| Chagas disease | |
|---|---|
| Other names | American trypanosomiasis |
 | |
| Photomicrograph of Giemsa-stained Trypanosoma cruzi | |
| Pronunciation |
|
| Specialty | Infectious disease |
| Symptoms | Fever, large lymph nodes, headache |
| Complications | Heart failure, enlarged esophagus, enlarged colon |
| Causes | Trypanosoma cruzi spread by kissing bugs |
| Diagnostic method | Finding the parasite, its DNA, or antibodies in the blood |
| Prevention | Eliminating kissing bugs and avoiding their bites |
| Medication | Benznidazole, nifurtimox |
| Frequency | 6.2 million (2017) |
| Deaths | 7,900 (2017) |
Chagas disease, also known as American trypanosomiasis, is a tropical parasitic disease caused by Trypanosoma cruzi. It is spread mostly by b-y insects known as Triatominae, or "kissing bugs". The symptoms change over the course of the infection. In the early stage, symptoms are typically either not present or mild, and may include fever, swollen lymph nodes, headaches, or swelling at the site of the bite. After four to eight weeks, untreated individuals enter the chronic phase of disease, which in most cases does not result in further symptoms. Up to 45% of people with chronic infection develop heart disease 10–30 years after the initial illness, which can lead to heart failure. Digestive complications, including an enlarged esophagus or an enlarged colon, may also occur in up to 21% of people, and up to 10% of people may experience nerve damage.
T. cruzi is commonly spread to humans and other mammals by the bite of a kissing bug. The disease may also be spread through blood transfusion, organ transplantation, eating food contaminated with the parasites, and vertical transmission (from a mother to her baby). Diagnosis of early disease is by finding the parasite in the blood using a microscope or detecting its DNA by polymerase chain reaction. Chronic disease is diagnosed by finding antibodies for T. cruzi in the blood. It affects more than 150 types of animals.
Prevention focuses on eliminating kissing bugs and avoiding their bites. This may involve the use of insecticides or bed-nets. Other preventive efforts include screening blood used for transfusions. As of 2019, a vaccine has not been developed. Early infections are treatable with the medications benznidazole or nifurtimox, which usually cure the disease if given shortly after the person is infected, but become less effective the longer a person has had Chagas disease. When used in chronic disease, medication may delay or prevent the development of end–stage symptoms. Benznidazole and nifurtimox often cause side effects, including skin disorders, digestive system irritation, and neurological symptoms, which can result in treatment being discontinued. As of 2019, new drugs for Chagas disease are under development, and experimental vaccines have been studied in animal models.
It is estimated that 6.2 million people, mostly in Mexico, Central America and South America, have Chagas disease as of 2017, resulting in an estimated 7,900 deaths. Most people with the disease are poor, and most do not realize they are infected. Large-scale population migrations have carried Chagas disease to new regions, which now include the United States and many European countries.
The disease was first described in 1909 by Brazilian physician Carlos Chagas, after whom it is named. Chagas disease is classified as a neglected tropical disease.
Signs and symptoms
Chagas disease occurs in two stages: an acute stage, which develops one to two weeks after the insect bite, and a chronic stage, which develops over many years. The acute stage is often symptom-free. When present, the symptoms are typically minor and not specific to any particular disease. Signs and symptoms include fever, malaise, headache, and enlargement of the liver, spleen, and lymph nodes. Rarely, people develop a swollen nodule at the site of infection, which is called "Romaña's sign" if it is on the eyelid, or a "chagoma" if it is elsewhere on the skin. In rare cases (less than 1–5%), infected individuals develop severe acute disease, which can cause life-threatening fluid accumulation around the heart, or inflammation of the heart or brain and surrounding tissues. The acute phase typically lasts four to eight weeks and resolves without treatment.
Unless treated with antiparasitic drugs, individuals remain chronically infected with T. cruzi after recovering from the acute phase. Most chronic infections are asymptomatic, which is referred to as indeterminate chronic Chagas disease. However, over decades with chronic Chagas disease, 30–40% of people develop organ dysfunction (determinate chronic Chagas disease), which most often affects the heart or digestive system.
The most common manifestation is heart disease, which occurs in 14–45% of people with chronic Chagas disease. People with Chagas heart disease often experience heart palpitations and sometimes fainting due to irregular heart function. By electrocardiogram, people with Chagas heart disease most frequently have arrhythmias. As the disease progresses, the heart's ventricles become enlarged (dilated cardiomyopathy), which reduces its ability to pump blood. In many cases the first sign of Chagas heart disease is heart failure, thromboembolism, or chest pain associated with abnormalities in the microvasculature.
