In biology, cell signaling (cell signalling in British English), or cell-cell communication, governs the basic activities of cells and coordinates multiple-cell actions. A signal is an entity that codes or conveys information. Biological processes are complex molecular interactions that involve many signals. The ability of cells to perceive and correctly respond to their microenvironment is the basis of development, tissue repair, and immunity, as well as normal tissue homeostasis. Errors in signaling interactions and cellular information processing may cause diseases such as cancer, autoimmunity, and diabetes. By understanding cell signaling, clinicians may treat diseases more effectively and, theoretically, researchers may develop artificial tissues.
All cells receive and respond to signals from their surroundings. This is accomplished by a variety of signal molecules that are secreted or expressed on the surface of one cell and bind to a receptor expressed by the other cells, thereby integrating and coordinating the function of the many individual cells that make up organisms. Each cell is programmed to respond to specific extracellular signal molecules. Extracellular signaling usually entails the following steps:
- Synthesis and release of the signaling molecule by the signaling cell;
- Transport of the signal to the target cell;
- Binding of the signal by a specific receptor leading to its activation;
- Initiation of signal-transduction pathways.
Signaling agents could be physical agents like mechanical pressure, voltage, temperature, and light or chemical agents like peptides, steroids, terpenoids, etc. It may be food material or pathogen-associated patterns, or it may be oxygen or carbon dioxide levels or specially biosynthesised signaling molecules like hormones and ferromones (ektohormones). Signaling molecules vary greatly in their physio-chemical properties such as solubility (hydrophobic or hydrophillic). Some of the signaling molecules are gaseous, such as nitric oxide. Additionally, proteins on the surface of neighboring cells could also be signals.
Synthesis involves various biosynthetic pathways, and happens in specific time and place. Signal molecules may be released from the cell and sometimes they are not released at all, such as cellular localization signals and DNA damage signals. Such intracellular signaling networks work within the cell. Signal molecules that can be released through various ways like membrane-diffusion, exocytosis or cell damage. In some cases the signal molecules remain attached with cell-surface, a mode that helps in juxtacrine signaling (discussed below). Sometimes signal molecules require activation, such as through proteolytic cleavage or covalent modifications.
The ultimate path of the signal may be intracellular or intercellular. The intercellular signaling is also called cell-to-cell communication. It can be short or long distance. Based on nature of this path of signal molecule from source to target cell; the signaling pathways are classified into autocrine, juxtacrine, intracrine, paracrine and endocrine.
Receptors play a key role in cell signaling. Receptors help in recognising the signal molecule (ligand). However some receptor molecules respond to physical agents (voltage, light, etc). Receptor molecules are generally proteins. Receptors may be located at cell surface, or interior of the cell such as cytosol, the organelles and nucleus (especially the transcription factors). Usually the cell surface receptors bind membrane-impermeable signal molecules, but sometimes they also interact with membrane permeable signal molecules. A key step in signaling is removal and degradation of the signal molecule. Sometimes the receptor is also degraded. Neurotransmitter reuptake is a mechanism of signaling molecule removal that is commonly seen in nervous system, and is a target of some class of prescription psychiatric medications.
Binding with the ligand causes conformational change in the receptor, which leads to further transmission of signaling. Due to conformational change, the receptor may either show an enzymic activity (called enzymic receptor), or a ion channel opening or closing activity (called a channel receptor). Sometimes the receptors themselves do not contain enzymatic or channel-like domains but they are linked with enzyme or transporter. Some receptors (like the nuclear-cytoplasmic superfamily) have a different mechanism. Once they bind with signal, they change their DNA binding properties and cellular localisation to the nucleus.
