From Wikipedia, the free encyclopedia
Carbon nanotubes (CNTs) are tubes made of carbon with diameters typically measured in nanometers.
Carbon nanotubes often refer to single-wall carbon nanotubes (SWCNTs) with diameters in the range of a nanometer. Single-wall carbon nanotubes are one of the allotropes of carbon, intermediate between fullerene cages and flat graphene.
Although not made this way, single-wall carbon nanotubes can be idealized as cutouts from a two-dimensional hexagonal lattice of carbon atoms rolled up along one of the Bravais lattice
vectors of the hexagonal lattice to form a hollow cylinder. In this
construction, periodic boundary conditions are imposed over the length
of this roll-up vector to yield a helical lattice of seamlessly bonded
carbon atoms on the cylinder surface.
Carbon nanotubes also often refer to multi-wall carbon nanotubes (MWCNTs) consisting of nested single-wall carbon nanotubes weakly bound together by van der Waals interactions
in a tree ring-like structure. If not identical, these tubes are very
similar to Oberlin, Endo, and Koyama's long straight and parallel
carbon layers cylindrically arranged around a hollow tube. Multi-wall carbon nanotubes are also sometimes used to refer to double- and triple-wall carbon nanotubes.
Carbon nanotubes can also refer to tubes with an undetermined
carbon-wall structure and diameters less than 100 nanometers. Such tubes
were discovered in 1952 by Radushkevich and Lukyanovich.
While nanotubes of other compositions
exist, most research has been focused on the carbon ones. Therefore,
the "carbon" qualifier is often left implicit in the acronyms, and the
names are abbreviated NT, SWNT, and MWNT.
The length of a carbon nanotube produced by common production
methods is often not reported, but is typically much larger than its
diameter. Thus, for many purposes, end effects are neglected and the
length of carbon nanotubes is assumed infinite.
Carbon nanotubes can exhibit remarkable electrical conductivity, while others are semiconductors. They also have exceptional tensile strength and thermal conductivity because of their nanostructure and strength of the bonds between carbon atoms. In addition, they can be chemically modified. These properties are expected to be valuable in many areas of technology, such as electronics, optics, composite materials (replacing or complementing carbon fibers), nanotechnology, and other applications of materials science.
Rolling up a hexagonal lattice along different directions to form
different infinitely long single-wall carbon nanotubes shows that all
of these tubes not only have helical but also translational symmetry
along the tube axis and many also have nontrivial rotational symmetry
about this axis. In addition, most are chiral,
meaning the tube and its mirror image cannot be superimposed. This
construction also allows single-wall carbon nanotubes to be labeled by a
pair of integers.
A special group of achiral single-wall carbon nanotubes are metallic, but all the rest are either small or moderate band gap semiconductors.
These electrical properties, however, do not depend on whether the
hexagonal lattice is rolled from its back to front or from its front to
back and hence are the same for the tube and its mirror image.
Structure of single-walled tubes
The
structure of an ideal (infinitely long) single-walled carbon nanotube
is that of a regular hexagonal lattice drawn on an infinite cylindrical
surface, whose vertices are the positions of the carbon atoms. Since
the length of the carbon-carbon bonds is fairly fixed, there are
constraints on the diameter of the cylinder and the arrangement of the
atoms on it.
The zigzag and armchair configurations
In the study of nanotubes, one defines a zigzag path on a graphene-like lattice as a path
that turns 60 degrees, alternating left and right, after stepping
through each bond. It is also conventional to define an armchair path as
one that makes two left turns of 60 degrees followed by two right turns
every four steps.
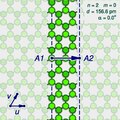
On some carbon nanotubes, there is a closed zigzag path that goes around the tube. One says that the tube is of the zigzag type or configuration, or simply is a zigzag nanotube. If the tube is instead encircled by a closed armchair path, it is said to be of the armchair type, or an armchair nanotube.
Zigzag nanotube, configuration (8, 0) |
|
Armchair nanotube, configuration (4, 4) |
An infinite nanotube that is of the zigzag (or armchair) type
consists entirely of closed zigzag (or armchair) paths, connected to
each other.
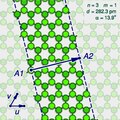
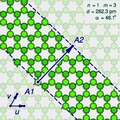
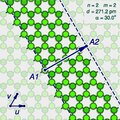
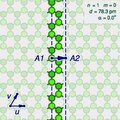
The (n,m) notation
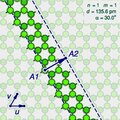
A
"sliced and unrolled" representation of a carbon nanotube as a strip of
a graphene molecule, overlaid on diagram of the full molecule (faint
background). The arrow shows the gap A2 where the atom A1 on one edge of the strip would fit in the opposite edge, as the strip is rolled up.
The zigzag and armchair configurations are not the only structures
that a single-walled nanotube can have. To describe the structure of a
general infinitely long tube, one should imagine it being sliced open by
a cut parallel to its axis, that goes through some atom A, and
then unrolled flat on the plane, so that its atoms and bonds coincide
with those of an imaginary graphene sheet—more precisely, with an
infinitely long strip of that sheet.
The two halves of the atom A will end up on opposite edges of the strip, over two atoms A1 and A2 of the graphene. The line from A1 to A2 will correspond to the circumference of the cylinder that went through the atom A, and will be perpendicular to the edges of the strip.
In the graphene lattice, the atoms can be split into two classes,
depending on the directions of their three bonds. Half the atoms have
their three bonds directed the same way, and half have their three bonds
rotated 180 degrees relative to the first half. The atoms A1 and A2, which correspond to the same atom A on the cylinder, must be in the same class.
It follows that the circumference of the tube and the angle of
the strip are not arbitrary, because they are constrained to the lengths
and directions of the lines that connect pairs of graphene atoms in the
same class.
