From Wikipedia, the free encyclopedia

Antibiotic resistance tests: Bacteria are added in streaks on the dish and antibiotics are added on the white disks. Bacteria in the culture on the left are susceptible to the antibiotic in each disk, as shown by the dark, clear rings where bacteria have not grown. Those on the right are fully susceptible to only three of the seven antibiotics tested.[1]
Antimicrobial resistance (AMR) occurs when a microbe and thus its progeny acquires a genetic mutation, either spontaneously or by gene transfer, rendering it resistant to the effect of one or more antimicrobial agents. In medical therapy, bacteria can become resistant to anti-bacterial agents, i.e. antibiotics, and in this setting the more specific term antibiotic resistance is used. Microbes that are resistant to multiple antimicrobials are termed multidrug resistant (MDR) (or, sometimes in the lay press, superbugs).[2] Common types of drug-resistant bacteria include MRSA (methicillin-resistant Staphylococcus aureus), VRSA (vancomycin-resistant S. aureus), ESBL (extended spectrum beta-lactamase), VRE (vancomycin-resistant Enterococcus) and MRAB (multidrug-resistant A. baumannii). Viruses, fungi and parasites can also become resistant to agents to which they were once susceptible. Infection by resistant microbes may be community-acquired infections or healthcare-associated infections - the term now preferred to hospital-acquired infections or nosocomial infections {from New Latin nosocomium ("hospital"), from Ancient Greek νοσοκομείον (nosokomeíon, "hospital"), from νόσος (nósos, "disease, illness") + κομέω (koméō, "to take care of")}.
Drug-resistant organisms may acquire resistance to first-line antibiotics, necessitating the use of a second-line agent to which the microbe is sensitive. In the case of some MDR pathogens, resistance to second- and even third-line antibiotics is sequentially acquired, as illustrated by Staphylococcus aureus and Pseudomonas aeruginosa.
Resistance may take the form of a spontaneous or induced genetic mutation, or the acquisition of resistance genes from other bacterial species by horizontal gene transfer via conjugation, transduction, or transformation. Many antibiotic resistance genes reside on transmissible plasmids, facilitating their transfer. Antibiotic-resistance plasmids frequently contain genes conferring resistance to several different antibiotics.
Genes for resistance to antibiotics, like the antibiotics themselves, are ancient.[3]:457–461 The increasing rates of antibiotic-resistant bacterial infections seen in clinical practice stems from antibiotic use both within human and veterinary medicine. Any use of antibiotics can increase selective pressure in a population of bacteria to allow the resistant bacteria to thrive and the susceptible bacteria to die off. As resistance to antibiotics becomes more common, a greater need for alternative treatments arises. However, despite a push for new antibiotic therapies, there has been a continued decline in the number of newly approved drugs.[4] Antibiotic resistance poses a grave and growing global problem: a World Health Organization report released April, 2014 stated, "this serious threat is no longer a prediction for the future, it is happening right now in every region of the world and has the potential to affect anyone, of any age, in any country. Antibiotic resistance –when bacteria change so antibiotics no longer work in people who need them to treat infections– is now a major threat to public health."[5]
Causes
There were few antibiotic-resistant bacteria before antibiotics existed,[6] [7] and widespread antibiotic use has caused more bacteria to become resistant, a process called evolutionary pressure. [8][9]
Reasons for the widespread use of antibiotics are manifold and include:
- their increasing global availability over time since the 1950's,
- their uncontrolled sale in many countries, where anyone can obtain them over the counter without a prescription,[citation needed] potentially resulting in antibiotics being used for too long or too short a time or when not indicated. This may result in emergence of resistance in any remaining bacteria.
- Prescribing or obtaining broad-spectrum antibiotics when not indicated: these are more likely to induce resistance than narrow-spectrum antibiotics.
Natural occurrence
Naturally occurring antibiotic resistance is not uncommon.[15] The genes that confer this resistance are known as the environmental resistome.[15] These genes may be transferred from non-disease-causing bacteria to those that do cause disease, leading to clinically significant antibiotic resistance.[15] In a 1952 experiment Joshua and Esther Lederberg showed that penicillin-resistant bacteria existed before penicillin treatment.[16] While experimenting at the University of Wisconsin-Madison, Joshua Lederberg and his graduate student Norton Zinder demonstrated preexistent bacterial resistance to streptomycin.[17] In 1962, the presence of penicillinase was detected in dormant endospores of Bacillus licheniformis, revived from dried soil on the roots of plants, preserved since 1689 in the British Museum.[18][19][20] Six strains of Clostridium, found in the bowels of William Braine and John Hartnell (members of the Franklin Expedition) showed resistance to cefoxitin and clindamycin.[21] Penicillinase may have emerged as a defense mechanism for bacteria in their habitats, such as the case of penicillinase-rich Staphylococcus aureus, living with penicillin-producing Trichophyton, however this was deemed circumstantial.[20] Search for a penicillinase ancestor has focused on the class of proteins that must be a priori capable of specific combination with penicillin.[22] The resistance to cefoxitin and clindamycin in turn was attributed to Braine's and Hartnell's contact with microorganisms that naturally produce them or random mutation in the chromosomes of Clostridium strains.[21] There is evidence that heavy metals and other pollutants may select for antibiotic-resistant bacteria, generating a constant source of them in small numbers.[23]:34[better source needed]Human medicine
