From Wikipedia, the free encyclopedia
The
kidneys are two
bean-shaped
organs present in left and right sides of the body in
vertebrates. They are located at the back of the
abdominal cavity. In adults they are about 11 centimetres (4.3 in) in length. They receive blood from the paired
renal arteries; blood exits into the paired
renal veins. Each kidney is attached to a
ureter, a tube that carries excreted
urine to the
bladder.
The
nephron
is the structural and functional unit of the kidney. Each adult kidney
contains around one million nephrons. The nephron utilizes four
processes to alter the blood plasma which flows to it:
filtration,
reabsorption,
secretion, and
excretion. The kidney participates in the control of the volume of various
body fluid compartments, fluid
osmolality,
acid-base balance, various
electrolyte concentrations, and removal of
toxins. Filtration occurs in the
glomerulus: one-fifth of the blood volume that enters the kidneys is filtered. Examples of substances reabsorbed are solute-free
water,
sodium,
bicarbonate,
glucose, and
amino acids. Examples of substances secreted are
hydrogen,
ammonium,
potassium and
uric acid. The kidneys also carry out functions independent of the nephron. For example, they convert a precursor of
vitamin D to its active form,
calcitriol; and synthesize the
hormones erythropoietin and
renin.
Renal physiology is the study of
kidney function.
Nephrology is the medical specialty which addresses diseases of kidney
function: these include
chronic kidney disease,
nephritic and
nephrotic syndromes,
acute kidney injury, and
pyelonephritis.
Urology addresses diseases of kidney (and urinary tract)
anatomy: these include
cancer,
renal cysts,
kidney stones and
ureteral stones, and
urinary tract obstruction.
Procedures used in the management of kidney disease include chemical and microscopic examination of the urine (
urinalysis), measurement of
kidney function by calculating the estimated
glomerular filtration rate (eGFR) using the
serum creatinine; and
kidney biopsy and
CT scan to evaluate for abnormal anatomy.
Dialysis and
kidney transplantation are used to treat
kidney failure; one (or both sequentially) of these are almost always used when renal function drops below 15%.
Nephrectomy is frequently used to cure
renal cell carcinoma.
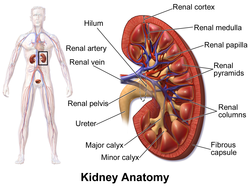
Structure

Surface projections of the organs of the
trunk, showing kidneys at the level of T12 to L3.
In humans, the kidneys are located high in the
abdominal cavity, one on each side of the
spine, and lie in a
retroperitoneal position at a slightly oblique angle. The asymmetry within the abdominal cavity, caused by the position of the
liver,
typically results in the right kidney being slightly lower and smaller
than the left, and being placed slightly more to the middle than the
left kidney. The left kidney is approximately at the vertebral level
T12 to
L3, and the right is slightly lower. The right kidney sits just below the
diaphragm and posterior to the
liver. The left sits below the diaphragm and posterior to the
spleen. On top of each kidney is an
adrenal gland. The upper parts of the kidneys are partially protected by the 11th and 12th
ribs. Each kidney, with its adrenal gland is surrounded by two layers of fat: the
perirenal fat present between renal fascia and renal capsule and
pararenal fat superior to the
renal fascia.
CT
scan of the kidneys. Left: cross section at upper abdomen level – the
liver is seen on the left side of scan (right side of body). Center:
longitudinal section though the center of the kidneys – the liver
partially covers the right kidney. Right: transverse section through the
left kidney.
The kidney is a
bean-shaped structure with a
convex and a
concave border. A recessed area on the concave border is the
renal hilum, where the
renal artery enters the kidney and the
renal vein and
ureter leave. The kidney is surrounded by tough fibrous tissue, the
renal capsule, which is itself surrounded by
perirenal fat,
renal fascia, and
pararenal fat. The anterior (front) surface of these tissues is the
peritoneum, while the posterior (rear) surface is the
transversalis fascia.
The superior pole of the right kidney is adjacent to the liver. For the left kidney, it is next to the
spleen. Both, therefore, move down upon inhalation.
In adult males, the kidney weighs between 125 and 170 grams. In females the weight of the kidney is between 115 and 155 grams.
A Danish study measured the median renal length to be 11.2 cm (4.4 in)
on the left side and 10.9 cm (4.3 in) on the right side in adults.
Median renal volumes were 146 cm
3 on the left and 134 cm
3 on the right.
