The CIE 1931 x,y chromaticity space, also showing the chromaticities of black-body light sources of various temperatures (Planckian locus), and lines of constant correlated color temperature.
The color temperature of a light source is the temperature of an ideal black-body radiator that radiates light of a color comparable to that of the light source. Color temperature is a characteristic of visible light that has important applications in lighting, photography, videography, publishing, manufacturing, astrophysics, horticulture,
and other fields. In practice, color temperature is meaningful only for
light sources that do in fact correspond somewhat closely to the
radiation of some black body, i.e., light in a range going from red to
orange to yellow to white
to blueish white; it does not make sense to speak of the color
temperature of, e.g., a green or a purple light. Color temperature is
conventionally expressed in kelvins, using the symbol K, a unit of measure for absolute temperature.
Color temperatures over 5000 K are called "cool colors" (bluish),
while lower color temperatures (2700–3000 K) are called "warm colors"
(yellowish). "Warm" in this context is an analogy to radiated heat flux
of traditional incandescent lighting
rather than temperature. The spectral peak of warm-coloured light is
closer to infrared, and most natural warm-coloured light sources emit
significant infrared radiation. The fact that "warm" lighting in this
sense actually has a "cooler" color temperature often leads to
confusion.
Categorizing different lighting
| Temperature | Source |
|---|---|
| 1700 K | Match flame, low pressure sodium lamps (LPS/SOX) |
| 1850 K | Candle flame, sunset/sunrise |
| 2400 K | Standard incandescent lamps |
| 2550 K | Soft white incandescent lamps |
| 2700 K | "Soft white" compact fluorescent and LED lamps |
| 3000 K | Warm white compact fluorescent and LED lamps |
| 3200 K | Studio lamps, photofloods, etc. |
| 3350 K | Studio "CP" light |
| 5000 K | Horizon daylight |
| 5000 K | Tubular fluorescent lamps or cool white / daylight compact fluorescent lamps (CFL) |
| 5500 – 6000 K | Vertical daylight, electronic flash |
| 6200 K | Xenon short-arc lamp |
| 6500 K | Daylight, overcast |
| 6500 – 9500 K | LCD or CRT screen |
| 15,000 – 27,000 K | Clear blue poleward sky |
| These temperatures are merely characteristic; there may be considerable variation | |
The black-body radiance (Bλ) vs. wavelength (λ) curves for the visible spectrum. The vertical axes of Planck's law
plots building this animation were proportionally transformed to keep
equal areas between functions and horizontal axis for wavelengths
380–780 nm. K indicates the color temperature in Kelvins, and M
indicates the color temperature in micro reciprocal degrees.
The color temperature of the electromagnetic radiation emitted from an ideal black body is defined as its surface temperature in kelvins, or alternatively in micro reciprocal degrees (mired). This permits the definition of a standard by which light sources are compared.
To the extent that a hot surface emits thermal radiation but is not an ideal black-body radiator, the color temperature of the light is not the actual temperature of the surface. An incandescent lamp's
light is thermal radiation, and the bulb approximates an ideal
black-body radiator, so its color temperature is essentially the
temperature of the filament. Thus a relatively low temperature emits a
dull red and a high temperature emits the almost white of the
traditional incandescent light bulb. Metal workers are able to judge the
temperature of hot metals by their color, from dark red to orange-white
and then white.
Many other light sources, such as fluorescent lamps, or LEDs
(light emitting diodes) emit light primarily by processes other than
thermal radiation. This means that the emitted radiation does not follow
the form of a black-body spectrum. These sources are assigned what is known as a correlated color temperature (CCT). CCT is the color temperature of a black-body radiator which to human color perception
most closely matches the light from the lamp. Because such an
approximation is not required for incandescent light, the CCT for an
incandescent light is simply its unadjusted temperature, derived from
comparison to a black-body radiator.
The Sun
The Sun
closely approximates a black-body radiator. The effective temperature,
defined by the total radiative power per square unit, is about 5780 K. The color temperature of sunlight above the atmosphere is about 5900 K.