Certain antibiotic classes induce resistance more than others. A multiresistant bacterium carries several resistance genes.[24] Increased rates of MRSA infections are seen when using glycopeptides, cephalosporins, and quinolones.[25][26] Cephalosporins, and particularly quinolones and clindamycin, are more likely to produce colonisation with Clostridium difficile[importance?] [27][28] Increasing bacterial resistance correlates with the volume of antibiotic prescribed, and not lack of compliance with taking antibiotics.[29] Inappropriate prescribing of antibiotics has been attributed to a number of causes, including people insisting on antibiotics, physicians prescribing them as they feel they do not have time to explain why they are not necessary, and physicians not knowing when to prescribe antibiotics or being overly cautious for medical and/or legal reasons.[30] For example, a third of people believe that antibiotics are effective for the common cold,[31] and the common cold is the most common reason antibiotics are prescribed[32] even though antibiotics are useless against viruses. A single regimen of antibiotics even in compliant individuals leads to a greater risk of resistant organisms to that antibiotic in the person for a month to possibly a year.[33][34]Antibiotic resistance increases with duration of treatment; therefore, as long as an effective minimum is kept shorter courses of antibiotics are likely to decrease rates of resistance, reduce cost, and have better outcomes due to fewer complications. Short course regimens exist for community-acquired pneumonia[35] spontaneous bacterial peritonitis,[36] suspected lung infections in ICU patients,[37] in the so called acute abdomen,[38] middle ear infection, sinusitis and throat infection,[39] and penetrating gut injury.[40][41] In some situations a short course is inferior to a long course.[42] A BMJ editorial recommended that antibiotics can often be safely stopped 72 hours after symptoms resolve.[43] Because individuals may feel better before the infection is eradicated, doctors must provide instructions to them so they know when it is safe to stop taking a prescription. Some researchers advocate doctors' using a very short course of antibiotics, reevaluating the patient after a few days, and stopping treatment if there are no clinical signs of infection.[44]
When less than the required dosage is taken or not taken within the prescribed time, antibiotic concentration in the tissues decreases to suboptimal levels increasing the frequency of antibiotic resistant organisms to develop and multiply. Factors within the intensive care unit setting such as mechanical ventilation and multiple underlying diseases also appear to contribute to bacterial resistance.[45]
Poor hand hygiene by hospital staff has been associated with the spread of resistant organisms,[46] and an increase in hand washing compliance results in decreased rates of these organisms.[47]
The improper use of antibiotics can often be attributed to the presence of structural violence in particular regions. Socioeconomic factors such as race and poverty affect accessibility of and adherence to drug therapy. The efficacy of treatment programs for drug-resistant strains depends on whether or not programmatic improvements take into account the effects of structural violence.[48]
Veterinary medicine
The use of antibiotics in animals is partly responsible for the emergence of antibiotic-resistant microorganisms in human medicine.[49] Antibiotic use in animals can be classified into therapeutic, prophylactic, metaphylactic, and growth promotion uses of antibiotics.[50] All four patterns select for bacterial resistance, since antibiotic resistance is a natural evolutionary process, but the non-therapeutic uses expose larger number of animals, and therefore of bacteria, for more extended periods, and at lower doses. They therefore greatly increase the cross-section for the evolution of resistance.Since the last third of the 20th century, antibiotics have been used extensively in animal husbandry. In 2013, 80% of antibiotics used in the US were used in animals and only 20% in humans; in 1997 half were used in humans and half in animals.[51] Some antibiotics are not used and not considered significant for use in humans, because they either lack efficacy or purpose in humans, such as ionophores in ruminants,[52]) or because the drug has gone out of use in humans. Others are used in both animals and humans, including penicillin and some forms of tetracycline.[53] Historically, regulation of antibiotic use in food animals has been limited to limiting drug residues in meat, egg, and milk products, rather than by direct concern over the development of antibiotic resistance. This mirrors the primary concerns in human medicine, where, in general, researchers and doctors were more concerned about effective but non-toxic doses of drugs rather than antibiotic resistance.[citation needed]
In 2001, the Union of Concerned Scientists estimated that greater than 70% of the antibiotics used in the U.S. are given to food animals (for example, chickens, pigs, and cattle), in the absence of disease.[51][54] The amounts given are termed "sub-therapeutic", i.e., insufficient to combat disease. Despite no diagnosis of disease, the administration of these drugs (most of which are not significant to human medicine) results in decreased mortality and morbidity and increased growth in the animals so treated. It is theorized that sub-therapeutic dosages kills some, but not all, of the bacterial organisms in the animal — likely leaving those that are naturally antibiotic-resistant[citation needed]. Studies have shown, however, that, in essence, the overall population levels of bacteria are unchanged; only the mix of bacteria is affected.[citation needed]The actual mechanism by which sub-therapeutic antibiotic feed additives serve as growth promoters is thus unclear. Some people have speculated that animals and fowl may have sub-clinical infections, which would be cured by low levels of antibiotics in feed, thereby allowing the creatures to thrive. No convincing evidence has been advanced for this theory, and the bacterial load in an animal is essentially unchanged by use of antibiotic feed additives. The mechanism of growth promotion is therefore probably something other than "killing off the bad bugs."
Antibiotics are used in U.S. animal feed to promote animal productivity.[11][55] In particular, poultry feed and water is a common route of administration of drugs, due to higher overall costs when drugs are administered by handling animals individually.