The basis vectors u and v
of the relevant sub-lattice, the (n,m) pairs that define non-isomorphic
carbon nanotube structures (red dots), and the pairs that define the
enantiomers of the chiral ones (blue dots).
Let u and v be two linearly independent vectors that connect the graphene atom A1
to two of its nearest atoms with the same bond directions. That is, if
one numbers consecutive carbons around a graphene cell with C1 to C6,
then u can be the vector from C1 to C3, and v be the vector from C1 to C5. Then, for any other atom A2 with same class as A1, the vector from A1 to A2 can be written as a linear combination n u + m v, where n and m are integers. And, conversely, each pair of integers (n,m) defines a possible position for A2.
Given n and m, one can reverse this theoretical operation by drawing the vector w on the graphene lattice, cutting a strip of the latter along lines perpendicular to w through its endpoints A1 and A2, and rolling the strip into a cylinder so as to bring those two points together. If this construction is applied to a pair (k,0), the result is a zigzag nanotube, with closed zigzag paths of 2k atoms. If it is applied to a pair (k,k), one obtains an armchair tube, with closed armchair paths of 4k atoms.
Nanotube types
Moreover, the structure of the nanotube is not changed if the strip is rotated by 60 degrees clockwise around A1 before applying the hypothetical reconstruction above. Such a rotation changes the corresponding pair (n,m) to the pair (−2m,n+m).
It follows that many possible positions of A2 relative to A1 — that is, many pairs (n,m)
— correspond to the same arrangement of atoms on the nanotube. That is
the case, for example, of the six pairs (1,2), (−2,3), (−3,1), (−1,−2),
(2,−3), and (3,−1). In particular, the pairs (k,0) and (0,k) describe the same nanotube geometry.
These redundancies can be avoided by considering only pairs (n,m) such that n > 0 and m ≥ 0; that is, where the direction of the vector w lies between those of u (inclusive) and v (exclusive). It can be verified that every nanotube has exactly one pair (n,m) that satisfies those conditions, which is called the tube's type.
Conversely, for every type there is a hypothetical nanotube. In fact,
two nanotubes have the same type if and only if one can be conceptually
rotated and translated so as to match the other exactly.
Instead of the type (n,m), the structure of a carbon nanotube can be specified by giving the length of the vector w (that is, the circumference of the nanotube), and the angle α between the directions of u and w, which may range from 0 (inclusive) to 60 degrees clockwise (exclusive). If the diagram is drawn with u horizontal, the latter is the tilt of the strip away from the vertical.
Here are some unrolled nanotube diagrams:
Chiral nanotube of the (3,1) type.
Chiral nanotube of the (1,3) type, mirror image of the (3,1) type.
Nanotube of the (2,2) type, the narrowest "armchair" one
Nanotube of the (3,0) type, the narrowest "zigzag" one
Chirality and mirror symmetry
A nanotube is chiral if it has type (n,m), with m > 0 and m ≠ n; then its enantiomer (mirror image) has type (m,n), which is different from (n,m). This operation corresponds to mirroring the unrolled strip about the line L through A1 that makes an angle of 30 degrees clockwise from the direction of the u vector (that is, with the direction of the vector u+v). The only types of nanotubes that are achiral are the (k,0) "zigzag" tubes and the (k,k) "armchair" tubes.
If two enantiomers are to be considered the same structure, then one may consider only types (n,m) with 0 ≤ m ≤ n and n > 0. Then the angle α between u and w, which may range from 0 to 30 degrees (inclusive both), is called the "chiral angle" of the nanotube.
Circumference and diameter
From n and m one can also compute the circumference c, which is the length of the vector w, which turns out to be

in picometres. The diameter  of the tube is then
of the tube is then  , that is
, that is

also in picometres. (These formulas are only approximate, especially for small n and m where the bonds are strained; and they do not take into account the thickness of the wall.)
The tilt angle α between u and w and the circumference c are related to the type indices n and m by

where arg(x,y) is the clockwise angle between the X-axis and the vector (x,y); a function that is available in many programming languages as atan2(y,x). Conversely, given c and α, one can get the type (n,m) by the formulas

which must evaluate to integers.
Physical limits
Narrowest nanotubes
If n and m are too small, the structure described by the pair (n,m)
will describe a molecule that cannot be reasonably called a "tube", and
may not even be stable. For example, the structure theoretically
described by the pair (1,0) (the limiting "zigzag" type) would be just a
chain of carbons. That is a real molecule, the carbyne;
which has some characteristics of nanotubes (such as orbital
hybridization, high tensile strength, etc.) — but has no hollow space,
and may not be obtainable as a condensed phase. The pair (2,0) would
theoretically yield a chain of fused 4-cycles; and (1,1), the limiting
"armchair" structure, would yield a chain of bi-connected 4-rings. These
structures may not be realizable.
The thinnest carbon nanotube proper is the armchair structure
with type (2,2), which has a diameter of 0.3 nm. This nanotube was grown
inside a multi-walled carbon nanotube. Assigning of the carbon nanotube
type was done by a combination of high-resolution transmission electron microscopy (HRTEM), Raman spectroscopy, and density functional theory (DFT) calculations.
The thinnest freestanding single-walled carbon nanotube is about 0.43 nm in diameter.
Researchers suggested that it can be either (5,1) or (4,2) SWCNT, but
the exact type of the carbon nanotube remains questionable.
(3,3), (4,3), and (5,1) carbon nanotubes (all about 0.4 nm in diameter)
were unambiguously identified using aberration-corrected high-resolution transmission electron microscopy inside double-walled CNTs.
Here are some tube types that are "degenerate" for being too narrow:
Degenerate "zigzag" tube type (1,0)
Degenerate "zigzag" tube type (2,0)
Degenerate "armchair" tube type (1,1)
Possibly degenerate chiral tube type (2,1)
Length
The observation of the longest carbon nanotubes grown so far, around 1/2 meter (550 mm long), was reported in 2013. These nanotubes were grown on silicon substrates using an improved chemical vapor deposition (CVD) method and represent electrically uniform arrays of single-walled carbon nanotubes.
