Metagenomics
allows the study of microbial communities like those present in this
stream receiving acid drainage from surface coal mining.
Metagenomics is the study of genetic material recovered directly from environmental samples. The broad field may also be referred to as environmental genomics, ecogenomics or community genomics.
While traditional microbiology and microbial genome sequencing and genomics rely upon cultivated clonal cultures, early environmental gene sequencing cloned specific genes (often the 16S rRNA gene) to produce a profile of diversity in a natural sample. Such work revealed that the vast majority of microbial biodiversity had been missed by cultivation-based methods.
Because of its ability to reveal the previously hidden diversity
of microscopic life, metagenomics offers a powerful lens for viewing the
microbial world that has the potential to revolutionize understanding
of the entire living world. As the price of DNA sequencing continues to fall, metagenomics now allows microbial ecology to be investigated at a much greater scale and detail than before. Recent studies use either "shotgun" or PCR directed sequencing to get largely unbiased samples of all genes from all the members of the sampled communities.
Etymology
The term "metagenomics" was first used by Jo Handelsman, Jon Clardy, Robert M. Goodman, Sean F. Brady, and others, and first appeared in publication in 1998.
The term metagenome referenced the idea that a collection of genes
sequenced from the environment could be analyzed in a way analogous to
the study of a single genome. In 2005, Kevin Chen and Lior Pachter (researchers at the University of California, Berkeley)
defined metagenomics as "the application of modern genomics technique
without the need for isolation and lab cultivation of individual
species".
History
Conventional sequencing begins with a culture of identical cells as a source of DNA.
However, early metagenomic studies revealed that there are probably
large groups of microorganisms in many environments that cannot be cultured and thus cannot be sequenced. These early studies focused on 16S ribosomal RNA sequences which are relatively short, often conserved within a species, and generally different between species. Many 16S rRNA sequences have been found which do not belong to any known cultured species,
indicating that there are numerous non-isolated organisms. These
surveys of ribosomal RNA (rRNA) genes taken directly from the
environment revealed that cultivation based methods find less than 1% of the bacterial and archaeal species in a sample.
Much of the interest in metagenomics comes from these discoveries that
showed that the vast majority of microorganisms had previously gone
unnoticed.
Early molecular work in the field was conducted by Norman R. Pace and colleagues, who used PCR to explore the diversity of ribosomal RNA sequences.
The insights gained from these breakthrough studies led Pace to propose
the idea of cloning DNA directly from environmental samples as early as
1985. This led to the first report of isolating and cloning bulk DNA from an environmental sample, published by Pace and colleagues in 1991 while Pace was in the Department of Biology at Indiana University. Considerable efforts ensured that these were not PCR
false positives and supported the existence of a complex community of
unexplored species. Although this methodology was limited to exploring
highly conserved, non-protein coding genes,
it did support early microbial morphology-based observations that
diversity was far more complex than was known by culturing methods. Soon
after that, Healy reported the metagenomic isolation of functional
genes from "zoolibraries" constructed from a complex culture of
environmental organisms grown in the laboratory on dried grasses in 1995. After leaving the Pace laboratory, Edward DeLong
continued in the field and has published work that has largely laid the
groundwork for environmental phylogenies based on signature 16S
sequences, beginning with his group's construction of libraries from marine samples.
In 2002, Mya Breitbart, Forest Rohwer,
and colleagues used environmental shotgun sequencing (see below) to
show that 200 liters of seawater contains over 5000 different viruses. Subsequent studies showed that there are more than a thousand viral species in human stool and possibly a million different viruses per kilogram of marine sediment, including many bacteriophages. Essentially all of the viruses in these studies were new species. In 2004, Gene Tyson, Jill Banfield, and colleagues at the University of California, Berkeley and the Joint Genome Institute sequenced DNA extracted from an acid mine drainage system. This effort resulted in the complete, or nearly complete, genomes for a handful of bacteria and archaea that had previously resisted attempts to culture them.