In research studies, occasional animal-to-human spread of drug-resistant organisms has been demonstrated. Resistant bacteria can be transmitted from animals to humans in three ways: by consuming animal products (milk, meat, eggs, etc.), from close or direct contact with animals or other humans, or through the environment.[56] In the first pathway, food preservation methods can help eliminate, decrease, or prevent the growth of bacteria in some food classes. Evidence for the transfer of antibiotic-resistant microorganisms from animals to humans has been scant, and most evidence shows that pathogens of concern in human populations originated in humans and are maintained there, with rare cases of transference to humans.[57][58]
The World Health Organization concluded that inappropriate use of antibiotics in animal husbandry is an underlying contributor to the emergence and spread of antibiotic-resistant germs, and that the use of antibiotics as growth promoters in animal feeds should be prohibited.[citation needed] Regarding this matter, the World Organisation for Animal Health has added to the Terrestrial Animal Health Code a series of guidelines with recommendations to its members for the creation and harmonization of national antimicrobial resistance surveillance and monitoring programs,[59][full citation needed] monitoring of the quantities of antibiotics used in animal husbandry,[60][full citation needed] and recommendations to ensure the proper and prudent use of antibiotic substances. Another guideline is to implement methodologies that help to establish associated risk factors and assess the risk of antibiotic resistance.[61][full citation needed]
In the world, antibiotics are widely used on animals. As in human medicine, antibiotics can often be bought without prescription and veterinary supervision for use on pets and livestock. Bacteria remaining in these animals are likely to be resistant to the antibiotics used, and may be passed into the environment by the excretion and secretion of materials such as milk, feces, urine, saliva, semen, lochia, etc. The actual impact of these resistant germs depends on their specific type and on the animal or organism they henceforth infect. Some germs, such as tetanus, are toxic regardless of their antibiotic-resistant status.[citation needed]
Environmental impact
Antibiotics have been polluting the environment since their introduction through human waste (medication, farming), animals, and the pharmaceutical industry.[62] Along with antibiotic waste, resistant bacteria follow, thus introducing antibiotic-resistant bacteria into the environment. As bacteria replicate quickly, the resistant bacteria that enter the environment replicate their resistance genes as they continue to divide. In addition, bacteria carrying resistance genes have the ability to spread those genes to other species via horizontal gene transfer. Therefore, even if the specific antibiotic is no longer introduced into the environment, antibiotic-resistance genes will persist through the bacteria that have since replicated without continuous exposure.[62]A study the Poudre River (Colorado, United States) implicated wastewater treatment plants, as well as animal-feeding operations in the dispersal of antibiotic-resistance genes into the environment.[63] This research was done using molecular signatures in order to determine the sources, and the location at the Poudre River was chosen due to lack of other anthropogenic influences upstream. The study indicates that monitoring of antibiotic-resistance genes may be useful in determining not only the point of origin of their release but also how these genes persist in the environment. In addition, studying physical and chemical methods of treatment may alleviate pressure of antibiotic-resistance genes in the environment, and thus their entry back into human contact.
Mechanisms

Schematic representation of how antibiotic resistance evolves via natural selection. The top section represents a population of bacteria before exposure to an antibiotic. The middle section shows the population directly after exposure, the phase in which selection took place. The last section shows the distribution of resistance in a new generation of bacteria. The legend indicates the resistance levels of individuals.
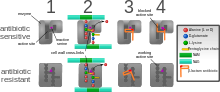
Diagram depicting antibiotic resistance through alteration of the antibiotic's target site, modeled after MRSA's resistance to penicillin. Beta-lactam antibiotics permanently inactivate PBP enzymes, which are essential for bacterial life, by permanently binding to their active sites. MRSA, however, expresses a PBP that does not allow the antibiotic into its active site.
The four main mechanisms by which microorganisms exhibit resistance to antimicrobials are:
- Drug inactivation or modification: for example, enzymatic deactivation of penicillin G in some penicillin-resistant bacteria through the production of β-lactamases. Most commonly, the protective enzymes produced by the bacterial cell will add an acetyl or phosphate group to a specific site on the antibiotic, which will reduce its ability to bind to the bacterial ribosomes and disrupt protein synthesis.[64]
- Alteration of target site: for example, alteration of PBP—the binding target site of penicillins—in MRSA and other penicillin-resistant bacteria. Another protective mechanism found among bacterial species is ribosomal protection proteins. These proteins protect the bacterial cell from antibiotics that target the cell’s ribosomes to inhibit protein synthesis. The mechanism involves the binding of the ribosomal protection proteins to the ribosomes of the bacterial cell, which in turn changes its conformational shape. This allows the ribosomes to continue synthesizing proteins essential to the cell while preventing antibiotics from binding to the ribosome to inhibit protein synthesis.
- Alteration of metabolic pathway: for example, some sulfonamide-resistant bacteria do not require para-aminobenzoic acid (PABA), an important precursor for the synthesis of folic acid and nucleic acids in bacteria inhibited by sulfonamides, instead, like mammalian cells, they turn to using preformed folic acid.