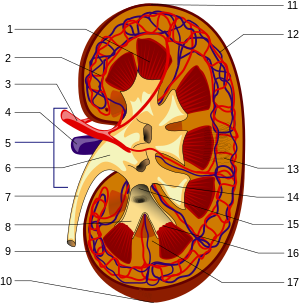
Gross anatomy
The substance, or
parenchyma, of the kidney is divided into two major structures: the outer
renal cortex and the inner
renal medulla. Grossly, these structures take the shape of eight to 18 cone-shaped
renal lobes, each containing renal cortex surrounding a portion of medulla called a
renal pyramid. Between the renal pyramids are projections of cortex called
renal columns.
Nephrons,
the urine-producing functional structures of the kidney, span the
cortex and medulla. The initial filtering portion of a nephron is the
renal corpuscle, which is located in the cortex. This is followed by a
renal tubule that passes from the cortex deep into the medullary pyramids. Part of the renal cortex, a
medullary ray is a collection of renal tubules that drain into a single
collecting duct.
The tip, or
papilla, of each pyramid empties urine into a
minor calyx; minor calyces empty into
major calyces, and major calyces empty into the
renal pelvis.
This becomes the ureter. At the hilum, the ureter and renal vein exit
the kidney and the renal artery enters. Hilar fat and lymphatic tissue
with lymph nodes surrounds these structures. The hilar fat is contiguous
with a fat-filled cavity called the
renal sinus.
The renal sinus collectively contains the renal pelvis and calyces and
separates these structures from the renal medullary tissue.
The kidneys possess no overtly moving structures
Blood supply
The
renal circulation supplies the blood to the kidneys via the
renal arteries, left and right, which branch directly from the
abdominal aorta. Despite their relatively small size, the kidneys receive approximately 20% of the
cardiac output.
Each renal artery branches into segmental arteries, dividing further into
interlobar arteries,
which penetrate the renal capsule and extend through the renal columns
between the renal pyramids. The interlobar arteries then supply blood to
the
arcuate arteries that run through the boundary of the cortex and the medulla. Each arcuate artery supplies several
interlobular arteries that feed into the
afferent arterioles that supply the
glomeruli.
After filtration occurs, the blood moves through a small network
of venules that converge into interlobular veins. As with the arteriole
distribution, the veins follow the same pattern: the interlobular
provide blood to the arcuate veins then back to the interlobar veins,
which come to form the renal vein exiting the kidney for transfusion for
blood.
The table below shows the path that blood takes when it travels
through the glomerulus, traveling "down" the arteries and "up" the
veins. However, this model is greatly simplified for clarity and
symmetry. Some of the other paths and complications are described at the
bottom of the table. The interlobar artery and vein (not to be confused
with interlobular) are between two renal lobes, also known as the renal
column (cortex region between two pyramids).
- Note 1: The renal artery also provides a branch to the inferior suprarenal artery to supply the adrenal gland.
- Note 2: Each renal artery partitions into an anterior and posterior
branch. The anterior branch further divides into the superior (apical),
anterosuperior, anteroinferior and inferior segmental arteries. The
posterior branch continues as the posterior segmental artery.
- Note 3: Also called the cortical radiate arteries. The interlobular artery also supplies to the stellate veins.
- Note 4: The efferent arterioles do not directly drain into the interlobular vein, but rather they go to the peritubular capillaries first. The efferent arterioles of the juxtamedullary nephron drain into the vasa recta.
Nerve supply
The kidney and
nervous system communicate via the
renal plexus, whose fibers course along the renal arteries to reach each kidney. Input from the
sympathetic nervous system triggers
vasoconstriction in the kidney, thereby reducing
renal blood flow. The kidney also receives input from the
parasympathetic nervous system, by way of the renal branches of the
vagus nerve; the function of this is yet unclear. Sensory input from the kidney travels to the T10-11 levels of the
spinal cord and is sensed in the corresponding
dermatome.
[10] Thus, pain in the flank region may be referred from corresponding kidney.
Microanatomy
Diagram of a long juxtamedullary nephron (left) and a short cortical nephron (right). All parts of the nephron are labelled except the (gray) connecting tubule located after the (dark red) distal convoluted tubule and before the large (gray) collecting duct (mislabeled collection duct).
Renal
histology is the study of the
microscopic structure of the kidney. Distinct
cell types include:
Gene and protein expression
About 20,000 protein coding genes are expressed in human cells and
almost 70% of these genes are expressed in normal, adult kidneys.
