| Bernhard Riemann | |
|---|---|

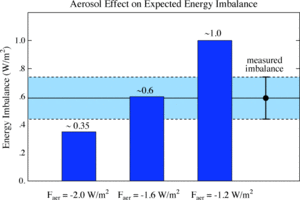
Bernhard Riemann in 1863.
|
|
| Born | Georg Friedrich Bernhard Riemann 17 September 1826 Breselenz, Kingdom of Hanover (modern-day Germany) |
| Died | 20 July 1866 (aged 39) Selasca, Kingdom of Italy |
| Residence | Kingdom of Hanover |
| Nationality | German |
| Alma mater | |
| Known for | See list |
| Scientific career | |
| Fields | |
| Institutions | University of Göttingen |
| Thesis | Grundlagen für eine allgemeine Theorie der Funktionen einer veränderlichen complexen Größe (1851) |
| Doctoral advisor | Carl Friedrich Gauss |
| Other academic advisors | |
| Notable students | Gustav Roch |
| Influences | J. P. G. L. Dirichlet |
| Signature | |
Biography
Early years
Riemann was born on September 17, 1826 in Breselenz, a village near Dannenberg in the Kingdom of Hanover. His father, Friedrich Bernhard Riemann, was a poor Lutheran pastor in Breselenz who fought in the Napoleonic Wars. His mother, Charlotte Ebell, died before her children had reached adulthood. Riemann was the second of six children, shy and suffering from numerous nervous breakdowns. Riemann exhibited exceptional mathematical skills, such as calculation abilities, from an early age but suffered from timidity and a fear of speaking in public.Education
During 1840, Riemann went to Hanover to live with his grandmother and attend lyceum (middle school). After the death of his grandmother in 1842, he attended high school at the Johanneum Lüneburg. In high school, Riemann studied the Bible intensively, but he was often distracted by mathematics. His teachers were amazed by his adept ability to perform complicated mathematical operations, in which he often outstripped his instructor's knowledge. In 1846, at the age of 19, he started studying philology and Christian theology in order to become a pastor and help with his family's finances.During the spring of 1846, his father, after gathering enough money, sent Riemann to the University of Göttingen, where he planned to study towards a degree in Theology. However, once there, he began studying mathematics under Carl Friedrich Gauss (specifically his lectures on the method of least squares). Gauss recommended that Riemann give up his theological work and enter the mathematical field; after getting his father's approval, Riemann transferred to the University of Berlin in 1847.[1] During his time of study, Jacobi, Lejeune Dirichlet, Steiner, and Eisenstein were teaching. He stayed in Berlin for two years and returned to Göttingen in 1849.
Academia
Riemann held his first lectures in 1854, which founded the field of Riemannian geometry and thereby set the stage for Einstein's general theory of relativity. In 1857, there was an attempt to promote Riemann to extraordinary professor status at the University of Göttingen. Although this attempt failed, it did result in Riemann finally being granted a regular salary. In 1859, following Lejeune Dirichlet's death, he was promoted to head the mathematics department at Göttingen. He was also the first to suggest using dimensions higher than merely three or four in order to describe physical reality.[2] In 1862 he married Elise Koch and had a daughter.Austro-Prussian War and death in Italy
Riemann's tombstone in Biganzolo
Riemann fled Göttingen when the armies of Hanover and Prussia clashed there in 1866.[3] He died of tuberculosis during his third journey to Italy in Selasca (now a hamlet of Verbania on Lake Maggiore) where he was buried in the cemetery in Biganzolo (Verbania). Riemann was a dedicated Christian, the son of a Protestant minister, and saw his life as a mathematician as another way to serve God. During his life, he held closely to his Christian faith and considered it to be the most important aspect of his life. At the time of his death, he was reciting the Lord’s Prayer with his wife and died before they finished saying the prayer.[4] Meanwhile, in Göttingen his housekeeper discarded some of the papers in his office, including much unpublished work. Riemann refused to publish incomplete work, and some deep insights may have been lost forever.[3]
Riemann's tombstone in Biganzolo (Italy) refers to Romans 8:28 ("And we know that all things work together for good to them that love God, to them who are called according to his purpose"):
Here rests in God Georg Friedrich Bernhard Riemann
Professor in Göttingen
born in Breselenz, Germany 17 September 1826
died in Selasca, Italy 20 July 1866
For those who love God, all things must work together for the best.[5]
Riemannian geometry
Riemann's published works opened up research areas combining analysis with geometry. These would subsequently become major parts of the theories of Riemannian geometry, algebraic geometry, and complex manifold theory. The theory of Riemann surfaces was elaborated by Felix Klein and particularly Adolf Hurwitz. This area of mathematics is part of the foundation of topology and is still being applied in novel ways to mathematical physics.In 1853, Gauss asked his student Riemann to prepare a Habilitationsschrift on the foundations of geometry. Over many months, Riemann developed his theory of higher dimensions and delivered his lecture at Göttingen in 1854 entitled "Ueber die Hypothesen welche der Geometrie zu Grunde liegen" ("On the hypotheses which underlie geometry"). It was only published twelve years later in 1868 by Dedekind, two years after his death. Its early reception appears to have been slow but it is now recognized as one of the most important works in geometry.