- Reduced drug accumulation: by decreasing drug permeability or increasing active efflux (pumping out) of the drugs across the cell surface[65] These specialized pumps can be found within the cellular membrane of certain bacterial species and are used to pump antibiotics out of the cell before they are able to do any damage. These efflux pumps are often activated by a specific substrate associated with an antibiotic.[66]
A mutation may produce a change in the binding site of the antibiotic, which may allow the site to continue proper functioning in the presence of the antibiotic or prevent the binding of the antibiotic to the site altogether. Research has shown the bacterial protein LexA may play a key role in the acquisition of bacterial mutations giving resistance to quinolones and rifampicin. DNA damage induces the SOS gene repressor LexA to undergo autoproteolytic activity. This includes the transcription of genes encoding Pol II, Pol IV, and Pol V, which are three nonessential DNA polymerases that are required for mutation in response to DNA damage.[68] The antibiotic action against the pathogen can be seen as an environmental pressure. Those bacteria with a mutation that allows them to survive live to reproduce. They then pass this trait to their offspring, which leads to the evolution of a fully resistant colony. Although these chromosomal mutations may seem to benefit the bacteria by providing antibiotic resistance, they also confer a cost of fitness. For example, a ribosomal mutation may protect a bacterial cell by changing the binding site of an antibiotic but it will also slow the process of protein synthesis.[64] Additionally, a particular study specifically compared the overall fitness of antibiotic resistant strains of Escherichia coli and Salmonella typhimurium to their drug-sensitive revertants. They observed a reduced overall fitness in the antibiotic resistant strains, especially in growth rate.[69]
There are three known mechanisms of fluoroquinolone resistance. Some types of efflux pumps can act to decrease intracellular quinolone concentration.[70] In Gram-negative bacteria, plasmid-mediated resistance genes produce proteins that can bind to DNA gyrase, protecting it from the action of quinolones. Finally, mutations at key sites in DNA gyrase or topoisomerase IV can decrease their binding affinity to quinolones, decreasing the drug's effectiveness.[71]
Antibiotic resistance can also be introduced artificially into a microorganism through laboratory protocols, sometimes used as a selectable marker to examine the mechanisms of gene transfer or to identify individuals that absorbed a piece of DNA that included the resistance gene and another gene of interest. A recent study demonstrated that the extent of horizontal gene transfer among Staphylococcus is much greater than previously expected—and encompasses genes with functions beyond antibiotic resistance and virulence, and beyond genes residing within the mobile genetic elements.[72]
For a long time, it has been thought that, for a microorganism to become resistant to an antibiotic, it must be in a large population. However, recent findings show that there is no necessity of large populations of bacteria for the appearance of antibiotic resistance. We know now that small populations of E.coli in an antibiotic gradient can become resistant. Any heterogeneous environment with respect to nutrient and antibiotic gradients may facilitate the development of antibiotic resistance in small bacterial populations and this is also true for the human body. Researchers hypothesize that the mechanism of resistance development is based on four SNP mutations in the genome of E.coli produced by the gradient of antibiotic. These mutations confer the bacteria emergence of antibiotic resistance.
A common misconception is that a person can become resistant to certain antibiotics. It is a strain of microorganism that can become resistant, not a person's body.[73]
Resistant bacteria
Staphylococcus aureus
Staphylococcus aureus (colloquially known as "Staph aureus" or a "Staph infection") is one of the major resistant pathogens. Found on the mucous membranes and the human skin of around a third of the population, it is extremely adaptable to antibiotic pressure. It was one of the earlier bacteria in which penicillin resistance was found—in 1947, just four years after the drug started being mass-produced. Methicillin was then the antibiotic of choice, but has since been replaced by oxacillin due to significant kidney toxicity. Methicillin-resistant Staphylococcus aureus (MRSA) was first detected in Britain in 1961, and is now "quite common" in hospitals. MRSA was responsible for 37% of fatal cases of sepsis in the UK in 1999, up from 4% in 1991. Half of all S. aureus infections in the US are resistant to penicillin, methicillin, tetracycline and erythromycin.This left vancomycin as the only effective agent available at the time. However, strains with intermediate (4-8 μg/ml) levels of resistance, termed glycopeptide-intermediate Staphylococcus aureus (GISA) or vancomycin-intermediate Staphylococcus aureus (VISA), began appearing in the late 1990s. The first identified case was in Japan in 1996, and strains have since been found in hospitals in England, France and the US. The first documented strain with complete (>16 μg/ml) resistance to vancomycin, termed vancomycin-resistant Staphylococcus aureus (VRSA) appeared in the United States in 2002.[74] However, in 2011, a variant of vancomycin has been tested that binds to the lactate variation and also binds well to the original target, thus reinstating potent antimicrobial activity.[75]
A new class of antibiotics, oxazolidinones, became available in the 1990s, and the first commercially available oxazolidinone, linezolid, is comparable to vancomycin in effectiveness against MRSA. Linezolid-resistance in S. aureus was reported in 2001.[76]
Community-acquired MRSA (CA-MRSA) has now emerged as an epidemic that is responsible for rapidly progressive, fatal diseases, including necrotizing pneumonia, severe sepsis, and necrotizing fasciitis.[77] MRSA is the most frequently identified antimicrobial drug-resistant pathogen in US hospitals. The epidemiology of infections caused by MRSA is rapidly changing. In the past 10 years[when?], infections caused by this organism have emerged in the community. The two MRSA clones in the United States most closely associated with community outbreaks, USA400 (MW2 strain, ST1 lineage) and USA300, often contain Panton-Valentine leukocidin (PVL) genes and, more frequently, have been associated with skin and soft tissue infections. Outbreaks of CA-MRSA infections have been reported in correctional facilities, among athletic teams, among military recruits, in newborn nurseries, and among men that have sex with men. CA-MRSA infections now appear endemic in many urban regions and cause most CA-S. aureus infections.[78]
