| Blunt trauma | |
|---|---|
| Other names | Blunt injury, non-penetrating trauma, blunt force trauma |
 | |
Blunt trauma is physical trauma to a body part, either by impact, injury or physical attack. The latter is often referred to as blunt force trauma, though it can also result from high-velocity impact. Blunt trauma is the initial trauma, from which develops more specific types such as contusions, abrasions, lacerations, and/or bone fractures. Blunt trauma is contrasted with penetrating trauma, in which an object such as a projectile or knife enters the body, though either can prove fatal.
Classification
Blunt abdominal trauma
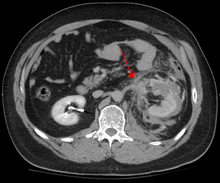
Abdominal CT showing left renal artery injury
Blunt abdominal trauma (BAT) represents 75% of all blunt trauma and is the most common example of this injury. The majority occurs in motor vehicle accidents, in which rapid deceleration may propel the driver into the steering wheel, dashboard, or seatbelt causing contusions in less serious cases, or rupture of internal organs from briefly increased intraluminal
pressure in the more serious, depending on the force applied.
Initially, there may be few indications that serious internal abdominal
injury has occurred, making assessment more challenging and requiring a
high degree of clinical suspicion.
There are two basic physical mechanisms at play with the potential of injury to intra-abdominal organs: compression and deceleration. The former occurs from a direct blow, such as a punch, or compression against a non-yielding object such as a seat belt or steering column.
This force may deform a hollow organ, increasing its intraluminal or internal pressure and possibly lead to rupture. Deceleration, on the other hand, causes stretching and shearing at the points where mobile contents in the abdomen, like bowel, are anchored. This can cause tearing of the mesentery of the bowel and injury to the blood vessels that travel within the mesentery. Classic examples of these mechanisms are a hepatic tear along the ligamentum teres and injuries to the renal arteries.
When blunt abdominal trauma is complicated by 'internal injury,' the liver and spleen (see blunt splenic trauma) are most frequently involved, followed by the small intestine.
In rare cases, this injury has been attributed to medical techniques such as the Heimlich Maneuver, attempts at CPR and manual thrusts to clear an airway.
Although these are rare examples, it has been suggested that they are
caused by applying excessive pressure when performing these life-saving
techniques. Finally, the occurrence of splenic rupture with mild blunt
abdominal trauma in those recovering from infectious mononucleosis or ‘mono’ is well reported.
Blunt abdominal trauma in sports
The supervised environment in which most sports injuries occur allows
for mild deviations from the traditional trauma treatment algorithms,
such as ATLS, due to the greater precision in identifying the mechanism
of injury. The priority in assessing blunt trauma in sports injuries is
separating contusions and musculo-tendinous injuries from injuries to
solid organs and the gut and recognizing potential for developing blood
loss, and reacting accordingly. Blunt injuries to the kidney from helmets, shoulder pads, and knees are described in American football, association football, martial arts, and all-terrain vehicle accidents.
A depiction of flail chest, a very serious blunt chest injury
Blunt thoracic trauma
The term blunt thoracic trauma or, put in a more familiar way, blunt chest injury, encompasses a variety of injuries to the chest. Broadly, this also includes damage caused by direct blunt force (such as a fist or a bat in an assault), acceleration or deceleration (such as that from a rear-end automotive accident), shear force (a combination of acceleration and deceleration), compression (such as a heavy object falling on a person), and blasts (such as an explosion of some sort). Common signs and symptoms include something as simple as bruising, but occasionally as complicated as hypoxia, ventilation-perfusion mismatch, hypovolemia, and reduced cardiac output due to the way the thoracic
organs may have been affected. Blunt thoracic trauma is not always
visible from the outside and such internal injuries may not show signs or symptoms
at the time the trauma initially occurs or even until hours after. A
high degree of clinical suspicion may sometimes be required to identify
such injuries, a CT scan
may prove useful in such instances. Those experiencing more obvious
complications from a blunt chest injury will likely undergo a focused
assessment with sonography for trauma (FAST)
which can reliably detect a significant amount of blood around the
heart or in the lung by using a special machine that visualizes sound
waves sent through the body. Only 10-15% of thoracic traumas require
surgery, but they can have serious impacts on the heart, lungs, and great vessels.
This table depicts mechanisms of blunt thoracic trauma and the most common injuries from each mechanism
The most immediate life-threatening injuries that may occur include tension pneumothorax, open pneumothorax, hemothorax, flail chest, cardiac tamponade, airway obstruction/rupture.