The Sun may appear red, orange, yellow, or white from Earth, depending on its position in the sky. The changing color of the Sun over the course of the day is mainly a result of the scattering of sunlight and is not due to changes in black-body radiation. Rayleigh scattering of sunlight by Earth's atmosphere causes the blue color of the sky, which tends to scatter blue light more than red light.
Some daylight in the early morning and late afternoon (the golden hours) has a lower ("warmer") color temperature due to increased scattering of shorter-wavelength sunlight by atmospheric particles – an optical phenomenon called the Tyndall effect.
Daylight has a spectrum similar to that of a black body with a correlated color temperature of 6500 K (D65 viewing standard) or 5500 K (daylight-balanced photographic film standard).
Hues of the Planckian locus on a linear scale
For colors based on black-body theory, blue occurs at higher
temperatures, whereas red occurs at lower temperatures. This is the
opposite of the cultural associations attributed to colors, in which
"red" is "hot", and "blue" is "cold".
Applications
Lighting
Color temperature comparison of common electric lamps
For lighting building interiors, it is often important to take into
account the color temperature of illumination. A warmer (i.e., a lower
color temperature) light is often used in public areas to promote
relaxation, while a cooler (higher color temperature) light is used to
enhance concentration, for example in schools and offices.
CCT dimming for LED technology is regarded as a difficult task,
since binning, age and temperature drift effects of LEDs change the
actual color value output. Here feedback loop systems are used, for
example with color sensors, to actively monitor and control the color
output of multiple color mixing LEDs.
Aquaculture
In fishkeeping, color temperature has different functions and foci in the various branches.
- In freshwater aquaria, color temperature is generally of concern only for producing a more attractive display. Lights tend to be designed to produce an attractive spectrum, sometimes with secondary attention paid to keeping the plants in the aquaria alive.
- In a saltwater/reef aquarium, color temperature is an essential part of tank health. Within about 400 to 3000 nanometers, light of shorter wavelength can penetrate deeper into water than longer wavelengths, providing essential energy sources to the algae hosted in (and sustaining) coral. This is equivalent to an increase of color temperature with water depth in this spectral range. Because coral typically live in shallow water and receive intense, direct tropical sunlight, the focus was once on simulating this situation with 6500 K lights. In the meantime higher temperature light sources have become more popular, first with 10000 K and more recently 16000 K and 20000 K. Actinic lighting at the violet end of the visible range (420–460 nm) is used to allow night viewing without increasing algae bloom or enhancing photosynthesis, and to make the somewhat fluorescent colors of many corals and fish "pop", creating brighter display tanks.
Digital photography
In digital photography,
the term color temperature sometimes refers to remapping of color
values to simulate variations in ambient color temperature. Most digital
cameras and raw image software provide presets simulating specific
ambient values (e.g., sunny, cloudy, tungsten, etc.) while others allow
explicit entry of white balance values in kelvins. These settings vary
color values along the blue–yellow axis, while some software includes
additional controls (sometimes labeled "tint") adding the magenta–green
axis, and are to some extent arbitrary and a matter of artistic
interpretation.
Photographic film
Photographic emulsion film does not respond to lighting color
identically to the human retina or visual perception. An object that
appears to the observer to be white may turn out to be very blue or
orange in a photograph. The color balance
may need to be corrected during printing to achieve a neutral color
print. The extent of this correction is limited since color film
normally has three layers sensitive to different colors and when used
under the "wrong" light source, every layer may not respond
proportionally, giving odd color casts in the shadows, although the
mid-tones may have been correctly white-balanced under the enlarger.
Light sources with discontinuous spectra, such as fluorescent tubes,
cannot be fully corrected in printing either, since one of the layers
may barely have recorded an image at all.
Photographic film is made for specific light sources (most commonly daylight film and tungsten film), and, used properly, will create a neutral color print. Matching the sensitivity of the film
to the color temperature of the light source is one way to balance
color. If tungsten film is used indoors with incandescent lamps, the
yellowish-orange light of the tungsten
incandescent lamps will appear as white (3200 K) in the photograph.