Streptococcus and Enterococcus
Streptococcus pyogenes (Group A Streptococcus: GAS) infections can usually be treated with many different antibiotics. Early treatment may reduce the risk of death from invasive group A streptococcal disease. However, even the best medical care does not prevent death in every case. For those with very severe illness, supportive care in an intensive-care unit may be needed. For persons with necrotizing fasciitis, surgery often is needed to remove damaged tissue.[79] Strains of S. pyogenes resistant to macrolide antibiotics have emerged; however, all strains remain uniformly susceptible to penicillin.[80]Resistance of Streptococcus pneumoniae to penicillin and other beta-lactams is increasing worldwide. The major mechanism of resistance involves the introduction of mutations in genes encoding penicillin-binding proteins. Selective pressure is thought to play an important role, and use of beta-lactam antibiotics has been implicated as a risk factor for infection and colonization. S. pneumoniae is responsible for pneumonia, bacteremia, otitis media, meningitis, sinusitis, peritonitis and arthritis.[80]
Multidrug-resistant Enterococcus faecalis and Enterococcus faecium are associated with nosocomial infections.[81] Among these strains, penicillin-resistant Enterococcus was seen in 1983, vancomycin-resistant Enterococcus in 1987, and linezolid-resistant Enterococcus in the late 1990s.[citation needed]
Pseudomonas aeruginosa
Pseudomonas aeruginosa is a highly prevalent opportunistic pathogen. One of the most worrisome characteristics of P. aeruginosa is its low antibiotic susceptibility, which is attributable to a concerted action of multidrug efflux pumps with chromosomally encoded antibiotic resistance genes (for example, mexAB-oprM, mexXY, etc.) and the low permeability of the bacterial cellular envelopes.[82] Pseudomonas aeruginosa has the ability to produce 4-hydroxy-2-alkylquinolines (HAQs) and it has been found that HAQs have prooxidant effects, and overexpressing modestly increased susceptibility to antibiotics. The study experimented with the Pseudomonas aeruginosa biofilms and found that a disruption of relA and spoT genes produced an inactivation of the Stringent response (SR) in cells with nutrient limitation, which provides cells be more susceptible to antibiotics.[83]Clostridium difficile
Clostridium difficile is a nosocomial pathogen that causes diarrheal disease in hospitals world wide.[84][85]C. difficile colitis is most strongly associated with fluoroquinolones, cephalosporins, carbapenems, and clindamycin.[86][87][88]
Some research suggests the overuse of antibiotics in the raising of livestock is contributing to outbreaks of bacterial infections such as C. difficile.[16]
Antibiotics, especially those with a broad activity spectrum (such as clindamycin) disrupt normal intestinal flora. This can lead to an overgrowth of C. difficile, which flourishes under these conditions. Pseudomembranous colitis can follow, creating generalized inflammation of the colon and the development of "pseudomembrane", a viscous collection of inflammatory cells, fibrin, and necrotic cells.[4] Clindamycin-resistant C. difficile was reported as the causative agent of large outbreaks of diarrheal disease in hospitals in New York, Arizona, Florida and Massachusetts between 1989 and 1992.[89] Geographically dispersed outbreaks of C. difficile strains resistant to fluoroquinolone antibiotics, such as ciprofloxacin and levofloxacin, were also reported in North America in 2005.[90]
Salmonella and E. coli
Infection with Escherichia coli and Salmonella can result from the consumption of contaminated food and water. Both of these bacteria are well known for causing nosocomial (hospital-linked) infections, and often, these strains found in hospitals are antibiotic resistant due to adaptations to wide spread antibiotic use.[91] When both bacteria are spread, serious health conditions arise. Many people are hospitalized each year after becoming infected, with some dying as a result. Since 1993, some strains of E. coli have become resistant to multiple types of fluoroquinolone antibiotics.[citation needed]Although mutation alone plays a huge role in the development of antibiotic resistance, there was a study done recently that found that high survival rates after exposure to antibiotics could not be accounted for by mutation alone.[92] This study focused specifically on Escherichia coli’s development of resistance to three antibiotic drugs: ampicillin, tetracycline, and nalidixic acid. At the conclusion of the study, these researchers found that some antibiotic resistance in E. coli developed due to epigenetic inheritance rather than by direct inheritance of a mutated gene. This was further supported by their data showing that reversion back to antibiotic sensitivity was relatively common as well. This could only be explained by epigenetics.[92] Epigenetics is a type of inheritance where gene expression is altered rather than the genetic code itself. There are many modes by which this alteration of gene expression can occur. This includes methylation of DNA and histone modification; however, the important idea is that both inheritance of random mutations and epigenetic markers can result in the expression of antibiotic resistance genes.[92]
Acinetobacter baumannii
On November 5, 2004, the Centers for Disease Control and Prevention (CDC) reported an increasing number of Acinetobacter baumannii bloodstream infections in patients at military medical facilities in which service members injured in the Iraq/Kuwait region during Operation Iraqi Freedom and in Afghanistan during Operation Enduring Freedom were treated. Most of these showed multidrug resistance (MRAB), with a few isolates resistant to all drugs tested.[93][94]Klebsiella pneumoniae
Klebsiella pneumoniae carbapenemase (KPC)-producing bacteria are a group of emerging highly drug-resistant Gram-negative bacilli causing infections associated with significant morbidity and mortality whose incidence is rapidly increasing in a variety of clinical settings around the world. Klebsiella pneumoniae includes numerous mechanisms for antibiotic resistance, many of which are located on highly mobile genetic elements.[95] Carbapenem antibiotics (heretofore often the treatment of last resort for resistant infections) are generally not effective against KPC-producing organisms.[96]Mycobacterium tuberculosis
Tuberculosis is increasing across the globe, especially in developing countries, over the past few years. TB resistant to antibiotics is called MDR TB (Multidrug Resistant TB). Globally, MDR TB causes 150,000 deaths annually.[97] The rise of the HIV/AIDS epidemic has contributed to this.[98]TB was considered one of the most prevalent diseases, and did not have a cure until the discovery of Streptomycin by Selman Waksman in 1943.[99] However, the bacteria soon developed resistance.