Also common in chronic Chagas disease is damage to the digestive system, particularly enlargement of the esophagus or colon, which affects 10–21% of people. Those with enlarged esophagus often experience pain (odynophagia) or trouble swallowing (dysphagia), acid reflux, cough, and weight loss. Individuals with enlarged colon often experience constipation, which can lead to severe blockage of the intestine or its blood supply. Up to 10% of chronically infected individuals develop nerve damage that can result in numbness and altered reflexes or movement. While chronic disease typically develops over decades, some individuals with Chagas disease (less than 10%) progress to heart damage directly after acute disease.
Signs and symptoms differ for people infected with T. cruzi through less common routes. People infected through ingestion of parasites tend to develop severe disease within three weeks of consumption, with symptoms including fever, vomiting, shortness of breath, cough, and pain in the chest, abdomen, and muscles. Those infected congenitally typically have few to no symptoms, but can have mild non-specific symptoms, or severe symptoms such as jaundice, respiratory distress, and heart problems. People infected through organ transplant or blood transfusion tend to have symptoms similar to those of vector-borne disease, but the symptoms may not manifest for anywhere from a week to five months. Chronically infected individuals who become immunosuppressed due to HIV infection can suffer particularly severe and distinct disease, most commonly characterized by inflammation in the brain and surrounding tissue or brain abscesses. Symptoms vary widely based on the size and location of brain abscesses, but typically include fever, headaches, seizures, loss of sensation, or other neurological issues that indicate particular sites of nervous system damage. Occasionally, these individuals also experience acute heart inflammation, skin lesions, and disease of the stomach, intestine, or peritoneum.
Cause
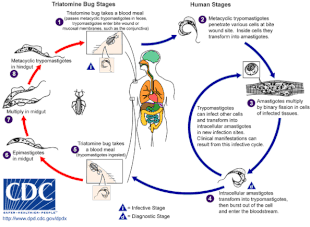
Chagas disease is caused by infection with the protozoan parasite T. cruzi, which is typically introduced into humans through the bite of triatomine bugs, also called "kissing bugs". At the bite site, motile T. cruzi forms called trypomastigotes invade various host cells. Inside a host cell, the parasite transforms into a replicative form called an amastigote, which undergoes several rounds of replication. The replicated amastigotes transform back into trypomastigotes, which burst the host cell and are released into the bloodstream. Trypomastigotes then disseminate throughout the body to various tissues, where they invade cells and replicate. Over many years, cycles of parasite replication and immune response can severely damage these tissues, particularly the heart and digestive tract.
Transmission
T. cruzi can be transmitted by various triatomine bugs in the genera Triatoma, Panstrongylus, and Rhodnius. The primary vectors for human infection are the species of triatomine bugs that inhabit human dwellings, namely Triatoma infestans, Rhodnius prolixus, Triatoma dimidiata and Panstrongylus megistus. These insects are known by a number of local names, including vinchuca in Argentina, Bolivia, Chile and Paraguay, barbeiro (the barber) in Brazil, pito in Colombia, chinche in Central America, and chipo in Venezuela. The bugs tend to feed at night, preferring moist surfaces near the eyes or mouth. A triatomine bug can become infected with T. cruzi when it feeds on an infected host. T. cruzi replicates in the insect's intestinal tract and is shed in the bug's feces. When an infected triatomine feeds, it pierces the skin and takes in a blood meal, defecating at the same time to make room for the new meal. The bite is typically painless, but causes itching. Scratching at the bite introduces the T. cruzi-laden feces into the bite wound, initiating infection.
In addition to classical vector spread, Chagas disease can be transmitted through food or drink contaminated with triatomine insects or their feces. Since heating or drying kills the parasites, drinks and especially fruit juices are the most frequent source of infection. This route of transmission has been implicated in several outbreaks, where it led to unusually severe symptoms, likely due to infection with a higher parasite load than from the bite of a triatomine bug.
T. cruzi can also be transmitted independent of the triatomine bug during blood transfusion, following organ transplantation, or across the placenta during pregnancy. Transfusion with the blood of an infected donor infects the recipient 10–25% of the time. To prevent this, blood donations are screened for T. cruzi in many countries with endemic Chagas disease, as well as the United States. Similarly, transplantation of solid organs from an infected donor can transmit T. cruzi to the recipient. This is especially true for heart transplant, which transmits T. cruzi 75–100% of the time, and less so for transplantation of the liver (0–29%) or a kidney (0–19%). An infected mother can also pass T. cruzi to her child through the placenta; this occurs in up to 15% of births by infected mothers. As of 2019, 22.5% of new infections occurred through congenital transmission.