Color negative film is almost always daylight-balanced, since it is
assumed that color can be adjusted in printing (with limitations, see
above). Color transparency film, being the final artefact in the
process, has to be matched to the light source or filters must be used
to correct color.
Filters on a camera lens, or color gels
over the light source(s) may be used to correct color balance. When
shooting with a bluish light (high color temperature) source such as on
an overcast day, in the shade, in window light, or if using tungsten
film with white or blue light, a yellowish-orange filter will correct
this. For shooting with daylight film (calibrated to 5600 K) under
warmer (low color temperature) light sources such as sunsets,
candlelight or tungsten lighting,
a bluish (e.g. #80A) filter may be used. More-subtle filters are needed
to correct for the difference between, say 3200 K and 3400 K tungsten
lamps or to correct for the slightly blue cast of some flash tubes,
which may be 6000 K.
If there is more than one light source with varied color
temperatures, one way to balance the color is to use daylight film and
place color-correcting gel filters over each light source.
Photographers sometimes use color temperature meters. These are
usually designed to read only two regions along the visible spectrum
(red and blue); more expensive ones read three regions (red, green, and
blue). However, they are ineffective with sources such as fluorescent or
discharge lamps, whose light varies in color and may be harder to
correct for. Because this light is often greenish, a magenta filter may
correct it. More sophisticated colorimetry tools can be used if such meters are lacking.
Desktop publishing
In the desktop publishing industry, it is important to know a
monitor’s color temperature. Color matching software, such as Apple's ColorSync
for Mac OS, measures a monitor's color temperature and then adjusts its
settings accordingly. This enables on-screen color to more closely
match printed color. Common monitor color temperatures, along with
matching standard illuminants in parentheses, are as follows:
- 5000 K (D50)
- 5500 K (D55)
- 6500 K (D65)
- 7500 K (D75)
- 9300 K
D50 is scientific shorthand for a standard illuminant:
the daylight spectrum at a correlated color temperature of 5000 K.
Similar definitions exist for D55, D65 and D75. Designations such as D50 are used to help classify color temperatures of light tables and viewing booths. When viewing a color slide
at a light table, it is important that the light be balanced properly
so that the colors are not shifted towards the red or blue.
Digital cameras, web graphics, DVDs, etc., are normally designed for a 6500 K color temperature. The sRGB standard commonly used for images on the Internet stipulates (among other things) a 6500 K display white point.
TV, video, and digital still cameras
The NTSC and PAL
TV norms call for a compliant TV screen to display an electrically
black and white signal (minimal color saturation) at a color temperature
of 6500 K. On many consumer-grade televisions, there is a very
noticeable deviation from this requirement. However, higher-end
consumer-grade televisions can have their color temperatures adjusted to
6500 K by using a preprogrammed setting or a custom calibration.
Current versions of ATSC
explicitly call for the color temperature data to be included in the
data stream, but old versions of ATSC allowed this data to be omitted.
In this case, current versions of ATSC cite default colorimetry
standards depending on the format. Both of the cited standards specify a
6500 K color temperature.
Most video and digital still cameras can adjust for color
temperature by zooming into a white or neutral colored object and
setting the manual "white balance" (telling the camera that "this object
is white"); the camera then shows true white as white and adjusts all
the other colors accordingly. White-balancing is necessary especially
when indoors under fluorescent lighting and when moving the camera from
one lighting situation to another. Most cameras also have an automatic
white balance function that attempts to determine the color of the light
and correct accordingly. While these settings were once unreliable,
they are much improved in today's digital cameras and produce an
accurate white balance in a wide variety of lighting situations.
Artistic application via control of color temperature
The
house above appears a light cream during midday, but seems to be bluish
white here in the dim light before full sunrise. Note the color
temperature of the sunrise in the background.
Video camera operators
can white-balance objects that are not white, downplaying the color of
the object used for white-balancing. For instance, they can bring more
warmth into a picture by white-balancing off something that is light
blue, such as faded blue denim; in this way white-balancing can replace a
filter or lighting gel when those are not available.