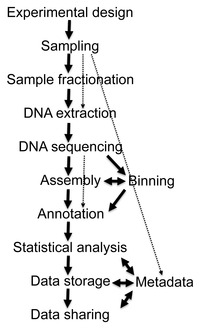
Flow diagram of a typical metagenome project
Beginning in 2003, Craig Venter, leader of the privately funded parallel of the Human Genome Project, has led the Global Ocean Sampling Expedition
(GOS), circumnavigating the globe and collecting metagenomic samples
throughout the journey. All of these samples are sequenced using shotgun
sequencing, in hopes that new genomes (and therefore new organisms)
would be identified. The pilot project, conducted in the Sargasso Sea, found DNA from nearly 2000 different species, including 148 types of bacteria never before seen. Venter has circumnavigated the globe and thoroughly explored the West Coast of the United States, and completed a two-year expedition to explore the Baltic, Mediterranean and Black
Seas. Analysis of the metagenomic data collected during this journey
revealed two groups of organisms, one composed of taxa adapted to
environmental conditions of 'feast or famine', and a second composed of
relatively fewer but more abundantly and widely distributed taxa
primarily composed of plankton.
In 2005 Stephan C. Schuster at Penn State University and colleagues published the first sequences of an environmental sample generated with high-throughput sequencing, in this case massively parallel pyrosequencing developed by 454 Life Sciences. Another early paper in this area appeared in 2006 by Robert Edwards, Forest Rohwer, and colleagues at San Diego State University.
Sequencing
Recovery of DNA sequences longer than a few thousand base pairs from environmental samples was very difficult until recent advances in molecular biological techniques allowed the construction of libraries in bacterial artificial chromosomes (BACs), which provided better vectors for molecular cloning.
Environmental
Shotgun Sequencing (ESS). (A) Sampling from habitat; (B) filtering
particles, typically by size; (C) Lysis and DNA extraction; (D) cloning
and library construction; (E) sequencing the clones; (F) sequence
assembly into contigs and scaffolds.
Shotgun metagenomics
Advances in bioinformatics,
refinements of DNA amplification, and the proliferation of
computational power have greatly aided the analysis of DNA sequences
recovered from environmental samples, allowing the adaptation of shotgun sequencing
to metagenomic samples (known also as whole metagenome shotgun or WMGS
sequencing). The approach, used to sequence many cultured microorganisms
and the human genome, randomly shears DNA, sequences many short sequences, and reconstructs them into a consensus sequence.
Shotgun sequencing reveals genes present in environmental samples.
Historically, clone libraries were used to facilitate this sequencing.
However, with advances in high throughput sequencing technologies, the
cloning step is no longer necessary and greater yields of sequencing
data can be obtained without this labour-intensive bottleneck step.
Shotgun metagenomics provides information both about which organisms are
present and what metabolic processes are possible in the community.
Because the collection of DNA from an environment is largely
uncontrolled, the most abundant organisms in an environmental sample are
most highly represented in the resulting sequence data. To achieve the
high coverage needed to fully resolve the genomes of under-represented
community members, large samples, often prohibitively so, are needed. On
the other hand, the random nature of shotgun sequencing ensures that
many of these organisms, which would otherwise go unnoticed using
traditional culturing techniques, will be represented by at least some
small sequence segments. An emerging approach combines shotgun sequencing and chromosome conformation capture (Hi-C), which measures the proximity of any two DNA sequences within the same cell, to guide microbial genome assembly.