Pathophysiology
In the acute phase of the disease, signs and symptoms are caused directly by the replication of T. cruzi and the immune system's response to it. During this phase, T. cruzi can be found in various tissues throughout the body and circulating in the blood. During the initial weeks of infection, parasite replication is brought under control by production of antibodies and activation of the host's inflammatory response, particularly cells that target intracellular pathogens such as NK cells and macrophages, driven by inflammation-signaling molecules like TNF-α and IFN-γ.
During chronic Chagas disease, long-term organ damage develops over years due to continued replication of the parasite and damage from the immune system. Early in the course of the disease, T. cruzi is found frequently in the striated muscle fibers of the heart. As disease progresses, the heart becomes generally enlarged, with substantial regions of cardiac muscle fiber replaced by scar tissue and fat. Areas of active inflammation are scattered throughout the heart, with each housing inflammatory immune cells, typically macrophages and T cells. Late in the disease, parasites are rarely detected in the heart, and may be present at only very low levels.
In the heart, colon, and esophagus, chronic disease also leads to a massive loss of nerve endings. In the heart, this may contribute to arrythmias and other cardiac dysfunction. In the colon and esophagus, loss of nervous system control is the major driver of organ dysfunction. Loss of nerves impairs the movement of food through the digestive tract, which can lead to blockage of the esophagus or colon and restriction of their blood supply.
Diagnosis
The presence of T. cruzi is diagnostic of Chagas disease. During the acute phase of infection, it can be detected by microscopic examination of fresh anticoagulated blood, or its buffy coat, for motile parasites; or by preparation of thin and thick blood smears stained with Giemsa, for direct visualization of parasites. Blood smear examination detects parasites in 34–85% of cases. Techniques such as microhematocrit centrifugation can be used to concentrate the blood, which makes the test more sensitive. On microscopic examination, T. cruzi trypomastigotes have a slender body, often in the shape of an S or U, with a flagellum connected to the body by an undulating membrane.
Alternatively, T. cruzi DNA can be detected by polymerase chain reaction (PCR). In acute and congenital Chagas disease, PCR is more sensitive than microscopy, and it is more reliable than antibody-based tests for the diagnosis of congenital disease because it is not affected by transfer of antibodies against T. cruzi from a mother to her baby (passive immunity). PCR is also used to monitor T. cruzi levels in organ transplant recipients and immunosuppressed people, which allows infection or reactivation to be detected at an early stage.
During the chronic phase, microscopic diagnosis is unreliable and PCR is less sensitive because the level of parasites in the blood is low. Chronic Chagas disease is usually diagnosed using serological tests, which detect immunoglobulin G antibodies against T. cruzi in the person's blood. The most common test methodologies are ELISA, indirect immunofluorescence, and indirect hemagglutination. Two positive serology results, using different test methods, are required to confirm the diagnosis. If the test results are inconclusive, additional testing methods such as Western blot can be used. T. cruzi antigens may also be detected in tissue samples using immunohistochemistry techniques.
Various rapid diagnostic tests for Chagas disease are available. These tests are easily transported and can be performed by people without special training. They are useful for screening large numbers of people and testing people who cannot access healthcare facilities, but their sensitivity is relatively low, and it is recommended that a second method is used to confirm a positive result.
T. cruzi can be isolated from samples through blood culture or xenodiagnosis, or by inoculating animals with the person's blood. In the blood culture method, the person's red blood cells are separated from the plasma and added to a specialized growth medium to encourage multiplication of the parasite. It can take up to six months to obtain the result. Xenodiagnosis involves feeding the person's blood to triatomine insects, then examining their feces for the parasite 30 to 60 days later. These methods are not routinely used, as they are slow and have low sensitivity.
Prevention
Efforts to prevent Chagas disease have largely focused on vector control to limit exposure to triatomine bugs. Insecticide-spraying programs have been the mainstay of vector control, consisting of spraying homes and the surrounding areas with residual insecticides. This was originally done with organochlorine, organophosphate, and carbamate insecticides, which were supplanted in the 1980s with pyrethroids. These programs have drastically reduced transmission in Brazil and Chile, and eliminated major vectors from certain regions: Triatoma infestans from Brazil, Chile, Uruguay, and parts of Peru and Paraguay, as well as Rhodnius prolixus from Central America. Vector control in some regions has been hindered by the development of insecticide resistance among triatomine bugs. In response, vector control programs have implemented alternative insecticides (e.g. fenitrothion and bendiocarb in Argentina and Bolivia), treatment of domesticated animals (which are also fed on by triatomine bugs) with pesticides, pesticide-impregnated paints, and other experimental approaches. In areas with triatomine bugs, transmission of T. cruzi can be prevented by sleeping under bed nets and by housing improvements that prevent triatomine bugs from colonizing houses.