The shortest carbon nanotube can be considered to be the organic compound cycloparaphenylene, which was synthesized in 2008 by Ramesh Jasti. Other small molecule carbon nanotubes have been synthesized since.
Density
The highest density of CNTs was achieved in 2013, grown on a conductive titanium-coated copper surface that was coated with co-catalysts cobalt and molybdenum at lower than typical temperatures of 450 °C. The tubes averaged a height of 380 nm and a mass density of 1.6 g cm−3. The material showed ohmic conductivity (lowest resistance ∼22 kΩ).
Variants
There
is no consensus on some terms describing carbon nanotubes in scientific
literature: both "-wall" and "-walled" are being used in combination
with "single", "double", "triple", or "multi", and the letter C is often
omitted in the abbreviation, for example, multi-walled carbon nanotube
(MWNT). International Standards Organization uses single-wall or multi-wall in its documents.
Multi-walled
Triple-walled armchair carbon nanotube
Multi-walled nanotubes (MWNTs) consist of multiple rolled layers
(concentric tubes) of graphene. There are two models that can be used to
describe the structures of multi-walled nanotubes. In the Russian Doll
model, sheets of graphite are arranged in concentric cylinders, e.g., a
(0,8) single-walled nanotube (SWNT) within a larger (0,17)
single-walled nanotube. In the Parchment
model, a single sheet of graphite is rolled in around itself,
resembling a scroll of parchment or a rolled newspaper. The interlayer
distance in multi-walled nanotubes is close to the distance between
graphene layers in graphite, approximately 3.4 Å. The Russian Doll
structure is observed more commonly. Its individual shells can be
described as SWNTs, which can be metallic or semiconducting. Because of
statistical probability and restrictions on the relative diameters of
the individual tubes, one of the shells, and thus the whole MWNT, is
usually a zero-gap metal.
Double-walled carbon nanotubes (DWNTs) form a special class of nanotubes because their morphology and properties are similar to those of SWNTs but they are more resistant to attacks by chemicals. This is especially important when it is necessary to graft chemical functions to the surface of the nanotubes (functionalization) to add properties to the CNT. Covalent functionalization of SWNTs will break some C=C double bonds,
leaving "holes" in the structure on the nanotube and thus modifying
both its mechanical and electrical properties. In the case of DWNTs,
only the outer wall is modified. DWNT synthesis on the gram-scale by the
CCVD technique was first proposed in 2003 from the selective reduction of oxide solutions in methane and hydrogen.
The telescopic motion ability of inner shells and their unique mechanical properties will permit the use of multi-walled nanotubes as the main movable arms in upcoming nanomechanical devices. The retraction force that occurs to telescopic motion is caused by the Lennard-Jones interaction between shells, and its value is about 1.5 nN.
Junctions and crosslinking
Transmission electron microscope image of carbon nanotube junction
Junctions between two or more nanotubes have been widely discussed theoretically. Such junctions are quite frequently observed in samples prepared by arc discharge as well as by chemical vapor deposition. The electronic properties of such junctions were first considered theoretically by Lambin et al.,
who pointed out that a connection between a metallic tube and a
semiconducting one would represent a nanoscale heterojunction. Such a
junction could therefore form a component of a nanotube-based electronic
circuit. The adjacent image shows a junction between two multiwalled
nanotubes.
Junctions between nanotubes and graphene have been considered theoretically and studied experimentally. Nanotube-graphene junctions form the basis of pillared graphene, in which parallel graphene sheets are separated by short nanotubes. Pillared graphene represents a class of three-dimensional carbon nanotube architectures.
Recently, several studies have highlighted the prospect of using
carbon nanotubes as building blocks to fabricate three-dimensional
macroscopic (>100 nm in all three dimensions) all-carbon devices.
Lalwani et al. have reported a novel radical-initiated thermal
crosslinking method to fabricate macroscopic, free-standing, porous,
all-carbon scaffolds using single- and multi-walled carbon nanotubes as
building blocks.
These scaffolds possess macro-, micro-, and nano-structured pores, and
the porosity can be tailored for specific applications. These 3D
all-carbon scaffolds/architectures may be used for the fabrication of
the next generation of energy storage, supercapacitors, field emission
transistors, high-performance catalysis, photovoltaics, and biomedical
devices, implants, and sensors.
Other morphologies
Carbon nanobuds are a newly created material combining two previously discovered allotropes of carbon: carbon nanotubes and fullerenes.
In this new material, fullerene-like "buds" are covalently bonded to
the outer sidewalls of the underlying carbon nanotube. This hybrid
material has useful properties of both fullerenes and carbon nanotubes.
In particular, they have been found to be exceptionally good field emitters. In composite materials,
the attached fullerene molecules may function as molecular anchors
preventing slipping of the nanotubes, thus improving the composite's
mechanical properties.
A carbon peapod
is a novel hybrid carbon material which traps fullerene inside a carbon
nanotube. It can possess interesting magnetic properties with heating
and irradiation. It can also be applied as an oscillator during
theoretical investigations and predictions.
In theory, a nanotorus is a carbon nanotube bent into a torus
(doughnut shape). Nanotori are predicted to have many unique
properties, such as magnetic moments 1000 times larger than that
previously expected for certain specific radii. Properties such as magnetic moment, thermal stability, etc. vary widely depending on the radius of the torus and the radius of the tube.
Graphenated carbon nanotubes are a relatively new hybrid that combines graphitic
foliates grown along the sidewalls of multiwalled or bamboo style CNTs.