Result of enzymatic activity of the receptor usually leads to recruitment of additional molecular changes thus causing a signal transduction "cascade". These intermediates often forms a second messenger system. Within the signal transduction cascade there may be enzymes and transporters which work similar way as receptors. Enzymatic activities include covalent modifications like proteolytic cleavage, phosphorylation/dephosphorylation, methylation/demethylation, ubiquitinylation/deubiquitinylation etc. These changes help regulate the propagation of the signal through the cell. An important phenomena that happens in the intracellular portion of signaling is signal amplification. During signal amplification, a few number of receptors are initially activated. The intracellular response results in multiple secondary messengers to be activated, thereby amplifying the initial signal.
Systems biology studies the underlying structure of cell-signaling networks and how changes in these networks may affect the transmission and flow of information (signal transduction). Such networks are complex systems in their organization and may exhibit a number of emergent properties, including bistability and ultrasensitivity. Analysis of cell-signaling networks requires a combination of experimental and theoretical approaches, including the development and analysis of simulations and modeling. Long-range allostery is often a significant component of cell-signaling events.
Between individual organisms of same species
Cell signaling has been most extensively studied in the context of human diseases and signaling between cells of a single organism. However, cell signaling may also occur between the cells of two different individuals of the same species. In many mammals, early embryo cells exchange signals with cells of the uterus. In the human gastrointestinal tract, bacteria exchange signals with each other and with human epithelial and immune system cells. For the yeast Saccharomyces cerevisiae during mating, some cells send a peptide signal (mating factor pheromones) into their environment. The mating factor peptide may bind to a cell surface receptor on other yeast cells and induce them to prepare for mating.
Classification
Cell signaling can be classified as either mechanical or biochemical based on the type of the signal. Mechanical signals are the forces exerted on the cell and the forces produced by the cell. These forces can both be sensed and responded to by the cells. Biochemical signals are biochemical molecules such as proteins, lipids, ions, and gases. These signals can be categorized based on the distance between signaling and responder cells. Signaling within, between, and amongst cells is subdivided into the following classifications:
- Intracrine signals are produced by the target cell that stay within the target cell.
- Autocrine signals are produced by the target cell, are secreted, and affect the target cell itself via receptors. Sometimes autocrine cells can target cells close by if they are the same type of cell as the emitting cell. An example of this are immune cells.
- Juxtacrine signals target adjacent (touching) cells. These signals are transmitted along cell membranes via protein or lipid components integral to the membrane and are capable of affecting either the emitting cell or cells immediately adjacent.
- Paracrine signals target cells in the vicinity of the emitting cell. Neurotransmitters represent an example.
- Endocrine signals target distant cells. Endocrine cells produce hormones that travel through the blood to reach all parts of the body.
Cells communicate with each other via direct contact (juxtacrine signaling), over short distances (paracrine signaling), or over large distances and/or scales (endocrine signaling).
Some cell–cell communication requires direct cell–cell contact. Some cells can form gap junctions that connect their cytoplasm to the cytoplasm of adjacent cells. In cardiac muscle, gap junctions between adjacent cells allow for action potential propagation from the cardiac pacemaker region of the heart to spread and coordinate the contraction of the heart.
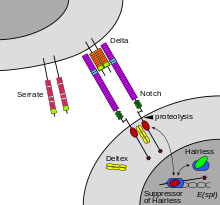
The notch signaling mechanism is an example of juxtacrine signaling (also known as contact-dependent signaling) in which two adjacent cells must make physical contact in order to communicate. This requirement for direct contact allows for very precise control of cell differentiation during embryonic development. In the worm Caenorhabditis elegans, two cells of the developing gonad each have an equal chance of terminally differentiating or becoming a uterine precursor cell that continues to divide. The choice of which cell continues to divide is controlled by competition of cell surface signals. One cell will happen to produce more of a cell surface protein that activates the Notch receptor on the adjacent cell. This activates a feedback loop or system that reduces Notch expression in the cell that will differentiate and that increases Notch on the surface of the cell that continues as a stem cell.