Blood transfusion was formerly the second-most common mode of transmission for Chagas disease. T. cruzi can survive in refrigerated stored blood, and can survive freezing and thawing, allowing it to persist in whole blood, packed red blood cells, granulocytes, cryoprecipitate, and platelets. The development and implementation of blood bank screening tests has dramatically reduced the risk of infection during blood transfusion. Nearly all blood donations in Latin American countries undergo Chagas screening. Widespread screening is also common in non-endemic nations with significant populations of immigrants from endemic areas including the United Kingdom (implemented in 1999), Spain (2005), the United States (2007), France and Sweden (2009), Switzerland (2012), and Belgium (2013). Blood is tested using serological tests, typically ELISAs, to detect antibodies against T. cruzi proteins.
Other modes of transmission have also been targeted by Chagas disease prevention programs. Treating T. cruzi-infected mothers during pregnancy reduces the risk of congenital transmission of the infection. To this end, many countries in Latin America have implemented routine screening of pregnant women and infants for T. cruzi infection, and the World Health Organization recommends screening all children born to infected mothers to prevent congenital infection from developing into chronic disease. Similarly to blood transfusions, many countries with endemic Chagas disease screen organs for transplantation with serological tests.
There is no vaccine against Chagas disease. Several experimental vaccines have been tested in animals infected with T. cruzi and were able to reduce parasite numbers in the blood and heart, but no vaccine candidates had undergone clinical trials in humans as of 2016.
Management
Chagas disease is managed using antiparasitic drugs to eliminate T. cruzi from the body and symptomatic treatment to address the effects of the infection. As of 2018, benznidazole and nifurtimox were the antiparasitic drugs of choice for treating Chagas disease, though benznidazole is the only drug available in most of Latin America. For either drug, treatment typically consists of two to three oral doses per day for 60 to 90 days. Antiparasitic treatment is most effective early in the course of infection: it eliminates T. cruzi from 50 to 80% of people in the acute phase, but only 20–60% of those in the chronic phase. Treatment of chronic disease is more effective in children than in adults, and the cure rate for congenital disease approaches 100% if treated in the first year of life. Antiparasitic treatment can also slow the progression of the disease and reduce the possibility of congenital transmission. Elimination of T. cruzi does not cure the cardiac and gastrointestinal damage caused by chronic Chagas disease, so these conditions must be treated separately. Antiparasitic treatment is not recommended for people who have already developed dilated cardiomyopathy.
Benznidazole is usually considered the first-line treatment because it has milder adverse effects than nifurtimox and its efficacy is better understood. Both benznidazole and nifurtimox have common side effects that can result in treatment being discontinued. The most common side effects of benznidazole are skin rash, digestive problems, decreased appetite, weakness, headache, and sleeping problems. These side effects can sometimes be treated with antihistamines or corticosteroids, and are generally reversed when treatment is stopped. However, benzidazole is discontinued in up to 29% of cases. Nifurtimox has more frequent side effects, affecting up to 97.5% of individuals taking the drug. The most common side effects are loss of appetite, weight loss, nausea and vomiting, and various neurological disorders including mood changes, insomnia, paresthesia and peripheral neuropathy. Treatment is discontinued in up to 75% of cases. Both drugs are contraindicated for use in pregnant women and people with liver or kidney failure. As of 2019, resistance to these drugs has been reported.
Complications
In the chronic stage, treatment involves managing the clinical manifestations of the disease. The treatment of Chagas cardiomyopathy is similar to that of other forms of heart disease. Beta blockers and ACE inhibitors may be prescribed, but some people with Chagas disease may not be able to take the standard dose of these drugs because they have low blood pressure or a low heart rate. To manage irregular heartbeats, people may be prescribed anti-arrhythmic drugs such as amiodarone, or have a pacemaker implanted. Blood thinners may be used to prevent thromboembolism and stroke. Chronic heart disease caused by Chagas is a common reason for heart transplantation surgery. Because transplant recipients take immunosuppressive drugs to prevent organ rejection, they are monitored using PCR to detect reactivation of the disease. People with Chagas disease who undergo heart transplantation have higher survival rates than the average heart transplant recipient.
