kirk Sorensen, former NASA scientist and chief nuclear technologist at Teledyne Brown Engineering, is a long-time promoter of the thorium fuel cycle, coining the term liquid fluoride thorium reactor. In 2011, Sorensen founded Flibe Energy, a company aimed at developing 20–50 MW LFTR reactor designs to power military bases. (It is easier to approve novel military designs than civilian power station designs in today's US nuclear regulatory environment).
Transatomic Power was created by Ph.D. students from MIT including CEO Leslie Dewan and Mark Massie, and Russ Wilcox of E Ink.[45] They are pursuing what they term a Waste-Annihilating Molten Salt Reactor (acronym WAMSR), intending to consume existing spent nuclear fuel.[46] Transatomic received venture capital funding in early 2015.[47]
In January 2016, the United States Department of Energy announced a $80m award fund to develop Generation IV reactor designs.[48] One of the two beneficiaries, Southern Company will use the funding to develop a Molten Chloride Fast Reactor (MCFR), a type of MSR developed earlier by British scientists.[12]
Variants
Liquid-salt very-high-temperature reactor
It is essentially a standard VHTR design that uses liquid salt as a coolant in the primary loop, rather than a single helium loop. It relies on "TRISO" fuel dispersed in graphite. Early AHTR research focused on graphite would be in the form of graphite rods that would be inserted in hexagonal moderating graphite blocks, but current studies focus primarily on pebble-type fuel. The LS-VHTR has many attractive features, including the ability to work at very high temperatures (the boiling point of most molten salt candidates is >1400 °C); low-pressure cooling that can be used to more easily match hydrogen production facility conditions (most thermochemical cycles require temperatures in excess of 750 °C); better electric conversion efficiency than a helium-cooled VHTR operating at similar conditions; passive safety systems and better retention of fission products in the event of an accident.[citation needed] This concept is now referred to as "fluoride salt-cooled high-temperature reactor" (FHR).[49]Liquid fluoride thorium reactor (LFTR)
Advocates estimate that five hundred metric tons of thorium could supply all U.S. energy needs for one year.[50] The U.S. Geological Survey estimates that the largest known U.S. thorium deposit, the Lemhi Pass district on the Montana-Idaho border, contains thorium reserves of 64,000 metric tons.[51]Molten-salt fueling options
The LFTR design was strongly supported by Alvin Weinberg, who patented the light-water reactor and was a director of the U.S.'s Oak Ridge National Laboratory. In 2016 Nobel prize winning physicist Carlo Rubbia, former Director General of CERN, claimed that one of the main reasons why research was cut is that thorium is difficult to turn into a nuclear weapon.[52]Alternatives to thorium include enriched uranium-235 or fissile material from dismantled nuclear weapons.[53]Thorium is not for tomorrow but unless you do any development, it will not get there. — Dr Carlo Rubbia, Nobel Laureate and former Director General of CERN, January 2016[52]
Molten-salt-cooled reactors
Molten-salt-cooled solid-fuel reactors are quite different from molten-salt-fueled reactors. They are called "molten salt reactor system" in the Generation IV proposal, also called Molten Salt Converter Reactor (MSCR). These reactors were additionally referred to as advanced high-temperature reactors (AHTRs), but since about 2010 the preferred DOE designation is fluoride high-temperature reactors (FHR).[54]The FHR concept cannot reprocess fuel easily and has fuel rods that need to be fabricated and validated, delaying deployment by up to twenty years[citation needed] from project inception. However, since it uses fabricated fuel, reactor manufacturers can still profit by selling fuel assemblies.
The FHR retains the safety and cost advantages of a low-pressure, high-temperature coolant, also shared by liquid metal cooled reactors. Notably, steam is not created in the core (as is present in BWRs), and no large, expensive steel pressure vessel (as required for PWRs). Since it can operate at high temperatures, the conversion of the heat to electricity can use an efficient, lightweight Brayton cycle gas turbine.