Many cell signals are carried by molecules that are released by one cell and move to make contact with another cell. Endocrine signals are called hormones. Hormones are produced by endocrine cells and they travel through the blood to reach all parts of the body. Specificity of signaling can be controlled if only some cells can respond to a particular hormone. Paracrine signals such as retinoic acid target only cells in the vicinity of the emitting cell. Neurotransmitters represent another example of a paracrine signal. Some signaling molecules can function as both a hormone and a neurotransmitter. For example, epinephrine and norepinephrine can function as hormones when released from the adrenal gland and are transported to the heart by way of the blood stream. Norepinephrine can also be produced by neurons to function as a neurotransmitter within the brain. Estrogen can be released by the ovary and function as a hormone or act locally via paracrine or autocrine signaling. Active species of oxygen and nitric oxide can also act as cellular messengers. This process is dubbed redox signaling.
In multicellular organisms
In a multicellular organism, signaling between cells occurs either through release into the extracellular space, divided in paracrine signaling (over short distances) and endocrine signaling (over long distances), or by direct contact, known as juxtacrine signaling. Autocrine signaling is a special case of paracrine signaling where the secreting cell has the ability to respond to the secreted signaling molecule. Synaptic signaling is a special case of paracrine signaling (for chemical synapses) or juxtacrine signaling (for electrical synapses) between neurons and target cells. Signaling molecules interact with a target cell as a ligand to cell surface receptors, and/or by entering into the cell through its membrane or endocytosis for intracrine signaling. This generally results in the activation of second messengers, leading to various physiological effects.
A particular molecule is generally used in diverse modes of signaling, and therefore a classification by mode of signaling is not possible. At least three important classes of signaling molecules are widely recognized, although non-exhaustive and with imprecise boundaries, as such membership is non-exclusive and depends on the context:
- Hormones are the major signaling molecules of the endocrine system, though they often regulate each other's secretion via local signaling (e.g. islet of Langerhans cells), and most are also expressed in tissues for local purposes (e.g. angiotensin) or failing that, structurally related molecules are (e.g. PTHrP).
- Neurotransmitters are signaling molecules of the nervous system, also including neuropeptides and neuromodulators. Neurotransmitters like the catecholamines are also secreted by the endocrine system into the systemic circulation.
- Cytokines are signaling molecules of the immune system, with a primary paracrine or juxtacrine role, though they can during significant immune responses have a strong presence in the circulation, with systemic effect (altering iron metabolism or body temperature). Growth factors can be considered as cytokines or a different class.
Signaling molecules can belong to several chemical classes: lipids, phospholipids, amino acids, monoamines, proteins, glycoproteins, or gases. Signaling molecules binding surface receptors are generally large and hydrophilic (e.g. TRH, Vasopressin, Acetylcholine), while those entering the cell are generally small and hydrophobic (e.g. glucocorticoids, thyroid hormones, cholecalciferol, retinoic acid), but important exceptions to both are numerous, and a same molecule can act both via surface receptor or in an intracrine manner to different effects. In intracrine signaling, once inside the cell, a signaling molecule can bind to intracellular receptors, other elements, or stimulate enzyme activity (e.g. gasses). The intracrine action of peptide hormones remains a subject of debate.
Hydrogen sulfide is produced in small amounts by some cells of the human body and has a number of biological signaling functions. Only two other such gases are currently known to act as signaling molecules in the human body: nitric oxide and carbon monoxide.
In plants
Signaling in plants happen through plant hormones, Phytochromes, Cryptochromes etc.
Important families of plant hormones are Auxin, cytokinin, gibberelline, ethyline, jasmonic acid, salicylic acid, strigolactones, polyamines, nitric oxide, peptide hormones etc. Translocation of RNA also has been reported.
Signaling receptors
Cells receive information from their neighbors through a class of proteins known as receptors. Receptors may bind with some molecules (ligands) or may interact with physical agents like light, mechanical temperature, pressure, etc. Some receptors are membrane bound and some receptors are cytosolic. A large number of cytosolic receptors belong to nuclear-cytoplasmic-superfamily.