Mild gastrointestinal disease can be treated symptomatically, such as by using laxatives for constipation, or taking a prokinetic drug like metoclopramide before meals to relieve esophageal symptoms. Surgery to sever the muscles of the lower esophageal sphincter (cardiomyotomy) is indicated in more severe cases of esophageal disease, and surgical removal of the affected part of the organ may be required for advanced megacolon and megaesophagus.
Epidemiology
In 2017, an estimated 6.2 million people worldwide had Chagas disease, with approximately 162,000 new infections and 7,900 deaths each year. This resulted in a global annual economic burden estimated at US$7.2 billion, 86% of which is borne by endemic countries. Chagas disease results in the loss of over 800,000 disability-adjusted life years each year.
Chagas is endemic to 21 countries in continental Latin America: Argentina, Belize, Bolivia, Brazil, Chile, Colombia, Costa Rica, Ecuador, El Salvador, French Guiana, Guatemala, Guyana, Honduras, Mexico, Nicaragua, Panama, Paraguay, Peru, Suriname, Uruguay, and Venezuela. The endemic area ranges from the southern United States to northern Chile and Argentina, with Bolivia (6.1%), Argentina (3.6%), and Paraguay (2.1%) exhibiting the highest prevalence of the disease. In endemic areas, due largely to vector control efforts and screening of blood donations, annual infections and deaths have fallen by 67% and more than 73% respectively from their peaks in the 1980s to 2010. Transmission by insect vector and blood transfusion has been completely interrupted in Uruguay (1997), Chile (1999), and Brazil (2006), and in Argentina, vectorial transmission has been interrupted in 13 of the 19 endemic provinces. During Venezuela's humanitarian crisis, vectorial transmission has begun occurring in areas where it had previously been interrupted and Chagas disease seroprevalence rates have increased. Transmission rates have also risen in the Gran Chaco region due to insecticide resistance and in the Amazon basin due to oral transmission.
While the rate of vector-transmitted Chagas disease has declined throughout most of Latin America, the rate of orally transmitted disease has risen, possibly due to increasing urbanization and deforestation bringing people into closer contact with triatomines and altering the distribution of triatomine species. Orally transmitted Chagas disease is of particular concern in Venezuela, where 16 outbreaks have been recorded between 2007 and 2018.
Chagas exists in two different ecological zones: In the Southern Cone region, the main vector lives in and around human homes. In Central America and Mexico, the main vector species lives both inside dwellings and in uninhabited areas. In both zones, Chagas occurs almost exclusively in rural areas, where T. cruzi also circulates in wild and domestic animals. T. cruzi commonly infects more than 100 species of mammals across Latin America including opossums, armadillos, marmosets, bats, and various rodents, all of which can be infected by the vectors or orally by eating triatomine bugs and other infected animals.
Non-endemic countries
Though Chagas is traditionally considered a disease of rural Latin America, international migration has dispersed those suffering from the disease to numerous non-endemic countries, primarily in North America and Europe. As of 2020, approximately 300,000 infected people are living in the United States, about 30,000 to 40,000 of whom have Chagas cardiomyopathy. The vast majority of Chagas infections in the United States occur in immigrants from Latin America, but local transmission is possible. Eleven triatomine species are native to the United States and some southern states have persistent cycles of disease transmission between insect vectors and animal reservoirs, which include woodrats, possums, raccoons, armadillos and skunks. However, locally acquired infection is very rare: only 28 cases were documented from 1955 to 2015. As of 2013, the cost of treatment in the United States was estimated to be US$900 million annually (global cost $7 billion), which included hospitalization and medical devices such as pacemakers.
Chagas disease affects approximately 68,000 to 123,000 people in Europe as of 2019. Spain, which has a high rate of immigration from Latin America, has the highest prevalence of the disease. It is estimated that 50,000 to 70,000 Spanish people are living with the disease, which accounts for 75% of European cases. The prevalence of Chagas varies widely within European countries due to differing immigration patterns. Italy has the second highest prevalence, followed by the Netherlands, the United Kingdom, and Germany.