The foliate density can vary as a function of deposition conditions
(e.g., temperature and time) with their structure ranging from a few
layers of graphene (< 10) to thicker, more graphite-like. The fundamental advantage of an integrated graphene-CNT
structure is the high surface area three-dimensional framework of the
CNTs coupled with the high edge density of graphene. Depositing a high
density of graphene foliates along the length of aligned CNTs can
significantly increase the total charge capacity per unit of nominal area as compared to other carbon nanostructures.
Cup-stacked carbon nanotubes (CSCNTs) differ from other quasi-1D
carbon structures, which normally behave as quasi-metallic conductors of
electrons. CSCNTs exhibit semiconducting behavior because of the
stacking microstructure of graphene layers.
Properties
Many properties of single-walled carbon nanotubes depend significantly on the (n,m) type, and this dependence is non-monotonic. In particular, the band gap can vary from zero to about 2 eV and the electrical conductivity can show metallic or semiconducting behavior.
Mechanical
Carbon nanotubes are the strongest and stiffest materials yet discovered in terms of tensile strength and elastic modulus. This strength results from the covalent sp2
bonds formed between the individual carbon atoms. In 2000, a
multiwalled carbon nanotube was tested to have a tensile strength of 63
gigapascals (9,100,000 psi).
(For illustration, this translates into the ability to endure tension
of a weight equivalent to 6,422 kilograms-force (62,980 N; 14,160 lbf)
on a cable with cross-section of 1 square millimetre (0.0016 sq in)).
Further studies, such as one conducted in 2008, revealed that individual
CNT shells have strengths of up to ≈100 gigapascals (15,000,000 psi),
which is in agreement with quantum/atomistic models. Because carbon nanotubes have a low density for a solid of 1.3 to 1.4 g/cm3, its specific strength of up to 48,000 kN·m·kg−1 is the best of known materials, compared to high-carbon steel's 154 kN·m·kg−1.
Although the strength of individual CNT shells is extremely high,
weak shear interactions between adjacent shells and tubes lead to
significant reduction in the effective strength of multiwalled carbon
nanotubes and carbon nanotube bundles down to only a few GPa.
This limitation has been recently addressed by applying high-energy
electron irradiation, which crosslinks inner shells and tubes, and
effectively increases the strength of these materials to ≈60 GPa for
multiwalled carbon nanotubes and ≈17 GPa for double-walled carbon nanotube bundles. CNTs are not nearly as strong under compression. Because of their hollow structure and high aspect ratio, they tend to undergo buckling when placed under compressive, torsional, or bending stress.
On the other hand, there was evidence that in the radial direction they are rather soft. The first transmission electron microscope observation of radial elasticity suggested that even van der Waals forces can deform two adjacent nanotubes. Later, nanoindentations with an atomic force microscope
were performed by several groups to quantitatively measure radial
elasticity of multiwalled carbon nanotubes and tapping/contact mode atomic force microscopy
was also performed on single-walled carbon nanotubes. Young's modulus
of on the order of several GPa showed that CNTs are in fact very soft in
the radial direction.
Electrical
Band
structures computed using tight binding approximation for (6,0) CNT
(zigzag, metallic), (10,2) CNT (semiconducting) and (10,10) CNT
(armchair, metallic).
Unlike graphene, which is a two-dimensional semimetal, carbon
nanotubes are either metallic or semiconducting along the tubular axis.
For a given (n,m) nanotube, if n = m, the nanotube is metallic; if n − m
is a multiple of 3 and n ≠ m, then the nanotube is quasi-metallic with a
very small band gap, otherwise the nanotube is a moderate semiconductor.
Thus, all armchair (n = m) nanotubes are metallic, and nanotubes (6,4), (9,1), etc. are semiconducting.
Carbon nanotubes are not semimetallic because the degenerate point (the
point where the π [bonding] band meets the π* [anti-bonding] band, at
which the energy goes to zero) is slightly shifted away from the K
point in the Brillouin zone because of the curvature of the tube
surface, causing hybridization between the σ* and π* anti-bonding bands,
modifying the band dispersion.
The rule regarding metallic versus semiconductor behavior has
exceptions because curvature effects in small-diameter tubes can
strongly influence electrical properties. Thus, a (5,0) SWCNT that
should be semiconducting in fact is metallic according to the
calculations. Likewise, zigzag and chiral SWCNTs with small diameters
that should be metallic have a finite gap (armchair nanotubes remain
metallic). In theory, metallic nanotubes can carry an electric current density of 4 × 109 A/cm2, which is more than 1,000 times greater than those of metals such as copper, where for copper interconnects, current densities are limited by electromigration. Carbon nanotubes are thus being explored as interconnects
and conductivity-enhancing components in composite materials, and many
groups are attempting to commercialize highly conducting electrical wire
assembled from individual carbon nanotubes. There are significant
challenges to be overcome however, such as undesired current saturation
under voltage,
and the much more resistive nanotube-to-nanotube junctions and
impurities, all of which lower the electrical conductivity of the
macroscopic nanotube wires by orders of magnitude, as compared to the
conductivity of the individual nanotubes.
Because of its nanoscale cross-section, electrons propagate only
along the tube's axis. As a result, carbon nanotubes are frequently
referred to as one-dimensional conductors. The maximum electrical conductance of a single-walled carbon nanotube is 2G0, where G0 = 2e2/h is the conductance of a single ballistic quantum channel.
Because of the role of the π-electron system in determining the electronic properties of graphene, doping
in carbon nanotubes differs from that of bulk crystalline
semiconductors from the same group of the periodic table (e.g.,
silicon). Graphitic substitution of carbon atoms in the nanotube wall by
boron or nitrogen dopants leads to p-type and n-type behavior,
respectively, as would be expected in silicon. However, some
non-substitutional (intercalated or adsorbed) dopants introduced into a
carbon nanotube, such as alkali metals and electron-rich metallocenes,
result in n-type conduction because they donate electrons to the
π-electron system of the nanotube. By contrast, π-electron acceptors
such as FeCl3 or electron-deficient metallocenes function as
p-type dopants because they draw π-electrons away from the top of the
valence band.