Cinematographers do not “white balance” in the same way as video camera operators; they use techniques such as filters, choice of film stock, pre-flashing, and, after shooting, color grading,
both by exposure at the labs and also digitally. Cinematographers also
work closely with set designers and lighting crews to achieve the
desired color effects.
For artists, most pigments and papers have a cool or warm cast,
as the human eye can detect even a minute amount of saturation. Gray
mixed with yellow, orange, or red is a “warm gray”. Green, blue, or
purple create “cool grays”. Note that this sense of temperature is the
reverse of that of real temperature; bluer is described as “cooler” even
though it corresponds to a higher-temperature black body.

| |
| "Warm" gray | "Cool" gray |
| Mixed with 6% yellow. | Mixed with 6% blue. |
Lighting designers sometimes select filters by color temperature, commonly to match light that is theoretically white. Since fixtures using discharge type lamps produce a light of a considerably higher color temperature than do tungsten lamps, using the two in conjunction could potentially produce a stark contrast, so sometimes fixtures with HID lamps,
commonly producing light of 6000–7000 K, are fitted with 3200 K filters
to emulate tungsten light. Fixtures with color mixing features or with
multiple colors, (if including 3200 K) are also capable of producing
tungsten-like light. Color temperature may also be a factor when
selecting lamps, since each is likely to have a different color temperature.
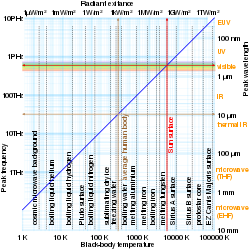
Log-log graphs of peak emission wavelength and radiant exitance vs black-body temperature – red arrows show that 5780 K black bodies have 501 nm peak wavelength and 63.3 MW/m² radiant exitance
The correlated color temperature (CCT, Tcp) is the temperature of the Planckian radiator whose perceived color most closely resembles that of a given stimulus at the same brightness and under specified viewing conditions
— CIE/IEC 17.4:1987, International Lighting Vocabulary (ISBN 3900734070)
Motivation
Black-body
radiators are the reference by which the whiteness of light sources is
judged. A black body can be described by its color temperature, whose
hues are depicted above. By analogy, nearly Planckian light sources such
as certain fluorescent or high-intensity discharge lamps
can be judged by their correlated color temperature (CCT), the color
temperature of the Planckian radiator that best approximates them. For
light source spectra that are not Planckian, color temperature is not a
well defined attribute; the concept of correlated color temperature was
developed to map such sources as well as possible onto the
one-dimensional scale of color temperature, where "as well as possible"
is defined in the context of an objective color space.
Background
Judd's (r,g) diagram. The concentric curves indicate the loci of constant purity.
Judd's
Maxwell triangle. Planckian locus in gray. Translating from trilinear
co-ordinates into Cartesian co-ordinates leads to the next diagram.
Judd's
uniform chromaticity space (UCS), with the Planckian locus and the
isotherms from 1000 K to 10000 K, perpendicular to the locus. Judd
calculated the isotherms in this space before translating them back into
the (x,y) chromaticity space, as depicted in the diagram at the top of
the article.
Close up of the Planckian locus in the CIE 1960 UCS, with the isotherms in mireds.
Note the even spacing of the isotherms when using the reciprocal
temperature scale and compare with the similar figure below. The even
spacing of the isotherms on the locus implies that the mired scale is a
better measure of perceptual color difference than the temperature
scale.
The notion of using Planckian radiators as a yardstick against which to judge other light sources is not new.
In 1923, writing about "grading of illuminants with reference to
quality of color ... the temperature of the source as an index of the
quality of color", Priest essentially described CCT as we understand it
today, going so far as to use the term "apparent color temperature", and
astutely recognized three cases:
- "Those for which the spectral distribution of energy is identical with that given by the Planckian formula."
- "Those for which the spectral distribution of energy is not identical with that given by the Planckian formula, but still is of such a form that the quality of the color evoked is the same as would be evoked by the energy from a Planckian radiator at the given color temperature."