High-throughput sequencing
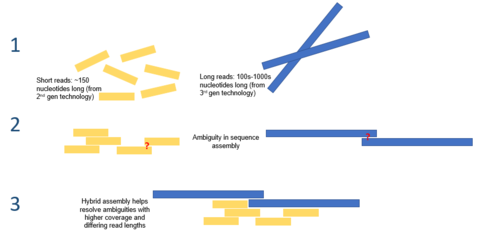
The first metagenomic studies conducted using high-throughput sequencing used massively parallel 454 pyrosequencing. Three other technologies commonly applied to environmental sampling are the Ion Torrent Personal Genome Machine, the Illumina MiSeq or HiSeq and the Applied Biosystems SOLiD system. These techniques for sequencing DNA generate shorter fragments than Sanger sequencing;
Ion Torrent PGM System and 454 pyrosequencing typically produces
~400 bp reads, Illumina MiSeq produces 400-700bp reads (depending on
whether paired end options are used), and SOLiD produce 25-75 bp reads.
Historically, these read lengths were significantly shorter than the
typical Sanger sequencing read length of ~750 bp, however the Illumina
technology is quickly coming close to this benchmark. However, this
limitation is compensated for by the much larger number of sequence
reads. In 2009, pyrosequenced metagenomes generate 200–500 megabases,
and Illumina platforms generate around 20–50 gigabases, but these
outputs have increased by orders of magnitude in recent years.
An additional advantage to high throughput sequencing is that this
technique does not require cloning the DNA before sequencing, removing
one of the main biases and bottlenecks in environmental sampling.
Bioinformatics
The
data generated by metagenomics experiments are both enormous and
inherently noisy, containing fragmented data representing as many as
10,000 species. The sequencing of the cow rumen metagenome generated 279 gigabases, or 279 billion base pairs of nucleotide sequence data, while the human gut microbiome gene catalog identified 3.3 million genes assembled from 567.7 gigabases of sequence data.
Collecting, curating, and extracting useful biological information from
datasets of this size represent significant computational challenges
for researchers.
Sequence pre-filtering
The
first step of metagenomic data analysis requires the execution of
certain pre-filtering steps, including the removal of redundant,
low-quality sequences and sequences of probable eukaryotic origin (especially in metagenomes of human origin). The methods available for the removal of contaminating eukaryotic genomic DNA sequences include Eu-Detect and DeConseq.
Assembly
DNA sequence data from genomic and metagenomic projects are essentially the same, but genomic sequence data offers higher coverage while metagenomic data is usually highly non-redundant.
Furthermore, the increased use of second-generation sequencing
technologies with short read lengths means that much of future
metagenomic data will be error-prone. Taken in combination, these
factors make the assembly of metagenomic sequence reads into genomes
difficult and unreliable. Misassemblies are caused by the presence of repetitive DNA sequences that make assembly especially difficult because of the difference in the relative abundance of species present in the sample. Misassemblies can also involve the combination of sequences from more than one species into chimeric contigs.
There are several assembly programs, most of which can use information from paired-end tags in order to improve the accuracy of assemblies. Some programs, such as Phrap or Celera Assembler, were designed to be used to assemble single genomes but nevertheless produce good results when assembling metagenomic data sets. Other programs, such as Velvet assembler, have been optimized for the shorter reads produced by second-generation sequencing through the use of de Bruijn graphs.
The use of reference genomes allows researchers to improve the assembly
of the most abundant microbial species, but this approach is limited by
the small subset of microbial phyla for which sequenced genomes are
available.
After an assembly is created, an additional challenge is "metagenomic
deconvolution", or determining which sequences come from which species
in the sample.
Gene prediction
Metagenomic analysis pipelines use two approaches in the annotation of coding regions in the assembled contigs. The first approach is to identify genes based upon homology with genes that are already publicly available in sequence databases, usually by BLAST searches. This type of approach is implemented in the program MEGAN4. The second, ab initio,
uses intrinsic features of the sequence to predict coding regions based
upon gene training sets from related organisms. This is the approach
taken by programs such as GeneMark and GLIMMER. The main advantage of ab initio
prediction is that it enables the detection of coding regions that lack
homologs in the sequence databases; however, it is most accurate when
there are large regions of contiguous genomic DNA available for
comparison.