Intrinsic superconductivity has been reported, although other experiments found no evidence of this, leaving the claim a subject of debate.
In 2021, Michael Strano, the Carbon P. Dubbs Professor of
Chemical Engineering at MIT, published department findings on the use of
carbon nanotubes to create an electrical current.
By immersing the structures in an organic solvent, the liquid drew
electrons out of the carbon particles. Strano was quoted as saying,
"This allows you to do electrochemistry, but with no wires," and
represents a significant breakthrough in the technology.
Future applications include powering micro- or nanoscale robots, as
well as driving alcohol oxidation reactions, which are important in the
chemicals industry.
Optical
Carbon nanotubes have useful absorption, photoluminescence (fluorescence), and Raman spectroscopy
properties. Spectroscopic methods offer the possibility of quick and
non-destructive characterization of relatively large amounts of carbon
nanotubes. There is a strong demand for such characterization from the
industrial point of view: numerous parameters of nanotube synthesis
can be changed, intentionally or unintentionally, to alter the nanotube
quality. As shown below, optical absorption, photoluminescence, and
Raman spectroscopies allow quick and reliable characterization of this
"nanotube quality" in terms of non-tubular carbon content, structure
(chirality) of the produced nanotubes, and structural defects. These
features determine nearly any other properties such as optical,
mechanical, and electrical properties.
Carbon nanotubes are unique "one-dimensional systems" which can be envisioned as rolled single sheets of graphite (or more precisely graphene).
This rolling can be done at different angles and curvatures resulting
in different nanotube properties. The diameter typically varies in the
range 0.4–40 nm (i.e., "only" ~100 times), but the length can vary
~100,000,000,000 times, from 0.14 nm to 55.5 cm. The nanotube aspect ratio, or the length-to-diameter ratio, can be as high as 132,000,000:1,
which is unequalled by any other material. Consequently, all the
properties of the carbon nanotubes relative to those of typical
semiconductors are extremely anisotropic (directionally dependent) and tunable.
Whereas mechanical, electrical, and electrochemical (supercapacitor) properties of the carbon nanotubes are well established and have immediate applications, the practical use of optical properties is yet unclear. The aforementioned tunability of properties is potentially useful in optics and photonics. In particular, light-emitting diodes (LEDs) and photo-detectors
based on a single nanotube have been produced in the lab. Their unique
feature is not the efficiency, which is yet relatively low, but the
narrow selectivity in the wavelength of emission and detection of light and the possibility of its fine tuning through the nanotube structure. In addition, bolometer and optoelectronic memory devices have been realised on ensembles of single-walled carbon nanotubes.
Crystallographic defects also affect the tube's electrical
properties. A common result is lowered conductivity through the
defective region of the tube. A defect in armchair-type tubes (which can
conduct electricity) can cause the surrounding region to become
semiconducting, and single monatomic vacancies induce magnetic
properties.
Thermal
All nanotubes are expected to be very good thermal conductors along the tube, exhibiting a property known as "ballistic conduction",
but good insulators lateral to the tube axis. Measurements show that an
individual SWNT has a room-temperature thermal conductivity along its
axis of about 3500 W·m−1·K−1; compare this to copper, a metal well known for its good thermal conductivity, which transmits 385 W·m−1·K−1. An individual SWNT has a room-temperature thermal conductivity lateral to its axis (in the radial direction) of about 1.52 W·m−1·K−1,
which is about as thermally conductive as soil. Macroscopic assemblies
of nanotubes such as films or fibres have reached up to 1500 W·m−1·K−1 so far.
Networks composed of nanotubes demonstrate different values of thermal
conductivity, from the level of thermal insulation with the thermal
conductivity of 0.1 W·m−1·K−1 to such high values.
That is dependent on the amount of contribution to the thermal
resistance of the system caused by the presence of impurities,
misalignments and other factors. The temperature stability of carbon
nanotubes is estimated to be up to 2800 °C in vacuum and about 750 °C in air.
Crystallographic defects strongly affect the tube's thermal properties. Such defects lead to phonon scattering, which in turn increases the relaxation rate of the phonons. This reduces the mean free path
and reduces the thermal conductivity of nanotube structures. Phonon
transport simulations indicate that substitutional defects such as
nitrogen or boron will primarily lead to scattering of high-frequency
optical phonons. However, larger-scale defects such as Stone Wales defects cause phonon scattering over a wide range of frequencies, leading to a greater reduction in thermal conductivity.
Synthesis
Techniques have been developed to produce nanotubes in sizable quantities, including arc discharge, laser ablation, chemical vapor deposition (CVD) and high-pressure carbon monoxide disproportionation (HiPCO). Among these arc discharge, laser ablation, chemical vapor deposition (CVD) are batch by batch process and HiPCO is gas phase continuous process.
Most of these processes take place in a vacuum or with process gases.
The CVD growth method is popular, as it yields high quantity and has a
degree of control over diameter, length and morphology. Using
particulate catalysts, large quantities of nanotubes can be synthesized
by these methods, but achieving the repeatability becomes a major
problem with CVD growth. The HiPCO process advances in catalysis and continuous growth are making CNTs more commercially viable.
The HiPCO process helps in producing high purity single walled carbon
nanotubes in higher quantity. The HiPCO reactor operates at high temperature 900-1100 °C and high pressure ~30-50 bar. It uses carbon monoxide as the carbon source and iron pentacarbonyl or nickel tetracarbonyl as a catalyst. These catalyst acts as the nucleation site for the nanotubes to grow.
Vertically aligned carbon nanotube arrays
are also grown by thermal chemical vapor deposition. A substrate
(quartz, silicon, stainless steel, etc.) is coated with a catalytic
metal (Fe, Co, Ni) layer. Typically that layer is iron, and is deposited
via sputtering to a thickness of 1–5 nm. A 10–50 nm underlayer of
alumina is often also put down on the substrate first. This imparts
controllable wetting and good interfacial properties.