Some important transmembrane receptors are Voltage gated ion channels , Ligand gated ion channels, Seven helix receptors or GPCRs, Two component receptors, Cytokine receptors, Receptor tyrosine kinase, Tyrosine kinase linked receptor, Receptor Serine threonine kinase, Receptor tyrosine phosphatase, Receptor guanylyl cyclase, Sphingomylinase linked receptor, Integrin, selectin, Cadherin, etc.
Notch is a cell surface protein that functions as a receptor. Animals have a small set of genes that code for signaling proteins that interact specifically with Notch receptors and stimulate a response in cells that express Notch on their surface. Molecules that activate (or, in some cases, inhibit) receptors can be classified as hormones, neurotransmitters, cytokines, and growth factors, in general called receptor ligands. Ligand receptor interactions such as that of the Notch receptor interaction, are known to be the main interactions responsible for cell signaling mechanisms and communication.
As shown in Figure 2 (above; left), notch acts as a receptor for ligands that are expressed on adjacent cells. While some receptors are cell-surface proteins, others are found inside cells. For example, estrogen is a hydrophobic molecule that can pass through the lipid bilayer of the membranes. As part of the endocrine system, intracellular estrogen receptors from a variety of cell types can be activated by estrogen produced in the ovaries.
A number of transmembrane receptors for small molecules and peptide hormones, as well as intracellular receptors for steroid hormones exist, giving cells the ability to respond to a great number of hormonal and pharmacological stimuli. In diseases, often, proteins that interact with receptors are aberrantly activated, resulting in constitutively activated downstream signals.
For several types of intercellular signaling molecules that are unable to permeate the hydrophobic cell membrane due to their hydrophilic nature, the target receptor is expressed on the membrane. When such a signaling molecule activates its receptor, the signal is carried into the cell usually by means of a second messenger such as cAMP.
The receptor-ligand interaction can be classified as:
- Agonism: it is when a ligand increases the activity of a ligand. Agonism is demonstrated in absence of any other competing ligand for the same receptor.
- Inverse agonism: When a receptor is constitutively active, and the constitutive activity is suppressed or inhibited by the ligand
- Antagonism: In presence of the agonist ligand, the antagonist molecule hinder the activation of the receptor by the ligand.
- Partial agonism: it is when a ligand shows agonism, but in spite of increasing dosage of the ligand, the receptor activation does not reach the full activation state.
- Partial inverse agonism: When a receptor is constitutively active, and in spite of increasing dosage of the ligand, the receptor activity decreases but does not become fully inactive.
- Protean agonism: Protean agonists can act both as an agonist or an inverse agonist based on whether the receptor is already inactive (quiescent) or already active.
- Biased agonism : when a receptor acts on more than one variants of next molecule in transduction chain; and binding with one agonist favours only one of the possible transduction pathway.