Species diversity
A 2016 representation of the tree of life
Gene annotations provide the "what", while measurements of species diversity provide the "who". In order to connect community composition and function in metagenomes, sequences must be binned. Binning is the process of associating a particular sequence with an organism. In similarity-based binning, methods such as BLAST
are used to rapidly search for phylogenetic markers or otherwise
similar sequences in existing public databases. This approach is
implemented in MEGAN. Another tool, PhymmBL, uses interpolated Markov models to assign reads. MetaPhlAn and AMPHORA
are methods based on unique clade-specific markers for estimating
organismal relative abundances with improved computational performances. Other tools, like mOTUs and MetaPhyler, use universal marker genes to profile prokaryotic species. With the mOTUs profiler is possible to profile species without a reference genome, improving the estimation of microbial community diversity. Recent methods, such as SLIMM,
use read coverage landscape of individual reference genomes to minimize
false-positive hits and get reliable relative abundances. In composition based binning, methods use intrinsic features of the sequence, such as oligonucleotide frequencies or codon usage bias. Once sequences are binned, it is possible to carry out comparative analysis of diversity and richness.
Data integration
The massive amount of exponentially growing sequence data is a daunting challenge that is complicated by the complexity of the metadata
associated with metagenomic projects. Metadata includes detailed
information about the three-dimensional (including depth, or height)
geography and environmental features of the sample, physical data about
the sample site, and the methodology of the sampling. This information is necessary both to ensure replicability
and to enable downstream analysis. Because of its importance, metadata
and collaborative data review and curation require standardized data
formats located in specialized databases, such as the Genomes OnLine
Database (GOLD).
Several tools have been developed to integrate metadata and
sequence data, allowing downstream comparative analyses of different
datasets using a number of ecological indices. In 2007, Folker Meyer and
Robert Edwards and a team at Argonne National Laboratory and the University of Chicago released the Metagenomics Rapid Annotation using Subsystem Technology server (MG-RAST) a community resource for metagenome data set analysis. As of June 2012 over 14.8 terabases (14x1012
bases) of DNA have been analyzed, with more than 10,000 public data
sets freely available for comparison within MG-RAST. Over 8,000 users
now have submitted a total of 50,000 metagenomes to MG-RAST. The Integrated Microbial Genomes/Metagenomes
(IMG/M) system also provides a collection of tools for functional
analysis of microbial communities based on their metagenome sequence,
based upon reference isolate genomes included from the Integrated Microbial Genomes (IMG) system and the Genomic Encyclopedia of Bacteria and Archaea (GEBA) project.
One of the first standalone tools for analysing high-throughput metagenome shotgun data was MEGAN (MEta Genome ANalyzer).
A first version of the program was used in 2005 to analyse the
metagenomic context of DNA sequences obtained from a mammoth bone.
Based on a BLAST comparison against a reference database, this tool
performs both taxonomic and functional binning, by placing the reads
onto the nodes of the NCBI taxonomy using a simple lowest common
ancestor (LCA) algorithm or onto the nodes of the SEED or KEGG classifications, respectively.
With the advent of fast and inexpensive sequencing instruments,
the growth of databases of DNA sequences is now exponential (e.g., the
NCBI GenBank database).
Faster and efficient tools are needed to keep pace with the
high-throughput sequencing, because the BLAST-based approaches such as
MG-RAST or MEGAN run slowly to annotate large samples (e.g., several
hours to process a small/medium size dataset/sample).
Thus, ultra-fast classifiers have recently emerged, thanks to more
affordable powerful servers. These tools can perform the taxonomic
annotation at extremely high speed, for example CLARK (according to CLARK's authors, it can classify accurately "32 million
metagenomic short reads per minute"). At such a speed, a very large
dataset/sample of a billion short reads can be processed in about 30
minutes.