When the substrate is heated to the growth temperature (~700 °C), the
continuous iron film breaks up into small islands... each island then
nucleates a carbon nanotube. The sputtered thickness controls the island
size, and this in turn determines the nanotube diameter. Thinner iron
layers drive down the diameter of the islands, and they drive down the
diameter of the nanotubes grown. The amount of time that the metal
island can sit at the growth temperature is limited, as they are mobile,
and can merge into larger (but fewer) islands. Annealing at the growth
temperature reduces the site density (number of CNT/mm2) while increasing the catalyst diameter.
The as-prepared carbon nanotubes always have impurities such as
other forms of carbon (amorphous carbon, fullerene, etc.) and
non-carbonaceous impurities (metal used for catalyst). These impurities need to be removed to make use of the carbon nanotubes in applications.
Functionalization
CNTs
are known to have weak dispersibility in many solvents such as water as
a consequence of strong intermolecular p–p interactions. This hinders
the processability of CNTs in industrial applications. In order to
tackle the issue, various techniques have been developed over the years
to modify the surface of CNTs in order to improve their stability and
solubility in water. This enhances the processing and manipulation of
insoluble CNTs, rendering them useful for synthesizing innovative CNT
nano-fluids with impressive properties that are tuneable for a wide
range of applications.
Chemical routes such as covalent functionalization have been studied
extensively, which involves the oxidation of CNTs via strong acids (e.g.
sulfuric acid, nitric acid, or a mixture of both) in order to set the
carboxylic groups onto the surface of the CNTs as the final product or
for further modification by esterification or amination. Free radical
grafting is a promising technique among covalent functionalization
methods, in which alkyl or aryl peroxides, substituted anilines, and
diazonium salts are used as the starting agents. Free radical grafting
of macromolecules
(as the functional group) onto the surface of CNTs can improve the
solubility of CNTs compared to common acid treatments which
involve the attachment of small molecules such as hydroxyl onto the
surface of CNTs. the solubility of CNTs can be improved
significantly by free-radical grafting because the large functional
molecules facilitate the dispersion of CNTs in a variety of solvents,
even at a low degree of functionalization. Recently, an innovative,
bio-based, environmentally friendly approach has been developed for the
covalent
functionalization of multi-walled carbon nanotubes (MWCNTs) using clove
buds. This approach is innovative and green
because it does not use toxic and hazardous acids which are typically
used in common carbon nanomaterial
functionalization procedures. The MWCNTs are functionalized in one pot
using a free radical grafting
reaction. The clove-functionalized MWCNTs are then dispersed in water,
producing a highly stable multi-walled carbon nanotubes aqueous
suspension (nanofluids).
Modeling
Computer simulated microstructures with agglomeration regions
Carbon nanotubes are modelled in a similar manner as traditional
composites in which a reinforcement phase is surrounded by a matrix
phase. Ideal models such as cylindrical, hexagonal and square models are
common. The size of the micromechanics model is highly function of the
studied mechanical properties. The concept of representative volume
element (RVE) is used to determine the appropriate size and
configuration of computer model to replicate the actual behavior of CNT
reinforced nanocomposite. Depending on the material property of interest
(thermal, electrical, modulus, creep), one RVE might predict the
property better than the alternatives. While the implementation of ideal
model is computationally efficient, they do not represent
microstructural features observed in scanning electron microscopy of
actual nanocomposites. To incorporate realistic modeling, computer
models are also generated to incorporate variability such as waviness,
orientation and agglomeration of multiwall or single wall carbon
nanotubes.
Metrology
There are many metrology standards and reference materials available for carbon nanotubes.
For single-wall carbon nanotubes, ISO/TS 10868 describes a measurement method for the diameter, purity, and fraction of metallic nanotubes through optical absorption spectroscopy,
while ISO/TS 10797 and ISO/TS 10798 establish methods to characterize
the morphology and elemental composition of single-wall carbon
nanotubes, using transmission electron microscopy and scanning electron microscopy respectively, coupled with energy dispersive X-ray spectrometry analysis.
NIST SRM 2483 is a soot of single-wall carbon nanotubes used as a reference material for elemental analysis, and was characterized using thermogravimetric analysis, prompt gamma activation analysis, induced neutron activation analysis, inductively coupled plasma mass spectroscopy, resonant Raman scattering, UV-visible-near infrared fluorescence spectroscopy and absorption spectroscopy, scanning electron microscopy, and transmission electron microscopy. The Canadian National Research Council
also offers a certified reference material SWCNT-1 for elemental
analysis using neutron activation analysis and inductively coupled
plasma mass spectroscopy. NIST RM 8281 is a mixture of three lengths of single-wall carbon nanotube.
For multiwall carbon nanotubes, ISO/TR 10929 identifies the basic properties and the content of impurities, while ISO/TS 11888 describes morphology using scanning electron microscopy, transmission electron microscopy, viscometry, and light scattering analysis. ISO/TS 10798 is also valid for multiwall carbon nanotubes.
Chemical modification
Carbon nanotubes can be functionalized to attain desired properties
that can be used in a wide variety of applications. The two main methods
of carbon nanotube functionalization are covalent and non-covalent
modifications. Because of their apparent hydrophobic nature,
carbon nanotubes tend to agglomerate hindering their dispersion in
solvents or viscous polymer melts. The resulting nanotube bundles or
aggregates reduce the mechanical performance of the final composite. The
surface of the carbon nanotubes can be modified to reduce the hydrophobicity and improve interfacial adhesion to a bulk polymer through chemical attachment.
The surface of carbon nanotubes can be chemically modified by coating spinel nanoparticles by hydrothermal synthesis and can be used for water oxidation purposes.