- "Those for which the spectral distribution of energy is such that the color can be matched only approximately by a stimulus of the Planckian form of spectral distribution."
Several important developments occurred in 1931. In chronological order:
- Raymond Davis published a paper on "correlated color temperature" (his term). Referring to the Planckian locus on the r-g diagram, he defined the CCT as the average of the "primary component temperatures" (RGB CCTs), using trilinear coordinates.
- The CIE announced the XYZ color space.
- Deane B. Judd published a paper on the nature of "least perceptible differences" with respect to chromatic stimuli. By empirical means he determined that the difference in sensation, which he termed ΔE for a "discriminatory step between colors ... Empfindung" (German for sensation) was proportional to the distance of the colors on the chromaticity diagram. Referring to the (r,g) chromaticity diagram depicted aside, he hypothesized that
-
- KΔE = |c1 − c2| = max(|r1 − r2|, |g1 − g2|).
These developments paved the way for the development of new
chromaticity spaces that are more suited to estimating correlated color
temperatures and chromaticity differences. Bridging the concepts of
color difference and color temperature, Priest made the observation that
the eye is sensitive to constant differences in "reciprocal"
temperature:
A difference of one micro-reciprocal-degree (μrd) is fairly representative of the doubtfully perceptible difference under the most favorable conditions of observation.
Priest proposed to use "the scale of temperature as a scale for
arranging the chromaticities of the several illuminants in a serial
order". Over the next few years, Judd published three more significant
papers:
The first verified the findings of Priest, Davis, and Judd, with a paper on sensitivity to change in color temperature.
The second proposed a new chromaticity space, guided by a principle that has become the holy grail of color spaces: perceptual uniformity (chromaticity distance should be commensurate with perceptual difference). By means of a projective transformation,
Judd found a more "uniform chromaticity space" (UCS) in which to find
the CCT. Judd determined the "nearest color temperature" by simply
finding the point on the Planckian locus nearest to the chromaticity of the stimulus on Maxwell's color triangle, depicted aside. The transformation matrix he used to convert X,Y,Z tristimulus values to R,G,B coordinates was:
From this, one can find these chromaticities:
The third depicted the locus of the isothermal chromaticities on the CIE 1931 x,y chromaticity diagram. Since the isothermal points formed normals
on his UCS diagram, transformation back into the xy plane revealed them
still to be lines, but no longer perpendicular to the locus.
MacAdam's "uniform chromaticity scale" diagram; a simplification of Judd's UCS.
Calculation
Judd's
idea of determining the nearest point to the Planckian locus on a
uniform chromaticity space is current. In 1937, MacAdam suggested a
"modified uniform chromaticity scale diagram", based on certain
simplifying geometrical considerations:
This (u,v) chromaticity space became the CIE 1960 color space, which is still used to calculate the CCT (even though MacAdam did not devise it with this purpose in mind). Using other chromaticity spaces, such as u'v', leads to non-standard results that may nevertheless be perceptually meaningful.
Close up of the CIE 1960 UCS.
The isotherms are perpendicular to the Planckian locus, and are drawn
to indicate the maximum distance from the locus that the CIE considers
the correlated color temperature to be meaningful:
The distance from the locus (i.e., degree of departure from a black body) is traditionally indicated in units of ; positive for points above the locus. This concept of distance has evolved to become Delta E, which continues to be used today.
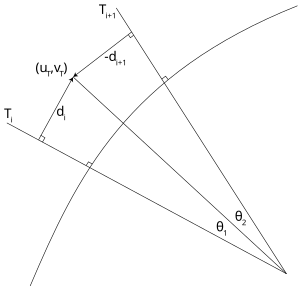
Robertson's method
Before the advent of powerful personal computers, it was common to estimate the correlated color temperature by way of interpolation from look-up tables and charts. The most famous such method is Robertson's, who took advantage of the relatively even spacing of the mired scale (see above) to calculate the CCT Tc using linear interpolation of the isotherm's mired values:
Computation of the CCT Tc corresponding to the chromaticity coordinate in the CIE 1960 UCS.
where and are the color temperatures of the look-up isotherms and i is chosen such that . (Furthermore, the test chromaticity lies between the only two adjacent lines for which .)