History
T. cruzi likely circulated in South American mammals long before the arrival of humans on the continent. T. cruzi has been detected in ancient human remains across South America, from a 9000-year-old Chinchorro mummy in the Atacama Desert, to remains of various ages in Minas Gerais, to an 1100-year-old mummy as far north as the Chihuahuan Desert near the Rio Grande. Many early written accounts describe symptoms consistent with Chagas disease, with early descriptions of the disease sometimes attributed to Miguel Diaz Pimenta (1707), Luís Gomes Ferreira (1735), and Theodoro J. H. Langgaard (1842).
The formal description of Chagas disease was made by Carlos Chagas in 1909 after examining a two-year-old girl with fever, swollen lymph nodes, and an enlarged spleen and liver. Upon examination of her blood, Chagas saw trypanosomes identical to those he had recently identified from the hindgut of triatomine bugs and named Trypanosoma cruzi in honor of his mentor, Brazilian physician Oswaldo Cruz. He sent infected triatomine bugs to Cruz in Rio de Janeiro, who showed the bite of the infected triatomine could transmit T. cruzi to marmoset monkeys as well. In just two years, 1908 and 1909, Chagas published descriptions of the disease, the organism that caused it, and the insect vector required for infection. Almost immediately thereafter, at the suggestion of Miguel Couto, then professor of the Faculdade de Medicina do Rio de Janeiro, the disease was widely referred to as "Chagas disease". Chagas' discovery brought him national and international renown, but in highlighting the inadequacies of the Brazilian government's response to the disease, Chagas attracted criticism to himself and to the disease that bore his name, stifling research on his discovery and likely frustrating his nomination for the Nobel Prize in 1921.
In the 1930s, Salvador Mazza rekindled Chagas disease research, describing over a thousand cases in Argentina's Chaco Province. In Argentina, the disease is known as mal de Chagas-Mazza in his honor. Serological tests for Chagas disease were introduced in the 1940s, demonstrating that infection with T. cruzi was widespread across Latin America. This, combined with successes eliminating the malaria vector through insecticide use, spurred the creation of public health campaigns focused on treating houses with insecticides to eradicate triatomine bugs. The 1950s saw the discovery that treating blood with crystal violet could eradicate the parasite, leading to its widespread use in transfusion screening programs in Latin America. Large-scale control programs began to take form in the 1960s, first in São Paulo, then various locations in Argentina, then national-level programs across Latin America. These programs received a major boost in the 1980s with the introduction of pyrethroid insecticides, which did not leave stains or odors after application and were longer-lasting and more cost-effective. Regional bodies dedicated to controlling Chagas disease arose through support of the Pan American Health Organization, with the Initiative of the Southern Cone for the Elimination of Chagas Diseases launching in 1991, followed by the Initiative of the Andean countries (1997), Initiative of the Central American countries (1997), and the Initiative of the Amazon countries (2004).
Research
Treatments
Fexinidazole, an antiparasitic drug approved for treating African trypanosomiasis, has shown activity against Chagas disease in animal models. As of 2019, it is undergoing phase II clinical trials for chronic Chagas disease in Spain. Other drug candidates include GNF6702, a proteasome inhibitor that is effective against Chagas disease in mice and is undergoing preliminary toxicity studies, and AN4169, which has had promising results in animal models.
A number of experimental vaccines have been tested in animals. Some approaches have used inoculation with dead or attenuated T. cruzi parasites or non-pathogenic organisms that share antigens with T. cruzi, such as Trypanosoma rangeli or Phytomonas serpens. DNA vaccination has also been explored. As of 2019, vaccine research has mainly been limited to small animal models, and further testing in large animals is needed.
Diagnostic tests
As of 2018, standard diagnostic tests for Chagas disease were limited in their ability to measure the response to antiparasitic treatment. Serological tests, for example, may remain positive for years after T. cruzi is eliminated from the body, and PCR may give false-negative results when parasitemia is low. Various potential biomarkers of treatment response are under investigation, such as immunoassays against specific T. cruzi antigens, flow cytometry testing to detect antibodies against different life stages of T. cruzi, and markers of physiological changes caused by the parasite, such as alterations in coagulation and lipid metabolism.
Another research area is the use of biomarkers to predict the progression of chronic Chagas disease. Serum levels of tumor necrosis factor alpha, brain and atrial natriuretic peptide, and angiotensin-converting enzyme 2, markers of heart damage and inflammation, have been found to correlate with the severity of Chagas cardiomyopathy. Endothelin 1 has been studied as a prognostic marker in animal models.
T. cruzi shed acute-phase antigen (SAPA), which can be detected in blood using ELISA or Western blot, has been used as an indicator of early acute and congenital infection. A novel assay for T. cruzi antigens in urine has been developed to diagnose congenital disease.