The subject founded by this work is Riemannian geometry. Riemann found the correct way to extend into n dimensions the differential geometry of surfaces, which Gauss himself proved in his theorema egregium. The fundamental object is called the Riemann curvature tensor. For the surface case, this can be reduced to a number (scalar), positive, negative, or zero; the non-zero and constant cases being models of the known non-Euclidean geometries.
Riemann's idea was to introduce a collection of numbers at every point in space (i.e., a tensor) which would describe how much it was bent or curved. Riemann found that in four spatial dimensions, one needs a collection of ten numbers at each point to describe the properties of a manifold, no matter how distorted it is. This is the famous construction central to his geometry, known now as a Riemannian metric.
Complex analysis
In his dissertation, he established a geometric foundation for complex analysis through Riemann surfaces, through which multi-valued functions like the logarithm (with infinitely many sheets) or the square root (with two sheets) could become one-to-one functions. Complex functions are harmonic functions (that is, they satisfy Laplace's equation and thus the Cauchy–Riemann equations) on these surfaces and are described by the location of their singularities and the topology of the surfaces. The topological "genus" of the Riemann surfaces is given by , where the surface has
, where the surface has  leaves coming together at
leaves coming together at  branch points. For
branch points. For  the Riemann surface has
the Riemann surface has  parameters (the "moduli").
parameters (the "moduli").His contributions to this area are numerous. The famous Riemann mapping theorem says that a simply connected domain in the complex plane is "biholomorphically equivalent" (i.e. there is a bijection between them that is holomorphic with a holomorphic inverse) to either
 or to the interior of the unit circle. The generalization of the theorem to Riemann surfaces is the famous uniformization theorem, which was proved in the 19th century by Henri Poincaré and Felix Klein. Here, too, rigorous proofs were first given after the development of
richer mathematical tools (in this case, topology). For the proof of the
existence of functions on Riemann surfaces he used a minimality
condition, which he called the Dirichlet principle. Weierstrass
found a gap in the proof: Riemann had not noticed that his working
assumption (that the minimum existed) might not work; the function space
might not be complete, and therefore the existence of a minimum was not
guaranteed. Through the work of David Hilbert in the Calculus of Variations, the Dirichlet principle was finally established. Otherwise, Weierstrass was very impressed with Riemann, especially with his theory of abelian functions. When Riemann's work appeared, Weierstrass withdrew his paper from Crelle's Journal
and did not publish it. They had a good understanding when Riemann
visited him in Berlin in 1859. Weierstrass encouraged his student Hermann Amandus Schwarz to find alternatives to the Dirichlet principle in complex analysis, in which he was successful. An anecdote from Arnold Sommerfeld[6]
shows the difficulties which contemporary mathematicians had with
Riemann's new ideas. In 1870, Weierstrass had taken Riemann's
dissertation with him on a holiday to Rigi and complained that it was
hard to understand. The physicist Hermann von Helmholtz assisted him in
the work over night and returned with the comment that it was "natural"
and "very understandable".