Since then, drugs such as isoniazid and rifampin have been used. M. tuberculosis develops resistance to drugs by spontaneous mutations in its genomes. Resistance to one drug is common, and this is why treatment is usually done with more than one drug. Extensively Drug-Resistant TB (XDR TB) is TB that is also resistant to the second line of drugs.[98][100]
Resistance of Mycobacterium tuberculosis to isoniazid, rifampin, and other common treatments has become an increasingly relevant clinical challenge. (For more on Drug-Resistant TB, visit the Multi-drug-resistant tuberculosis page.) Evidence is lacking for whether these bacteria have plasmids.[101] Also M. tuberculosis lack the opportunity to interact with other bacteria in order to share plasmids.[101][102]
Neisseria gonorrhoeae
Neisseria gonorrhoeae is a sexually transmitted pathogen that can cause pelvic pain, pain on urination, penile and vaginal discharge, as well as systemic symptoms. The bacteria was first identified in 1879,[103] although some Biblical scholars believe that references to the disease can be found as early as Parshat Metzora of the Old Testament.[104]In the 1940s effective treatment with penicillin became available, but by the 1970s resistant strains predominated. Resistance to penicillin has developed through two mechanisms: chomasomally mediated resistance (CMRNG) and penicillinase-mediated resistance (PPNG). CMRNG involves stepwise mutation of penA, which codes for the penicilin-binding protein (PBP-2); mtr, which encodes an efflux pump to remove penicilin from the cell; and penB, which encodes the bacterial cell wall porins. PPNG involves the acquisition of a plasmid-borne beta-lactamase.[105]
Fluoroquinolones were a useful next-line treatment until resistance was achieved through efflux pumps and mutations to the gyrA gene, which encodes DNA gyrase.[105] Third-generation cephalosporins have been used to treat gonorrhoea since 2007, but resistant strains have emerged. Strains of Neisseria gonorrhoea have also been found to be resistant to tetracyclines and aminoglycosides. Neisseria gonorrheoea has a high affinity for horizontal gene transfer, and as a result, the existence of any strain resistant to a given drug could spread easily across strains.
As of 2010, the recommended treatment is a single 250 mg intramuscular injection of ceftriaxone, sometimes in combination with azithromycin or doxycycline.[106][107]
Viruses
Specific antiviral drugs are used to treat some viral infections. These drugs prevent viruses from reproducing by inhibiting essential stages of the virus's replication cycle in infected cells. Antivirals are used to treat HIV, hepatitis B, hepatitis C, influenza, herpes viruses including varicella zoster virus, cytomegalovirus and Epstein-Barr virus. With each virus, some strains have become resistant to the administered drugs.[108]Resistance to HIV antivirals is problematic, and even multi-drug resistant strains have evolved.[109] Resistant strains of the HIV virus emerge rapidly if only one antiviral drug is used.[110] Using three or more drugs together has helped to control this problem, but new drugs are needed because of the continuing emergence of drug-resistant HIV strains.[111]
Fungi
Infections by fungi are a cause of high morbidity and mortality in immunocompromised persons, such as those with HIV/AIDS, tuberculosis or receiving chemotherapy.[112] The fungi, candida, Cryptococcus neoformans and Aspergillus fumigatus cause most of these infections and antifungal resistance occurs in all of them.[113] Multidrug resistance in fungi is increasing because of the widespread use of antifungal drugs to treat infections in immunocompromised individuals.[114]Parasites
The protozoan parasites that cause the diseases malaria, trypanosomiasis, toxoplasmosis, cryptosporidiosis and leishmaniasis are important human pathogens.[115]Malarial parasites that are resistant to the drugs that are currently available to infections are common and this has led to increased efforts to develop new drugs.[116] Resistance to recently developed drugs such as artemisinin has also been reported. The problem of drug resistance in malaria has driven efforts to develop vaccines.[117]
Trypanosomes are parasitic protozoa that cause African trypanosomiasis and Chagas disease (American trypanosomiasis).[118][119] There are no vaccines to prevent these infections so drugs such as pentamidine and suramin, benznidazole and nifurtimox and used to treat infections. These drugs are effective but infections caused by resistant parasites have been reported.[115]
Leishmaniasis is caused by protozoa and is an important public health problem worldwide, especially in sub-tropical and tropical countries. Drug resistance has "become a major concern".[120]
Reducing antibiotic use in humans
World Health Organization recommendations
An April 30, 2014, report by the WHO addressed this issue, and a summary was described in a WHO press release as follows:[5]- People can help tackle resistance by:
- using antibiotics only when prescribed by a doctor;
- completing the full prescription, even if they feel better;
- never sharing antibiotics with others or using leftover prescriptions.
- Health workers and pharmacists can help tackle resistance by:
- enhancing infection prevention and control;
- only prescribing and dispensing antibiotics when they are truly needed;
- prescribing and dispensing the right antibiotic(s) to treat the illness.
- Policymakers can help tackle resistance by:
- strengthening resistance tracking and laboratory capacity;
- regulating and promoting appropriate use of medicines.
- Policymakers and industry can help tackle resistance by:
- fostering innovation and research and development of new tools;
- promoting cooperation and information sharing among all stakeholders.