With the increasing availability of samples containing ancient
DNA and due to the uncertainty associated with the nature of those
samples (ancient DNA damage), FALCON,
a fast tool capable of producing conservative similarity estimates has
been made available. According to FALCON's authors, it can use relaxed
thresholds and edit distances without affecting the memory and speed
performance.
Comparative metagenomics
Comparative
analyses between metagenomes can provide additional insight into the
function of complex microbial communities and their role in host health. Pairwise or multiple comparisons between metagenomes can be made at the level of sequence composition (comparing GC-content
or genome size), taxonomic diversity, or functional complement.
Comparisons of population structure and phylogenetic diversity can be
made on the basis of 16S and other phylogenetic marker genes, or—in the
case of low-diversity communities—by genome reconstruction from the
metagenomic dataset. Functional comparisons between metagenomes may be made by comparing sequences against reference databases such as COG or KEGG, and tabulating the abundance by category and evaluating any differences for statistical significance. This gene-centric approach emphasizes the functional complement of the community
as a whole rather than taxonomic groups, and shows that the functional
complements are analogous under similar environmental conditions.
Consequently, metadata on the environmental context of the metagenomic
sample is especially important in comparative analyses, as it provides
researchers with the ability to study the effect of habitat upon
community structure and function.
Additionally, several studies have also utilized oligonucleotide
usage patterns to identify the differences across diverse microbial
communities. Examples of such methodologies include the dinucleotide
relative abundance approach by Willner et al. and the HabiSign approach of Ghosh et al.
This latter study also indicated that differences in tetranucleotide
usage patterns can be used to identify genes (or metagenomic reads)
originating from specific habitats. Additionally some methods as
TriageTools or Compareads detect similar reads between two read sets. The similarity measure they apply on reads is based on a number of identical words of length k shared by pairs of reads.
A key goal in comparative metagenomics is to identify microbial
group(s) which are responsible for conferring specific characteristics
to a given environment. However, due to issues in the sequencing
technologies artifacts need to be accounted for like in metagenomeSeq. Others have characterized inter-microbial interactions between the resident microbial groups. A GUI-based comparative metagenomic analysis application called Community-Analyzer has been developed by Kuntal et al.
which implements a correlation-based graph layout algorithm that not
only facilitates a quick visualization of the differences in the
analyzed microbial communities (in terms of their taxonomic
composition), but also provides insights into the inherent
inter-microbial interactions occurring therein. Notably, this layout
algorithm also enables grouping of the metagenomes based on the probable
inter-microbial interaction patterns rather than simply comparing
abundance values of various taxonomic groups. In addition, the tool
implements several interactive GUI-based functionalities that enable
users to perform standard comparative analyses across microbiomes.
Data analysis
Community metabolism
In many bacterial communities, natural or engineered (such as bioreactors), there is significant division of labor in metabolism (Syntrophy), during which the waste products of some organisms are metabolites for others. In one such system, the methanogenic bioreactor, functional stability requires the presence of several syntrophic species (Syntrophobacterales and Synergistia) working together in order to turn raw resources into fully metabolized waste (methane). Using comparative gene studies and expression experiments with microarrays or proteomics
researchers can piece together a metabolic network that goes beyond
species boundaries. Such studies require detailed knowledge about which
versions of which proteins are coded by which species and even by which
strains of which species. Therefore, community genomic information is
another fundamental tool (with metabolomics and proteomics) in the quest to determine how metabolites are transferred and transformed by a community.
Metatranscriptomics
Metagenomics allows researchers to access the functional and
metabolic diversity of microbial communities, but it cannot show which
of these processes are active. The extraction and analysis of metagenomic mRNA (the metatranscriptome) provides information on the regulation and expression profiles of complex communities. Because of the technical difficulties (the short half-life of mRNA, for example) in the collection of environmental RNA there have been relatively few in situ metatranscriptomic studies of microbial communities to date. While originally limited to microarray technology, metatranscriptomics studies have made use of transcriptomics technologies to measure whole-genome expression and quantification of a microbial community, first employed in analysis of ammonia oxidation in soils.