If the isotherms are tight enough, one can assume , leading to
The distance of the test point to the i-th isotherm is given by
where is the chromaticity coordinate of the i-th isotherm on the Planckian locus and mi is the isotherm's slope. Since it is perpendicular to the locus, it follows that where li is the slope of the locus at .
Precautions
Although
the CCT can be calculated for any chromaticity coordinate, the result
is meaningful only if the light sources are nearly white.
The CIE recommends that "The concept of correlated color temperature
should not be used if the chromaticity of the test source differs more
than [] from the Planckian radiator."
Beyond a certain value of , a chromaticity co-ordinate may be equidistant to two points on the locus, causing ambiguity in the CCT.
Approximation
If
a narrow range of color temperatures is considered—those encapsulating
daylight being the most practical case—one can approximate the Planckian
locus in order to calculate the CCT in terms of chromaticity
coordinates. Following Kelly's observation that the isotherms intersect
in the purple region near (x = 0.325, y = 0.154), McCamy proposed this cubic approximation:
where n = (x − xe)/(y - ye) is the inverse slope line, and (xe = 0.3320, ye = 0.1858)
is the "epicenter"; quite close to the intersection point mentioned by
Kelly. The maximum absolute error for color temperatures ranging from
2856 K (illuminant A) to 6504 K (D65) is under 2 K.
A more recent proposal, using exponential terms, considerably
extends the applicable range by adding a second epicenter for high color
temperatures:
where n is as before and the other constants are defined below:
|
|
3–50 kK | 50–800 kK |
|---|---|---|
| xe | 0.3366 | 0.3356 |
| ye | 0.1735 | 0.1691 |
| A0 | −949.86315 | 36284.48953 |
| A1 | 6253.80338 | 0.00228 |
| t1 | 0.92159 | 0.07861 |
| A2 | 28.70599 | 5.4535×10−36 |
| t2 | 0.20039 | 0.01543 |
| A3 | 0.00004 |
|
| t3 | 0.07125 |
|
The author suggests that one use the low-temperature equation to determine whether the higher-temperature parameters are needed.
Color rendering index
The CIE color rendering index
(CRI) is a method to determine how well a light source's illumination
of eight sample patches compares to the illumination provided by a
reference source. Cited together, the CRI and CCT give a numerical
estimate of what reference (ideal) light source best approximates a
particular artificial light, and what the difference is.
Spectral power distribution

Characteristic spectral power distributions (SPDs) for an incandescent lamp (left) and a fluorescent lamp (right). The horizontal axes are wavelengths in nanometers, and the vertical axes show relative intensity in arbitrary units.
Light sources and illuminants may be characterized by their spectral power distribution (SPD). The relative SPD curves provided by many manufacturers may have been produced using 10 nm increments or more on their spectroradiometer. The result is what would seem to be a smoother ("fuller spectrum")
power distribution than the lamp actually has. Owing to their spiky
distribution, much finer increments are advisable for taking
measurements of fluorescent lights, and this requires more expensive
equipment.
Color temperature in astronomy
In astronomy,
the color temperature is defined by the local slope of the SPD at a
given wavelength, or, in practice, a wavelength range. Given, for
example, the color magnitudes B and V which are calibrated to be equal for an A0V star (e.g. Vega), the stellar color temperature is given by the temperature for which the color index of a black-body radiator fits the stellar one. Besides the ,
other color indices can be used as well. The color temperature (as well
as the correlated color temperature defined above) may differ largely
from the effective temperature given by the radiative flux of the
stellar surface. For example, the color temperature of an A0V star is
about 15000 K compared to an effective temperature of about 9500 K.
- Characteristic spectral power distribution of an A0V star (Teff = 9500 K, cf. Vega) compared to black-body spectra. The 15000 K black-body spectrum (dashed line) matches the visible part of the stellar SPD much better than the black body of 9500 K. All spectra are normalized to intersect at 555 nanometers.