Signaling pathways
| Receptor Family | Example of Ligands/ activators (Bracket: receptor for it) | Example of effectors | Further downstream effects |
|---|---|---|---|
| Ligand Gated Ion Channels | Acetylcholine (Such as Nicotinic acetylcholine receptor), |
Changes in membrane permeability | Change in membrane potential |
| Seven Helix Receptor | Light(Rhodopsin), Dopamine (Dopamine receptor), GABA (GABA receptor), Prostaglandin (prostaglandin receptor) etc. |
Trimeric G protein | Adenylate Cyclase, cGMP phosphodiesterase, G-protein gated ion channel, etc. |
| Frizzled (Special type of 7Helix receptor) | Wnt | Dishevelled, axin - APC, GSK3-beta - Beta catenin | Gene expression |
| Two Component | Diverse activators | Histidine Kinase | Response Regulator - flagellar movement, Gene expression |
| Receptor Tyrosine Kinase | Insulin (insulin receptor), EGF (EGF receptor), FGF-Alpha, FGF-Beta, etc (FGF-receptors) |
Ras, MAP-kinases, PLC, PI3-Kinase | Gene expression change |
| Cytokine receptors | Erythropoietin, Growth Hormone (Growth Hormone Receptor), IFN-Gamma (IFN-Gamma receptor) etc |
JAK kinase | STAT transcription factor - Gene expression |
| Tyrosine kinase Linked- receptors | MHC-peptide complex - TCR, Antigens - BCR | Cytoplasmic Tyrosine Kinase | Gene expression |
| Receptor Serine/Threonine Kinase | Activin(activin receptor), Inhibin, Bone-morphogenetic protein(BMP Receptor), TGF-beta |
Smad transcription factors | Control of gene expression |
| Membrane Guanylyl Cyclase | Atrial natriuretic peptide, Sea urching egg peptide etc. |
cGMP | Regulation of Kinases and channels- Diverse actions |
| Cytoplasmic Guanylyl cyclase | Nitric Oxide(Nitric oxide receptor) | cGMP | Regulation of cGMP Gated channels, Kinases |
| Sphingomyelinase linked receptors | IL-1(IL-1 receptor), TNF (TNF-receptors) |
Ceramide activated kinases | Gene expression |
| Integrins | Fibronectins, other extracellular matrix proteins | Nonreceptor tyrosine kinase | Diverse response |
| Cytoplasmic Steroid receptors | Steroid hormones, Thyroid hormones, Retinoic acid etc |
Work as/ interact with transcription factors | Gene expression |
In some cases, receptor activation caused by ligand binding to a receptor is directly coupled to the cell's response to the ligand. For example, the neurotransmitter GABA can activate a cell surface receptor that is part of an ion channel. GABA binding to a GABAA receptor on a neuron opens a chloride-selective ion channel that is part of the receptor. GABAA receptor activation allows negatively charged chloride ions to move into the neuron, which inhibits the ability of the neuron to produce action potentials. However, for many cell surface receptors, ligand-receptor interactions are not directly linked to the cell's response. The activated receptor must first interact with other proteins inside the cell before the ultimate physiological effect of the ligand on the cell's behavior is produced. Often, the behavior of a chain of several interacting cell proteins is altered following receptor activation. The entire set of cell changes induced by receptor activation is called a signal transduction mechanism or pathway.
In the case of Notch-mediated signaling, the signal transduction mechanism can be relatively simple. As shown in Figure 2, the activation of Notch can cause the Notch protein to be altered by a protease. Part of the Notch protein is released from the cell surface membrane and takes part in gene regulation. Cell signaling research involves studying the spatial and temporal dynamics of both receptors and the components of signaling pathways that are activated by receptors in various cell types. Emerging methods for single-cell mass-spectrometry analysis promise to enable studying signal transduction with single-cell resolution.
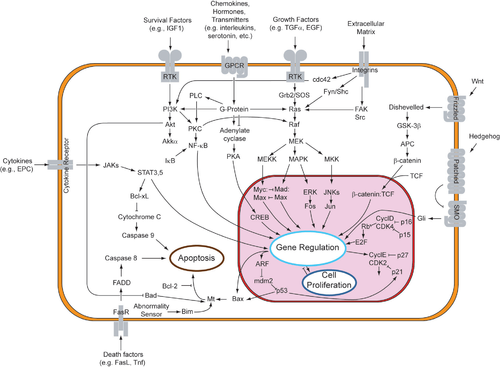
A more complex signal transduction pathway is shown in Figure 3. This pathway involves changes of protein–protein interactions inside the cell, induced by an external signal. Many growth factors bind to receptors at the cell surface and stimulate cells to progress through the cell cycle and divide. Several of these receptors are kinases that start to phosphorylate themselves and other proteins when binding to a ligand. This phosphorylation can generate a binding site for a different protein and thus induce protein–protein interaction. In Figure 3, the ligand (called epidermal growth factor, or EGF) binds to the receptor (called EGFR). This activates the receptor to phosphorylate itself. The phosphorylated receptor binds to an adaptor protein (GRB2), which couples the signal to further downstream signaling processes. For example, one of the signal transduction pathways that are activated is called the mitogen-activated protein kinase (MAPK) pathway. The signal transduction component labeled as "MAPK" in the pathway was originally called "ERK," so the pathway is called the MAPK/ERK pathway. The MAPK protein is an enzyme, a protein kinase that can attach phosphate to target proteins such as the transcription factor MYC and, thus, alter gene transcription and, ultimately, cell cycle progression. Many cellular proteins are activated downstream of the growth factor receptors (such as EGFR) that initiate this signal transduction pathway.