Viruses
Metagenomic sequencing is particularly useful in the study of viral
communities. As viruses lack a shared universal phylogenetic marker (as 16S RNA for bacteria and archaea, and 18S RNA
for eukarya), the only way to access the genetic diversity of the viral
community from an environmental sample is through metagenomics. Viral
metagenomes (also called viromes) should thus provide more and more
information about viral diversity and evolution. For example, a metagenomic pipeline called Giant Virus Finder showed the first evidence of existence of giant viruses in a saline desert and in Antarctic dry valleys
.
Applications
Metagenomics
has the potential to advance knowledge in a wide variety of fields. It
can also be applied to solve practical challenges in medicine, engineering, agriculture, sustainability and ecology.
Agriculture
The soils in which plants grow are inhabited by microbial communities, with one gram of soil containing around 109-1010 microbial cells which comprise about one gigabase of sequence information.
The microbial communities which inhabit soils are some of the most
complex known to science, and remain poorly understood despite their
economic importance. Microbial consortia perform a wide variety of ecosystem services necessary for plant growth, including fixing atmospheric nitrogen, nutrient cycling, disease suppression, and sequester iron and other metals.
Functional metagenomics strategies are being used to explore the
interactions between plants and microbes through cultivation-independent
study of these microbial communities.
By allowing insights into the role of previously uncultivated or rare
community members in nutrient cycling and the promotion of plant growth,
metagenomic approaches can contribute to improved disease detection in crops and livestock and the adaptation of enhanced farming practices which improve crop health by harnessing the relationship between microbes and plants.
Biofuel
Bioreactors allow the observation of microbial communities as they convert biomass into cellulosic ethanol.
Biofuels are fuels derived from biomass conversion, as in the conversion of cellulose contained in corn stalks, switchgrass, and other biomass into cellulosic ethanol. This process is dependent upon microbial consortia(association) that transform the cellulose into sugars, followed by the fermentation of the sugars into ethanol. Microbes also produce a variety of sources of bioenergy including methane and hydrogen.
The efficient industrial-scale deconstruction of biomass requires novel enzymes with higher productivity and lower cost. Metagenomic approaches to the analysis of complex microbial communities allow the targeted screening of enzymes with industrial applications in biofuel production, such as glycoside hydrolases.
Furthermore, knowledge of how these microbial communities function is
required to control them, and metagenomics is a key tool in their
understanding. Metagenomic approaches allow comparative analyses between
convergent microbial systems like biogas fermenters or insect herbivores such as the fungus garden of the leafcutter ants.
Biotechnology
Microbial communities produce a vast array of biologically active chemicals that are used in competition and communication.
Many of the drugs in use today were originally uncovered in microbes;
recent progress in mining the rich genetic resource of non-culturable
microbes has led to the discovery of new genes, enzymes, and natural
products. The application of metagenomics has allowed the development of commodity and fine chemicals, agrochemicals and pharmaceuticals where the benefit of enzyme-catalyzed chiral synthesis is increasingly recognized.
Two types of analysis are used in the bioprospecting
of metagenomic data: function-driven screening for an expressed trait,
and sequence-driven screening for DNA sequences of interest.
Function-driven analysis seeks to identify clones expressing a desired
trait or useful activity, followed by biochemical characterization and
sequence analysis. This approach is limited by availability of a
suitable screen and the requirement that the desired trait be expressed
in the host cell. Moreover, the low rate of discovery (less than one per
1,000 clones screened) and its labor-intensive nature further limit
this approach. In contrast, sequence-driven analysis uses conserved DNA sequences to design PCR primers to screen clones for the sequence of interest.
In comparison to cloning-based approaches, using a sequence-only
approach further reduces the amount of bench work required. The
application of massively parallel sequencing also greatly increases the
amount of sequence data generated, which require high-throughput
bioinformatic analysis pipelines.