In addition, the surface of carbon nanotubes can be fluorinated
or halofluorinated by heating while in contact with a fluoroorganic
substance, thereby forming partially fluorinated carbons (so called
Fluocar materials) with grafted (halo)fluoroalkyl functionality.
Applications
A
primary obstacle for applications of carbon nanotubes has been their
cost. Prices for single-walled nanotubes declined from around $1500 per
gram as of 2000 to retail prices of around $50 per gram of as-produced
40–60% by weight SWNTs as of March 2010. As of 2016, the retail price of
as-produced 75% by weight SWNTs was $2 per gram.
Current
Current use and application of nanotubes has mostly been limited to
the use of bulk nanotubes, which is a mass of rather unorganized
fragments of nanotubes. Bulk nanotube materials may never achieve a
tensile strength similar to that of individual tubes, but such
composites may, nevertheless, yield strengths sufficient for many
applications. Bulk carbon nanotubes have already been used as composite
fibers in polymers to improve the mechanical, thermal and electrical properties of the bulk product.
- Easton-Bell Sports, Inc. have been in partnership with Zyvex Performance Materials, using CNT technology in a number of their bicycle components – including flat and riser handlebars, cranks, forks, seatposts, stems and aero bars.
- Amroy Europe Oy manufactures Hybtonite carbon nanoepoxy resins where carbon nanotubes have been chemically activated to bond to epoxy,
resulting in a composite material that is 20% to 30% stronger than
other composite materials. It has been used for wind turbines, marine
paints and a variety of sports gear such as skis, ice hockey sticks,
baseball bats, hunting arrows, and surfboards.
- Surrey NanoSystems synthesises carbon nanotubes to create vantablack.
Other current applications include:
- "Gecko tape" (also called "nano tape") is often commercially sold as double-sided adhesive tape.
It can be used to hang lightweight items such as pictures and
decorative items on smooth walls without punching holes in the wall. The
carbon nanotube arrays comprising the synthetic setae leave no residue after removal and can stay sticky in extreme temperatures.
- tips for atomic force microscope probes
- in tissue engineering, carbon nanotubes can act as scaffolding for bone growth.
Under development
Current research for modern applications include:
- Utilizing carbon nanotubes as the channel material of carbon nanotube field-effect transistors.
- Using carbon nanotubes as a scaffold for diverse microfabrication techniques.
- Energy dissipation in self-organized nanostructures under influence of an electric field.
- Using carbon nanotubes for environmental monitoring due to their active surface area and their ability to absorb gases.
- Jack Andraka used carbon nanotubes in his pancreatic cancer test.
His method of testing won the Intel International Science and
Engineering Fair Gordon E. Moore Award in the spring of 2012.
- The Boeing Company has patented the use of carbon nanotubes for structural health monitoring
of composites used in aircraft structures. This technology will greatly
reduce the risk of an in-flight failure caused by structural
degradation of aircraft.
- Zyvex Technologies has also built a 54' maritime vessel, the Piranha Unmanned Surface Vessel,
as a technology demonstrator for what is possible using CNT technology.
CNTs help improve the structural performance of the vessel, resulting
in a lightweight 8,000 lb boat that can carry a payload of 15,000 lb
over a range of 2,500 miles.
Carbon nanotubes can serve as additives to various structural
materials. For instance, nanotubes form a tiny portion of the
material(s) in some (primarily carbon fiber) baseball bats, golf clubs, car parts, or damascus steel.
IBM expected carbon nanotube transistors to be used on Integrated Circuits by 2020.
Potential
The strength and flexibility of carbon nanotubes makes them of
potential use in controlling other nanoscale structures, which suggests
they will have an important role in nanotechnology engineering. The highest tensile strength of an individual multi-walled carbon nanotube has been tested to be 63 GPa. Carbon nanotubes were found in Damascus steel from the 17th century, possibly helping to account for the legendary strength of the swords made of it.
Recently, several studies have highlighted the prospect of using carbon
nanotubes as building blocks to fabricate three-dimensional macroscopic
(>1mm in all three dimensions) all-carbon devices. Lalwani et al.
have reported a novel radical initiated thermal crosslinking method to
fabricated macroscopic, free-standing, porous, all-carbon scaffolds
using single- and multi-walled carbon nanotubes as building blocks.
These scaffolds possess macro-, micro-, and nano- structured pores and
the porosity can be tailored for specific applications. These 3D
all-carbon scaffolds/architectures may be used for the fabrication of
the next generation of energy storage, supercapacitors, field emission
transistors, high-performance catalysis, photovoltaics, and biomedical devices and implants.
CNTs are potential candidates for future via and wire material in
nano-scale VLSI circuits. Eliminating electromigration reliability
concerns that plague today's Cu interconnects, isolated (single and multi-wall) CNTs can carry current densities in excess of 1000 MA/cm2 without electromigration damage.
Single-walled nanotubes are likely candidates for miniaturizing
electronics. The most basic building block of these systems is an
electric wire, and SWNTs with diameters of an order of a nanometer can
be excellent conductors. One useful application of SWNTs is in the development of the first intermolecular field-effect transistors (FET). The first intermolecular logic gate using SWCNT FETs was made in 2001.
A logic gate requires both a p-FET and an n-FET. Because SWNTs are
p-FETs when exposed to oxygen and n-FETs otherwise, it is possible to
expose half of an SWNT to oxygen and protect the other half from it. The
resulting SWNT acts as a not logic gate with both p- and n-type FETs in the same molecule.
Large quantities of pure CNTs can be made into a freestanding
sheet or film by surface-engineered tape-casting (SETC) fabrication
technique which is a scalable method to fabricate flexible and foldable
sheets with superior properties. Another reported form factor is CNT fiber (a.k.a. filament) by wet spinning. The fiber is either directly spun from the synthesis pot or spun from
pre-made dissolved CNTs. Individual fibers can be turned into a yarn. Apart from its strength and flexibility, the main advantage is making an electrically conducting yarn.