Some signaling transduction pathways respond differently, depending on the amount of signaling received by the cell. For instance, the hedgehog protein activates different genes, depending on the amount of hedgehog protein present.
Complex multi-component signal transduction pathways provide opportunities for feedback, signal amplification, and interactions inside one cell between multiple signals and signaling pathways.
Intra- and inter-species signaling
Molecular signaling can occur between different organisms, whether unicellular or multicellular. The emitting organism produces the signaling molecule, secretes it into the environment, where it diffuses, and it is sensed or internalized by the receiving organism. In some cases of interspecies signaling, the emitting organism can actually be a host of the receiving organism, or vice versa.
Intraspecies signaling occurs especially in bacteria, yeast, social insects, but also many vertebrates. The signaling molecules used by multicellular organisms are often called pheromones. They can have such purposes as alerting against danger, indicating food supply, or assisting in reproduction. In unicellular organisms such as bacteria, signaling can be used to 'activate' peers from a dormant state, enhance virulence, defend against bacteriophages, etc. In quorum sensing, which is also found in social insects, the multiplicity of individual signals has the potentiality to create a positive feedback loop, generating coordinated response. In this context, the signaling molecules are called autoinducers. This signaling mechanism may have been involved in evolution from unicellular to multicellular organisms. Bacteria also use contact-dependent signaling, notably to limit their growth.
Molecular signaling can also occur between individuals of different species. This has been particularly studied in bacteria. Different bacterial species can coordinate to colonize a host and participate in common quorum sensing. Therapeutic strategies to disrupt this phenomenon are being investigated. Interactions mediated through signaling molecules are also thought to occur between the gut flora and their host, as part of their commensal or symbiotic relationship. Gram negative microbes deploy bacterial outer membrane vesicles for intra- and inter-species signaling in natural environments and at the host-pathogen interface.
Additionally, interspecies signaling occurs between multicellular organisms. In Vespa mandarinia, individuals release a scent that directs the colony to a food source.
In plants, inter-species signaling is particularly important in mycorrhizal symbiosis and root nodule symbiosis. In both symbioses, Receptor-like kinase (RLK), G-proteins, MAP-kinases and Ca2+ plays a very important role.
Computational models
Recent approaches to better understand elements of pathway crosstalk, complex ligand-receptor binding, and signaling network dynamics have been aided by the use of systems biology approaches. Computational models often take aim at compiling information from published literature to generate a coherent set of signaling components and their associated interactions. The development of computational models allows for a more in-depth probing of cell signaling pathways at a global level by manipulating different variables and systemically evaluating the resulting response. The use of analytical models for the study of signal transduction has been heavily applied in the fields of pharmacology and drug discovery to assess receptor-ligand interactions and pharmacokinetics as well as the flow of metabolites in large networks. A commonly applied strategy to model cell signaling mechanisms is through the use of ordinary differential equation (ODE) models by expressing the time-dependent concentration of a signaling molecule as a function of other molecules downstream and/or upstream within the pathway. ODE models have already been applied for dynamic analysis of the Mitogen-activated protein kinase, Estrogen receptor alpha, and MTOR signaling pathways among numerous others.