Strategies
Excessive antibiotic use has become one of the top contributors to the development of antibiotic resistance. Since the beginning of the antibiotic era, antibiotics have been used to treat a wide range of disease and illness.[121] Overuse of antibiotics has become the primary cause rising levels of antibiotic resistance. The main problem is that doctors are willing to prescribe antibiotics to ill-informed individuals who believe that antibiotics can cure nearly all illnesses, including viral infections like the common cold. In fact, in a recent analysis of drug prescriptions, it was found that 35.7% of individuals with a cold or an upper respiratory infection (both viral in origin) were given prescriptions for antibiotics.[122] These prescriptions accomplished nothing other than increase the risk for further evolution of antibiotic resistant bacteria.Rational use
Rational use of antimicrobials may reduce the chances of development of opportunistic infection by antibiotic-resistant bacteria due to dysbacteriosis. The immune systems will cure minor bacterial infections on its own. It is also important to note that antibiotics will not cure viral infections such as colds and the flu, and taking an antibiotic unnecessarily to treat a viral infection can lead to increased resistance.[123]Rapid viral testing
It is unclear if rapid viral testing affects antibiotic use in children.[124]Vaccines
Vaccines do not have the problem of resistance because a vaccine enhances the body's immune system, whereas an antibiotic operates separately from the body's normal defenses. Nevertheless, new strains that escape immunity induced by vaccines may evolve; for example, an updated influenza vaccine is needed each year.While theoretically promising, antistaphylococcal vaccines have shown limited efficacy, because of immunological variation between Staphylococcus species, and the limited duration of effectiveness of the antibodies produced. Development and testing of more effective vaccines is underway.[125]
Phage therapy
Phage therapy, an approach that has been extensively researched and used as a therapeutic agent for over 60 years, especially in the Soviet Union, represents a potentially significant but currently underdeveloped approach to the treatment of bacterial disease.[126] Phage therapy was widely used in the United States until the discovery of antibiotics, in the early 1940s. Bacteriophages or "phages" are viruses that invade bacterial cells and, in the case of lytic phages, disrupt bacterial metabolism and cause the bacterium to lyse. Phage therapy is the therapeutic use of lytic bacteriophages to treat pathogenic bacterial infections.[127] [128] [129]Bacteriophage therapy is a potentially important alternative to antibiotics in the current era of multidrug-resistant pathogens. A review of studies that dealt with the therapeutic use of phages from 1966 to 1996 and few latest ongoing phage therapy projects via internet showed: Phages were used topically, orally or systemically in Polish and Soviet studies. The success rate found in these studies was 80–95%, with few gastrointestinal or allergic side-effects. British studies also demonstrated significant efficacy of phages against Escherichia coli, Acinetobacter spp., Pseudomonas spp., and Staphylococcus aureus. US studies dealt with improving the bioavailability of phage. Phage therapy may prove as an important alternative to antibiotics for treating multidrug-resistant pathogens.[130]
Probiotics
By definition, probiotics are "A live microbial feed supplement which beneficially affects the host animal by improving its intestinal microbial balance."[131] Treatment works by ingesting an encapsulated pill filled with helpful microbes. Once these organisms reach the gut, they replenish the beneficial bacteria that were wiped out due to antibiotic use. The microbes also apply niche competition to the harmful microbes trying to colonize the body, helping to prevent the occurrence of an infection.Fecal transplants
A stool transplant works by taking the feces from a healthy individual and inserting it into an individual who has an infection. There are three methods in which this treatment can be administered. The mixture can be applied to the top of the small intestine by insertion of a tube through the mouth or nose, applied in the colon during a colonoscopy, or by using an enema in the lower end of the colon. This allows for healthy bacteria to be reintroduced to the gut microbiome.[132] This is another method of introducing natural, beneficial bacteria back into the body and eradicating the harmful microbes.Cytokines
The Australian Commonwealth Scientific and Industrial Research Organisation (CSIRO), realizing the need for the reduction of antibiotic use, has been working on two alternatives. One alternative is to prevent diseases by adding cytokines instead of antibiotics to animal feed.[133] These proteins are made in the animal body "naturally" after a disease and are not antibiotics, so they do not contribute to the problem of antibiotic resistance. Furthermore, studies on using cytokines have shown they also enhance the growth of animals like the antibiotics now used, but without the drawbacks of antibiotic use. Cytokines have the potential to achieve the animal growth rates traditionally sought by the use of antibiotics without the contribution of antibiotic resistance associated with the widespread nontherapeutic uses of antibiotics currently used in the food animal production industries. In addition, CSIRO is working on vaccines for diseases.[133]Alternating therapy
Alternating therapy is a proposed method in which two or three antibiotics are taken in a rotation versus taking just one antibiotic such that bacteria resistant to one antibiotic are killed when the next antibiotic is taken. Studies have found that this method reduces the rate at which antibiotic resistant bacteria emerge in vitro relative to a single drug for the entire duration.[134]Develop new drugs
Since the discovery of antibiotics, research and development (R&D) efforts have provided new drugs in time to treat bacteria that became resistant to older antibiotics, but in the 2000s there has been concern that development has slowed enough that seriously ill people may run out of treatment options.[135] Another concern is that doctors may become reluctant to perform routine surgeries due to the increased risk of harmful infection.[136] Backup treatments can have serious side-effects; for example, treatment of multi-drug-resistant tuberculosis can cause deafness and insanity.[137] The potential crisis at hand is the result of a marked decrease in industry R&D.[138] Poor financial investment in antibiotic research has exacerbated the situation.[51][138] In 2011, Pfizer, one of the last major pharmaceutical companies developing new antibiotics, shut down its primary research effort, citing poor shareholder returns relative to drugs for chronic illnesses.[139]In the United States, drug companies and the administration of President Barack Obama have been proposing changing the standards by which the FDA approves antibiotics targeted at resistant organisms.[136][140] On 12 December 2013, the Antibiotic Development to Advance Patient Treatment (ADAPT) Act of 2013 was introduced in the U.S. Congress. The ADAPT Act aims to fast-track the drug development in order to combat the growing public health threat of 'superbugs'. Under this Act, the FDA can approve antibiotics and antifungals needed for life-threatening infections based on data from smaller clinical trials. The CDC will reinforce the monitoring of the use of antibiotics that treat serious and life-threatening infections and the emerging resistance, and make the data publicly available. The FDA antibiotics labeling process, 'Susceptibility Test Interpretive Criteria for Microbial Organisms' or 'breakpoints' is also streamlined to allow the most up-to-date and cutting-edge data available to healthcare professionals under the new Act.[141][142]
On 18 September 2014 Obama signed an executive order [143] to implement the recommendations proposed in a report [144] by the President's Council of Advisors on Science and Technology (PCAST) which outlines strategies to stream-line clinical trials and speed up the R&D of new antibiotics. Among the proposals:
- Create a 'robust, standing national clinical trials network for antibiotic testing' which will promptly enroll patients once identified to be suffering from dangerous bacterial infections. The network will allow testing multiple new agents from different companies simultaneously for their safety and efficacy.