Much of the current research on FHRs is focused on small, compact heat exchangers that reduce molten salt volumes and associated costs.[55]
Molten salts can be highly corrosive and corrosivity increases with temperature. For the primary cooling loop, a material is needed that can withstand corrosion at high temperatures and intense radiation. Experiments show that Hastelloy-N and similar alloys are suited to these tasks at operating temperatures up to about 700 °C. However, operating experience is limited. Still higher operating temperatures are desirable – at 850 °C thermochemical production of hydrogen becomes possible. Materials for this temperature range have not been validated, though carbon composites, molybdenum alloys (e.g. TZM), carbides, and refractory metal based or ODS alloys might be feasible.
Dual-fluid molten salt reactors
A prototypical example of a dual fluid reactor is the lead-cooled, salt-fueled reactor.Fused salt selection
Molten FLiBe
The salt mixtures are chosen to make the reactor safer and more practical. Fluoride salts are favored, because fluorine has only one stable isotope (F-19), and does not easily become radioactive under neutron bombardment. Both of these make fluorine better than chlorine, which has two stable isotopes (Cl-35 and Cl-37), as well as a slow-decaying isotope between them which facilitates neutron absorption by Cl-35. Compared to chlorine and other halides, fluorine also absorbs fewer neutrons and slows ("moderates") neutrons better. Low-valence fluorides boil at high temperatures, though many pentafluorides and hexafluorides boil at low temperatures. They also must be very hot before they break down into their constituent elements. Such molten salts are "chemically stable" when maintained well below their boiling points.
On the other hand, some salts are so useful that isotope separation of the halide is worthwhile. Chlorides permit fast breeder reactors to be constructed using molten salts. Much less research has been done on reactor designs using chloride salts. Chlorine, unlike fluorine, must be purified to isolate the heavier stable isotope, chlorine-37, thus reducing production of sulfur tetrafluoride that occurs when chlorine-35 absorbs a neutron to become chlorine-36, then degrades by beta decay to sulfur-36.
Similarly, any lithium present in a salt mixture must be in the form of purified lithium-7, because lithium-6 effectively captures neutrons and produces tritium. Even if pure 7Li is used, salts containing lithium will cause significant tritium production, comparable with heavy water reactors.
Reactor salts are usually close to eutectic mixtures to reduce their melting point. A low melting point simplifies melting the salt at startup and reduces the risk of the salt freezing as it is cooled in the heat exchanger.
Due to the high "redox window" of fused fluoride salts, the redox potential of the fused salt system can be changed. Fluorine-Lithium-Beryllium ("FLiBe") can be used with beryllium additions to lower the redox potential and almost eliminate corrosion. However, since beryllium is extremely toxic, special precautions must be engineered into the design to prevent its release into the environment. Many other salts can cause plumbing corrosion, especially if the reactor is hot enough to make highly reactive hydrogen.
To date, most research has focused on FLiBe, because lithium and beryllium are reasonably effective moderators and form a eutectic salt mixture with a lower melting point than each of the constituent salts. Beryllium also performs neutron doubling, improving the neutron economy. This process occurs when the beryllium nucleus re-emits two neutrons after absorbing a single neutron. For the fuel carrying salts, generally 1% or 2% (by mole) of UF4 is added. Thorium and plutonium fluorides have also been used.