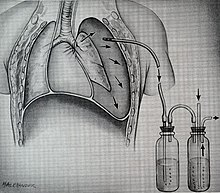
An example of a chest tube
The injuries may necessitate a procedure, with the most common being the insertion of an intercostal drain,
more commonly referred to as a chest tube. This tube is typically
placed because it helps restore a certain balance in pressures (usually
due to misplaced air or surrounding blood) that are impeding the lungs
ability to inflate and thus exchange vital gases that allow the body to
function. A less common procedure that may be employed is a pericardiocentesis which by removing blood surrounding the heart, permits the heart to regain some ability to appropriately pump blood. In certain dire circumstances an emergent thoracotomy may be employed.
Blunt cranial trauma
The
primary clinical concern when blunt trauma to the head occurs is damage
to the brain, although other structures, including the skull, face, orbits, and neck are also at risk. Following assessment of the patient's airway, circulation, and breathing, a cervical collar
may be placed if there is suspicion of trauma to the neck. Evaluation
of blunt trauma to the head continues with the secondary survey in which
evidence of cranial trauma, including bruises, contusions, lacerations,
and abrasions are noted. In addition to noting external injury, a
comprehensive neurologic exam is typically performed to assess for
damage to the brain. Depending on the mechanism of injury and
examination, a CT scan of the skull and brain may be ordered. This is
typically done to assess for blood within the skull, or fracture of the skull bones.
A CT-scan showing an epidural hematoma, a variety of intracranial bleeding commonly associated with blunt trauma to the temple region
Traumatic brain injury
Traumatic brain injury
(TBI) is a significant cause of morbidity and mortality and is most
commonly caused by falls, motor vehicle accidents, sports- and
work-related injuries, and assaults. It is the most common cause of
death in patients under the age of 25. TBI is graded from mild to
severe, with greater severity correlating with increased morbidity and
mortality.
Most patients with more severe traumatic brain injury have of a combination of intracranial injuries, which can include diffuse axonal injury, cerebral contusions, as well as intracranial bleeding, including subarachnoid hemorrhage, subdural hematoma, epidural hematoma, and intraparenchymal hemorrhage.
The recovery of brain function following a traumatic accident is highly
variable and depends upon the specific intracranial injuries that
occur, however there is significant correlation between the severity of
the initial insult as well as the level of neurologic function during
the initial assessment and the level of lasting neurologic deficits. Initial treatment may be targeted at reducing the intracranial pressure if there is concern for swelling or bleeding within this skull, which may require surgery such as a hemicraniectomy in which part of the skull is removed.
A fracture, an injury to the skeletal component of the upper extremity.
Blunt trauma to extremities
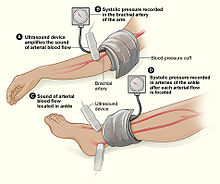
The Ankle-Brachial Index is depicted here. Note: ultrasound enhancement of pulses is not required but may be helpful.
Injury to extremities (like arms, legs, hands, feet) is extremely common. Falls are the most common etiology, making up as much as 30% of upper & 60% of lower extremity injuries. The most common mechanism for solely upper extremity injuries is machine operation or tool use. Work related accidents and vehicle crashes are also common causes. The injured extremity is examined for four major functional components which include soft tissues, nerves, vessels, and bones. Vessels are examined for expanding hematoma, bruit, distal pulse exam, and signs/symptoms of ischemia.
Essentially asking the question, “Does blood seem to be getting through
the injured area in a way that enough is getting to the parts past the
injury?” When it is not obvious that the answer to this question is, “yes,” an injured extremity index or ankle-brachial index may be used to help guide whether further evaluation with computed tomography arteriography.
This uses a special scanner and a substance that makes it easier to
examine the vessels in finer detail than what the human hand can feel or
the human eye can see. Soft tissue damage can lead to rhabdomyolysis (a rapid breakdown of injured muscle that can overwhelm the kidneys) or may potentially develop compartment syndrome (when pressure builds up in muscle compartments damages the nerves and vessels in the same compartment). Bones are evaluated with plain film x-ray or computed tomography if deformity (misshapen), bruising, or joint laxity (looser or more flexible than usual) are observed. Neurologic evaluation involves testing of the major nerve functions of the axillary, radial, and median nerves in the upper extremity as well as the femoral, sciatic, deep peroneal, and tibial nerves in the lower extremity. Surgical treatment may be necessary depending on the extent of injury and involved structures, but many are managed nonoperatively.
Blunt pelvic trauma
The most common causes of blunt pelvic trauma are motor vehicle accidents
and multiple-story falls, and thus pelvic injuries are commonly
associated with additional traumatic injuries in other locations. In the pelvis specifically, the structures at risk include the pelvic bones, the proximal femur, major blood vessels such as the iliac arteries, the urinary tract, reproductive organs, and the rectum.