- Establish a 'Special Medical Use (SMU)' pathway for FDA to approve new antimicrobial agents for use in limited patient populations, shorten the approval timeline for new drug so patients with severe infections could benefit as quickly as possible.
- Provide economic incentives, especially for development of new classes of antibiotics, to offset the steep R&D costs which drive away the industry to develop antibiotics.
The U.S. National Institutes of Health plans to fund a new research network on the issue up to $62 million from 2013 to 2019.[146] Using authority created by the Pandemic and All Hazards Preparedness Act of 2006, the Biomedical Advanced Research and Development Authority in the U.S. Department of Health and Human Services announced that it will spend between $40 million and $200 million in funding for R&D on new antibiotic drugs under development by GlaxoSmithKline.[147]
One major cause of antibiotic resistance is the increased pumping activity of microbial ABC transporters, which diminishes the effective drug concentration inside the microbial cell. ABC transporter inhibitors that can be used in combination with current antimicrobials are being tested in clinical trials and are available for therapeutic regimens.[148]
Reducing antibiotic use in animals
in Europe
In 1997, European Union health ministers voted to ban avoparcin and four additional antibiotics used to promote animal growth in 1999.[149] In 2006 a ban on the use of antibiotics in European feed, with the exception of two antibiotics in poultry feeds, became effective.[150] In Scandinavia, there is evidence that the ban has led to a lower prevalence of antibiotic resistance in (nonhazardous) animal bacterial populations.[151] A corresponding change in antibiotic-resistance cases among humans has not yet been demonstrated.[citation needed]in the United States
The United States Department of Agriculture (USDA) and the Food and Drug Administration (FDA) collect data on antibiotic use in humans and in a more limited fashion in animals.[152]In 2000, the FDA announced their intention to revoke approval of fluoroquinolone use in poultry production because of substantial evidence linking it to the emergence of fluoroquinolone-resistant Campylobacter infections in humans. Legal challenges from the food animal and pharmaceutical industries delayed the final decision to do so until 2006.[153] Fluroquinolones have been banned from extra-label use in food animals in the USA since 2007. However, they remain widely used in companion and exotic animals.
In 2001, National Hog Farmer magazine warned U.S. producers that C. difficile "is sweeping the industry, killing many piglets" (Neutkens D; "New Clostridium Claiming Baby Pigs"). In 2006, a study by the USDA's National Animal Health Monitoring System further investigated the prevalence of C. difficile in hog farms. The study, which covered hog farms of a size typical of those producing 94% of US swine, found the prevalence of C. difficile "relatively low (11.4%)" and that there was no difference in region or in size of farm.[154] Human infection with C. difficile (either drug-resistant or not) is most commonly associated with the use of strong antibiotics in hospitalized humans, and is not associated with humans in contact with farm animals.
During 2007, two federal bills (S. 549[155] and H.R. 962[156]) aimed at phasing out "nontherapeutic" antibiotics in U.S. food animal production. The Senate bill, introduced by Sen. Edward "Ted" Kennedy, died. The House bill, introduced by Rep. Louise Slaughter, died after being referred to Committee.
The FDA first determined in 1977 that there is evidence of emergence of antibiotic-resistant bacterial strains in livestock. The long-established practice of permitting OTC sales of antibiotics (including penicillin and other drugs) to lay animal owners for administration to their own animals nonetheless continued in all states. In March 2012, the United States District Court for the Southern District of New York, ruling in an action brought by the Natural Resources Defense Council and others, ordered the FDA to revoke approvals for the use of antibiotics in livestock that violated FDA regulations.[157] On April 11, 2012 the FDA announced a voluntary program to phase out unsupervised use of drugs as feed additives and convert approved over-the-counter uses for antibiotics to prescription use only, requiring veterinarian supervision of their use and a prescription.[158][159] In December 2013, the FDA announced the commencement of these steps to phase out the use of antibiotics for the purposes of promoting livestock growth.[51][160]
Growing U.S. consumer concern about using antibiotics in animal feed has led to a niche market of "antibiotic-free" animal products, but this small market is unlikely to change entrenched, industry-wide practices.[161]
Applications
Antibiotic resistance is an important tool for genetic engineering. By constructing a plasmid that contains an antibiotic-resistance gene as well as the gene being engineered or expressed, a researcher can ensure that, when bacteria replicate, only the copies that carry the plasmid survive. This ensures that the gene being manipulated passes along when the bacteria replicates.In general, the most commonly used antibiotics in genetic engineering are "older" antibiotics that have largely fallen out of use in clinical practice. These include:
In industry, the use of antibiotic resistance is disfavored, since maintaining bacterial cultures would require feeding them large quantities of antibiotics. Instead, the use of auxotrophic bacterial strains (and function-replacement plasmids) is preferred.