The electronic properties of individual CNT fibers (i.e. bundle of
individual CNT) are governed by the two-dimensional structure of CNTs.
The fibers were measured to have a resistivity
only one order of magnitude higher than metallic conductors at 300K. By
further optimizing the CNTs and CNT fibers, CNT fibers with improved
electrical properties could be developed.
CNT-based yarns are suitable for applications in energy and electrochemical water treatment when coated with an ion-exchange membrane. Also, CNT-based yarns could replace copper as a winding material. Pyrhönen et al. (2015) have built a motor using CNT winding.
Safety and health
The National Institute for Occupational Safety and Health
(NIOSH) is the leading United States federal agency conducting research
and providing guidance on the occupational safety and health
implications and applications of nanotechnology. Early scientific
studies have indicated that some of these nanoscale particles may pose a
greater health risk than the larger bulk form of these materials. In
2013, NIOSH published a Current Intelligence Bulletin detailing the
potential hazards and recommended exposure limit for carbon nanotubes
and fibers.
As of October 2016, single wall carbon nanotubes have been registered through the European Union's Registration, Evaluation, Authorization and Restriction of Chemicals
(REACH) regulations, based on evaluation of the potentially hazardous
properties of SWCNT. Based on this registration, SWCNT commercialization
is allowed in the EU up to 10 metric tons. Currently, the type of SWCNT
registered through REACH is limited to the specific type of single wall
carbon nanotubes manufactured by OCSiAl, which submitted the application.
History
The true identity of the discoverers of carbon nanotubes is a subject of some controversy. A 2006 editorial written by Marc Monthioux and Vladimir Kuznetsov in the journal Carbon described the origin of the carbon nanotube.
A large percentage of academic and popular literature attributes the
discovery of hollow, nanometer-size tubes composed of graphitic carbon
to Sumio Iijima of NEC
in 1991. His paper initiated a flurry of excitement and could be
credited with inspiring the many scientists now studying applications of
carbon nanotubes. Though Iijima has been given much of the credit for
discovering carbon nanotubes, it turns out that the timeline of carbon
nanotubes goes back much further than 1991.
In 1952, L. V. Radushkevich and V. M. Lukyanovich published clear images of 50 nanometer diameter tubes made of carbon in the Journal of Physical Chemistry Of Russia.
This discovery was largely unnoticed, as the article was published in
Russian, and Western scientists' access to Soviet press was limited
during the Cold War. Monthioux and Kuznetsov mentioned in their Carbon editorial:
The
fact is, Radushkevich and Lukyanovich [...] should be credited for the
discovery that carbon filaments could be hollow and have a nanometer-
size diameter, that is to say for the discovery of carbon nanotubes.
In 1976, Morinobu Endo of CNRS observed hollow tubes of rolled up graphite sheets synthesised by a chemical vapour-growth technique. The first specimens observed would later come to be known as single-walled carbon nanotubes (SWNTs).
Endo, in his early review of vapor-phase-grown carbon fibers (VPCF),
also reminded us that he had observed a hollow tube, linearly extended
with parallel carbon layer faces near the fiber core. This appears to be the observation of multi-walled carbon nanotubes at the center of the fiber. The mass-produced MWCNTs today are strongly related to the VPGCF developed by Endo. In fact, they call it the "Endo-process", out of respect for his early work and patents.
In 1979, John Abrahamson presented evidence of carbon nanotubes at the 14th Biennial Conference of Carbon at Pennsylvania State University.
The conference paper described carbon nanotubes as carbon fibers that
were produced on carbon anodes during arc discharge. A characterization
of these fibers was given, as well as hypotheses for their growth in a
nitrogen atmosphere at low pressures.
In 1981, a group of Soviet scientists published the results of
chemical and structural characterization of carbon nanoparticles
produced by a thermocatalytical disproportionation of carbon monoxide.
Using TEM images and XRD
patterns, the authors suggested that their "carbon multi-layer tubular
crystals" were formed by rolling graphene layers into cylinders. They
speculated that via this rolling, many different arrangements of
graphene hexagonal nets are possible. They suggested two such possible
arrangements: circular arrangement (armchair nanotube); and a spiral,
helical arrangement (chiral tube).
In 1987, Howard G. Tennent of Hyperion Catalysis was issued a
U.S. patent for the production of "cylindrical discrete carbon fibrils"
with a "constant diameter between about 3.5 and about 70 nanometers...,
length 102 times the diameter, and an outer region of
multiple essentially continuous layers of ordered carbon atoms and a
distinct inner core...."
Helping to create the initial excitement associated with carbon
nanotubes were Iijima's 1991 discovery of multi-walled carbon nanotubes
in the insoluble material of arc-burned graphite rods;
and Mintmire, Dunlap, and White's independent prediction that if
single-walled carbon nanotubes could be made, they would exhibit
remarkable conducting properties. Nanotube research accelerated greatly following the independent discoveries by Iijima and Ichihashi at NEC, and Bethune et al. at IBM, of single-walled
carbon nanotubes, and methods to specifically produce them by adding
transition-metal catalysts to the carbon in an arc discharge. The arc
discharge technique was well known to produce the famed Buckminster
fullerene on a preparative scale,
and these results appeared to extend the run of accidental discoveries
relating to fullerenes. The discovery of nanotubes remains a contentious
issue. Many believe that Iijima's report in 1991 is of particular
importance because it brought carbon nanotubes into the awareness of the
scientific community as a whole.
In 2020, during archaeological excavation of Keezhadi in Tamilnadu, India,
~2500-year-old pottery was discovered whose coatings appear to contain
carbon nanotubes. The robust mechanical properties of the nanotubes are
partially why the coatings have lasted for so many years, say the
scientists.