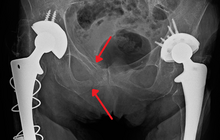
An X-ray showing a fracture of the inferior and superior pubic rami in a patient with previous hip replacements
One of the primary concerns is the risk of pelvic fracture, which itself is associated with a myriad of complications including bleeding, damage to the urethra and bladder, and nerve damage. If pelvic trauma is suspected, emergency medical services personnel may place a pelvic binder
on patients to stabilize the patient's pelvis and prevent further
damage to these structures while patients are transported to a hospital.
During the evaluation of trauma patients in an emergency department,
the stability of the pelvis is typically assessed by the healthcare
provider to determine whether fracture may have occurred. Providers may
then decide to order imaging such as an X-ray or CT scan
to detect fractures; however, if there is concern for life-threatening
bleeding, patients should receive an X-ray of the pelvis.
Following initial treatment of the patient, fractures may be need to be
treated surgically if significant, while some minor fractures may heal
without requiring surgery.
A life-threatening concern is hemorrhage, which may result from damage to the aorta, iliac arteries or veins in the pelvis. The majority of bleeding due to pelvic trauma is due to injury to the veins. Fluid (often blood) may be detected in the pelvis via ultrasound during the FAST scan
that is often performed following traumatic accidents. Should a patient
appear hemodynamically unstable in the absence of obvious blood on the
FAST scan, there may be concern for bleeding into the retroperitoneal space, known as retroperitoneal hematoma. Stopping the bleeding may require endovascular intervention or surgery, depending on the location and severity.
Diagnosis
In
most settings, the initial evaluation and stabilization of traumatic
injury follows the same general principles of identifying and treating
immediately life-threatening injuries. In the US, the American College of Surgeons
publishes the Advanced Trauma Life Support guidelines, which provide a
step-by-step approach to the initial assessment, stabilization,
diagnostic reasoning, and treatment of traumatic injuries that codifies
this general principle.
The assessment typically begins by ensuring that the subject's airway
is open and competent, that breathing is unlabored, and that
circulation—i.e. pulses that can be felt—is present. This is sometimes
described as the "A, B, C's"—Airway, Breathing, and Circulation—and is
the first step in any resuscitation or triage. Then, the history of the
accident or injury is amplified with any medical, dietary (timing of
last oral intake) and past history, from whatever sources such as
family, friends, previous treating physicians that might be available.
This method is sometimes given the mnemonic "SAMPLE".
The amount of time spent on diagnosis should be minimized and
expedited by a combination of clinical assessment and appropriate use of
technology, such as diagnostic peritoneal lavage (DPL), or bedside ultrasound examination (FAST) before proceeding to laparotomy if required. If time and the patient's stability permits, CT examination may be carried out if available.
Its advantages include superior definition of the injury, leading to
grading of the injury and sometimes the confidence to avoid or postpone
surgery. Its disadvantages include the time taken to acquire images,
although this gets shorter with each generation of scanners, and the
removal of the patient from the immediate view of the emergency or
surgical staff. Many providers use the aid of a algorithm such as the ATLS
guidelines to determine which images to obtain following the initial
assessment. These algorithms take into account the mechanism of injury, physical examination, and patient's vital signs to determine whether patients should have imaging or proceed directly to surgery.
Recently, criteria have been defined that might allow patients
with blunt abdominal trauma to be discharged safely without further
evaluation. The characteristics of such patients would include:
- absence of intoxication
- no evidence of lowered blood pressure or raised pulse rate
- no abdominal pain or tenderness
- no blood in the urine.
To be considered low risk, patients would need to meet all low-risk criteria.
Treatment
When
blunt trauma is significant enough to require evaluation by a
healthcare provider, treatment is typically aimed at treating
life-threatening injuries, which requires ensuring the patient is able
to breathe and preventing ongoing blood loss. If there is evidence that
the patient has lost blood, one or more intravenous lines may be placed and crystalloid solutions and/or blood will be administered at rates sufficient to maintain the circulation.
In the United States, surgical treatment of trauma typically follows the advanced trauma life support guidelines, which are developed by the American College of Surgeons.
These guidelines use evidence-based algorithms to determine whether
immediate surgery is warranted based on the patient's vital signs and
whether or not there is evidence of ongoing internal or external
bleeding. Further treatment depends on the severity of organ damage
estimated by the exam and any diagnostic studies. Ultimately treatment
will vary from close observation with the ability to intervene quickly,
to surgery, which may be open or laparoscopic. In the case of blunt abdominal trauma, there is no shown benefit from surgery unless bleeding or peritonitis is present.