or to the interior of the unit circle. The generalization of the theorem to Riemann surfaces is the famous uniformization theorem, which was proved in the 19th century by Henri Poincaré and Felix Klein. Here, too, rigorous proofs were first given after the development of
richer mathematical tools (in this case, topology). For the proof of the
existence of functions on Riemann surfaces he used a minimality
condition, which he called the Dirichlet principle. Weierstrass
found a gap in the proof: Riemann had not noticed that his working
assumption (that the minimum existed) might not work; the function space
might not be complete, and therefore the existence of a minimum was not
guaranteed. Through the work of David Hilbert in the Calculus of Variations, the Dirichlet principle was finally established. Otherwise, Weierstrass was very impressed with Riemann, especially with his theory of abelian functions. When Riemann's work appeared, Weierstrass withdrew his paper from Crelle's Journal
and did not publish it. They had a good understanding when Riemann
visited him in Berlin in 1859. Weierstrass encouraged his student Hermann Amandus Schwarz to find alternatives to the Dirichlet principle in complex analysis, in which he was successful. An anecdote from Arnold Sommerfeld[6]
shows the difficulties which contemporary mathematicians had with
Riemann's new ideas. In 1870, Weierstrass had taken Riemann's
dissertation with him on a holiday to Rigi and complained that it was
hard to understand. The physicist Hermann von Helmholtz assisted him in
the work over night and returned with the comment that it was "natural"
and "very understandable".Other highlights include his work on abelian functions and theta functions on Riemann surfaces. Riemann had been in a competition with Weierstrass since 1857 to solve the Jacobian inverse problems for abelian integrals, a generalization of elliptic integrals. Riemann used theta functions in several variables and reduced the problem to the determination of the zeros of these theta functions. Riemann also investigated period matrices and characterized them through the "Riemannian period relations" (symmetric, real part negative). By Ferdinand Georg Frobenius and Solomon Lefschetz the validity of this relation is equivalent with the embedding of
 (where
(where  is the lattice of the period matrix) in a projective space by means of theta functions. For certain values of
is the lattice of the period matrix) in a projective space by means of theta functions. For certain values of  , this is the Jacobian variety of the Riemann surface, an example of an abelian manifold.
, this is the Jacobian variety of the Riemann surface, an example of an abelian manifold.Many mathematicians such as Alfred Clebsch furthered Riemann's work on algebraic curves. These theories depended on the properties of a function defined on Riemann surfaces. For example, the Riemann–Roch theorem (Roch was a student of Riemann) says something about the number of linearly independent differentials (with known conditions on the zeros and poles) of a Riemann surface.
According to Laugwitz,[7] automorphic functions appeared for the first time in an essay about the Laplace equation on electrically charged cylinders. Riemann however used such functions for conformal maps (such as mapping topological triangles to the circle) in his 1859 lecture on hypergeometric functions or in his treatise on minimal surfaces.
Real analysis
In the field of real analysis, he discovered the Riemann integral in his habilitation. Among other things, he showed that every piecewise continuous function is integrable. Similarly, the Stieltjes integral goes back to the Göttinger mathematician, and so they are named together the Riemann–Stieltjes integral.In his habilitation work on Fourier series, where he followed the work of his teacher Dirichlet, he showed that Riemann-integrable functions are "representable" by Fourier series. Dirichlet has shown this for continuous, piecewise-differentiable functions (thus with countably many non-differentiable points). Riemann gave an example of a Fourier series representing a continuous, almost nowhere-differentiable function, a case not covered by Dirichlet. He also proved the Riemann–Lebesgue lemma: if a function is representable by a Fourier series, then the Fourier coefficients go to zero for large n.
Riemann's essay was also the starting point for Georg Cantor's work with Fourier series, which was the impetus for set theory.
He also worked with hypergeometric differential equations in 1857 using complex analytical methods and presented the solutions through the behavior of closed paths about singularities (described by the monodromy matrix). The proof of the existence of such differential equations by previously known monodromy matrices is one of the Hilbert problems.
Number theory
He made some famous contributions to modern analytic number theory. In a single short paper, the only one he published on the subject of number theory, he investigated the zeta function that now bears his name, establishing its importance for understanding the distribution of prime numbers. The Riemann hypothesis was one of a series of conjectures he made about the function's properties.In Riemann's work, there are many more interesting developments. He proved the functional equation for the zeta function (already known to Euler), behind which a theta function lies. Also, it gives a better approximation for the prime-counting function
 than Gauss's function
than Gauss's function  [citation needed].
Through the summation of this approximation function over the
non-trivial zeros on the line with real portion 1/2, he gave an exact,
"explicit formula" for
[citation needed].
Through the summation of this approximation function over the
non-trivial zeros on the line with real portion 1/2, he gave an exact,
"explicit formula" for  .
.Riemann knew Chebyshev's work on the Prime Number Theorem. He had visited Dirichlet in 1852. But Riemann's methods were very different.