The sequence-driven approach to screening is limited by the breadth and
accuracy of gene functions present in public sequence databases. In
practice, experiments make use of a combination of both functional and
sequence-based approaches based upon the function of interest, the
complexity of the sample to be screened, and other factors. An example of success using metagenomics as a biotechnology for drug discovery is illustrated with the malacidin antibiotics.
Ecology
Metagenomics can provide valuable insights into the functional ecology of environmental communities.
Metagenomic analysis of the bacterial consortia found in the
defecations of Australian sea lions suggests that nutrient-rich sea lion
faeces may be an important nutrient source for coastal ecosystems. This
is because the bacteria that are expelled simultaneously with the
defecations are adept at breaking down the nutrients in the faeces into a
bioavailable form that can be taken up into the food chain.
DNA sequencing can also be used more broadly to identify species present in a body of water, debris filtered from the air, or sample of dirt. This can establish the range of invasive species and endangered species, and track seasonal populations.
Environmental remediation
Metagenomics can improve strategies for monitoring the impact of pollutants on ecosystems
and for cleaning up contaminated environments. Increased understanding
of how microbial communities cope with pollutants improves assessments
of the potential of contaminated sites to recover from pollution and
increases the chances of bioaugmentation or biostimulation trials to succeed.
Gut Microbe Characterization
Microbial communities play a key role in preserving human health, but their composition and the mechanism by which they do so remains mysterious.
Metagenomic sequencing is being used to characterize the microbial
communities from 15-18 body sites from at least 250 individuals. This is
part of the Human Microbiome initiative with primary goals to determine if there is a core human microbiome,
to understand the changes in the human microbiome that can be
correlated with human health, and to develop new technological and bioinformatics tools to support these goals.
Another medical study as part of the MetaHit (Metagenomics of the
Human Intestinal Tract) project consisted of 124 individuals from
Denmark and Spain consisting of healthy, overweight, and irritable bowel
disease patients. The study attempted to categorize the depth and
phylogenetic diversity of gastrointestinal bacteria. Using Illumina GA
sequence data and SOAPdenovo, a de Bruijn graph-based tool specifically
designed for assembly short reads, they were able to generate 6.58
million contigs greater than 500 bp for a total contig length of 10.3 Gb
and a N50 length of 2.2 kb.
The study demonstrated that two bacterial divisions,
Bacteroidetes and Firmicutes, constitute over 90% of the known
phylogenetic categories that dominate distal gut bacteria. Using the
relative gene frequencies found within the gut these researchers
identified 1,244 metagenomic clusters that are critically important for
the health of the intestinal tract. There are two types of functions in
these range clusters: housekeeping and those specific to the intestine.
The housekeeping gene clusters are required in all bacteria and are
often major players in the main metabolic pathways including central
carbon metabolism and amino acid synthesis. The gut-specific functions
include adhesion to host proteins and the harvesting of sugars from
globoseries glycolipids. Patients with irritable bowel syndrome were
shown to exhibit 25% fewer genes and lower bacterial diversity than
individuals not suffering from irritable bowel syndrome indicating that
changes in patients’ gut biome diversity may be associated with this
condition.
While these studies highlight some potentially valuable medical
applications, only 31-48.8% of the reads could be aligned to 194 public
human gut bacterial genomes and 7.6-21.2% to bacterial genomes available
in GenBank which indicates that there is still far more research
necessary to capture novel bacterial genomes.
Infectious disease diagnosis
Differentiating
between infectious and non-infectious illness, and identifying the
underlying etiology of infection, can be quite challenging. For example,
more than half of cases of encephalitis
remain undiagnosed, despite extensive testing using state-of-the-art
clinical laboratory methods. Metagenomic sequencing shows promise as a
sensitive and rapid method to diagnose infection by comparing genetic
material found in a patient's sample to a database of thousands of
bacteria, viruses, and other pathogens