| Material | Total neutron capture relative to graphite (per unit volume) |
Moderating ratio (Avg. 0.1 to 10 eV) |
|---|---|---|
| Heavy water | 0.2 | 11449 |
| ZrH[57][58][59] | ~0.2 | ~0 if <0 .14="" ev="" if="">0.14 eV |
| Light water | 75 | 246 |
| Graphite | 1 | 863 |
| Sodium | 47 | 2 |
| UCO | 285 | 2 |
| UO2 | 3583 | 0.1 |
| 2LiF–BeF2 | 8 | 60 |
| LiF–BeF2–ZrF4 (64.5–30.5–5) | 8 | 54 |
| NaF–BeF2 (57–43) | 28 | 15 |
| LiF–NaF–BeF2 (31–31–38) | 20 | 22 |
| LiF–ZrF4 (51–49) | 9 | 29 |
| NaF–ZrF4 (59.5–40.5) | 24 | 10 |
| LiF-NaF–ZrF4 (26–37–37) | 20 | 13 |
| KF–ZrF4 (58–42) | 67 | 3 |
| RbF–ZrF4 (58–42) | 14 | 13 |
| LiF–KF (50–50) | 97 | 2 |
| LiF–RbF (44–56) | 19 | 9 |
| LiF–NaF–KF (46.5–11.5–42) | 90 | 2 |
| LiF–NaF–RbF (42–6–52) | 20 | 8 |
Fused salt purification
Techniques for preparing and handling molten salt were first developed at Oak Ridge National Lab.[60] The purpose of salt purification was to eliminate oxides, sulfur and metal impurities. Oxides could result in the deposition of solid particles in reactor operation. Sulfur had to be removed because of its corrosive attack on nickel-based alloys at operational temperature. Structural metal such as chromium, nickel, and iron had to be removed for corrosion control.A water content reduction purification stage using HF and helium sweep gas was specified to run at 400 °C. Oxide and sulfur contamination in the salt mixtures were removed using gas sparging of HF – H2 mixture, with the salt heated to 600 °C.[60](p8) Structural metal contamination in the salt mixtures were removed using hydrogen gas sparging, at 700 °C.[60](p26) Solid ammonium hydrofluoride was proposed as a safer alternative for oxide removal.[61]
Fused salt processing
The possibility of online processing can be an MSR advantage. Continuous processing would reduce the inventory of fission products, control corrosion and improve neutron economy by removing fission products with high neutron absorption cross-section, especially xenon. This makes the MSR particularly suited to the neutron-poor thorium fuel cycle. Online fuel processing can introduce risks of fuel processing accidents,[62](p15) which can trigger release of radio isotopes.In some thorium breeding scenarios, the intermediate product protactinium-233 would be removed from the reactor and allowed to decay into highly pure uranium-233, an attractive bomb-making material. More modern designs propose to use a lower specific power or a separate large thorium breeding blanket. This dilutes the protactinium to such an extent that few protactinium atoms absorb a second neutron or, via a (n, 2n) reaction (in which an incident neutron is not absorbed but instead knocks a neutron out of the nucleus), generate uranium-232. Because U-232 has a short half-life and its decay chain contains hard gamma emitters, it makes the isotopic mix of uranium less attractive for bomb-making. This benefit would come with the added expense of a larger fissile inventory or a 2-fluid design with a large quantity of blanket salt.
The necessary fuel salt reprocessing technology has been demonstrated, but only at laboratory scale. A prerequisite to full-scale commercial reactor design is the R&D to engineer an economically competitive fuel salt cleaning system.
Fissile fuel reprocessing issues
Changes in the composition of a MSR fast neutron (kg/GW)
Reprocessing refers to the chemical separation of fissionable uranium and plutonium from spent nuclear fuel.[63] The recovery of uranium or plutonium could increase the risk of nuclear proliferation. In the United States the regulatory regime has varied dramatically in different administrations.[63]
In the original 1971 Molten Salt Breeder Reactor proposal, uranium reprocessing was scheduled every ten days as part of reactor operation.[64](p181) Subsequently, a once-through fueling design was proposed that limited uranium reprocessing to every thirty years at the end of useful salt life.[65](p98) A mixture of uranium-238 was called for to make sure recovered uranium would not be weapons grade. This design is referred to as denatured molten salt reactor.[66] If reprocessing were to be prohibited then the uranium would be disposed with other fission products.
Comparison to light water reactors
MSRs, especially those with the fuel dissolved in the salt differ considerably from conventional reactors. Reactor core pressure can be low and the temperature much higher. In this respect an MSR is more similar to a liquid metal cooled reactor than to a conventional light water cooled reactor. MSRs are often planned as breeding reactors with a closed fuel cycle – as opposed to the once-through fuel currently used in U.S. nuclear reactors.Safety concepts rely on a negative temperature coefficient of reactivity and a large possible temperature rise to limit reactivity excursions. As an additional method for shutdown, a separate, passively cooled container below the reactor can be included. In case of problems and for regular maintenance the fuel is drained from the reactor. This stops the nuclear reaction and acts as another second cooling system. Neutron-producing accelerators have been proposed for some super-safe subcritical experimental designs.[67]
Cost estimates from the 1970s were slightly lower than for conventional light-water reactors.[68]
The temperatures of some proposed designs are high enough to produce process heat for hydrogen production or other chemical reactions. Because of this, they are included in the GEN-IV roadmap for further study.[69]