Just over 300 genes are more specifically expressed in the kidney, with
only some 50 genes being highly specific for the kidney. Many of the
corresponding kidney specific proteins are expressed in the cell
membrane and function as transporter proteins. The highest expressed
kidney specific protein is
uromodulin,
the most abundant protein in urine with functions that prevent
calcification and growth of bacteria. Specific proteins are expressed in
the different compartments of the kidney with
podocin and
nephrin expressed in glomeruli, Solute carrier family protein
SLC22A8 expressed in proximal tubules,
calbindin expressed in distal tubules and
aquaporin 2 expressed in the collecting duct cells.
Development
The mammalian kidney develops from
intermediate mesoderm.
Kidney development, also called
nephrogenesis,
proceeds through a series of three successive developmental phases: the
pronephros, mesonephros, and metanephros. The metanephros are primordia
of the permanent kidney.
Function
Schematic diagram of the nephron (yellow), relevant circulation (red/blue), and the four methods of altering the filtrate.
The microscopic structural and functional unit of the kidney is the
nephron.
It processes the blood supplied to it via filtration, reabsorption,
secretion and excretion; the consequence of those processes is the
production of
urine.
Mechanism
Filtration
Filtration, which takes place at the
renal corpuscle, is the process by which cells and large proteins are retained while materials of smaller molecular weights are filtered from the blood to make an
ultrafiltrate
that eventually becomes urine. The kidney generates 180 liters of
filtrate a day. The process is also known as hydrostatic filtration due
to the hydrostatic pressure exerted on the capillary walls.
Reabsorption
Secretion and reabsorption of various substances throughout the nephron
Reabsorption is the transport of molecules from this ultrafiltrate
and into the peritubular capillary. It is accomplished via selective
receptors
on the luminal cell membrane. Water is 65% reabsorbed in the proximal
tubule. Glucose at normal plasma levels is completely reabsorbed in the
proximal tubule. The mechanism for this is the Na
+/glucose
cotransporter. A plasma level of 350 mg/dL will fully saturate the
transporters and glucose will be lost in the urine. A plasma glucose
level of approximately 160 is sufficient to allow glucosuria, which is
an important clinical clue to diabetes mellitus.
Amino acids are reabsorbed by sodium dependent transporters in the
proximal tubule.
Hartnup disease is a deficiency of the tryptophan amino acid transporter, which results in
pellagra.
| Location of Reabsorption |
Reabsorbed nutrient |
Notes
|
| Early proximal tubule |
Glucose (100%), amino acids (100%), bicarbonate (90%), Na+ (65%), Cl− (65%), phosphate (65%) and H2O (65%) |
- PTH will inhibit phosphate reabsorption.
- AT II stimulates Na+, H2O and HCO3− reabsorption.
|
| Thin descending loop of Henle |
H2O |
- Reabsorbs via medullary hypertonicity and makes urine hypertonic.
|
| Thick ascending loop of Henle |
Na+ (10–20%), K+, Cl−; indirectly induces para cellular reabsorption of Mg2+, Ca2+ |
- This region is impermeable to H2O and the urine becomes less concentrated as it ascends.
|
| Early distal convoluted tubule |
Na+, Cl− |
- PTH causes Ca2+ reabsorption.
|
| Collecting tubules |
Na+(3–5%), H2O |
- Na+ is reabsorbed in exchange for K+, and H+, which is regulated by aldosterone.
- ADH acts on the V2 receptor and inserts aquaporins on the luminal side
|
| Source:
|
Secretion
Secretion
is the reverse of reabsorption: molecules are transported from the
peritubular capillary through the interstitial fluid, then through the
renal tubular cell and into the ultrafiltrate.
Excretion
The last step in the processing of the ultrafiltrate is
excretion: the ultrafiltrate passes out of the nephron and travels through a tube called the
collecting duct, which is part of the
collecting duct system, and then to the ureters where it is renamed
urine. In addition to transporting the ultrafiltrate, the collecting duct also takes part in reabsorption.
Homeostasis
The kidney participates in whole-body
homeostasis, regulating
acid-base balance,
electrolyte concentrations,
extracellular fluid volume, and
blood pressure.
The kidney accomplishes these homeostatic functions both independently
and in concert with other organs, particularly those of the
endocrine system. Various endocrine hormones coordinate these endocrine functions; these include
renin,
angiotensin II,
aldosterone,
antidiuretic hormone, and
atrial natriuretic peptide, among others.
The kidneys excrete a variety of waste products produced by
metabolism into the urine. These include the nitrogenous wastes
urea, from protein
catabolism, and
uric acid, from
nucleic acid
metabolism. The ability of mammals and some birds to concentrate
wastes into a volume of urine much smaller than the volume of blood from
which the wastes were extracted is dependent on an elaborate
countercurrent multiplication
mechanism. This requires several independent nephron characteristics to
operate: a tight hairpin configuration of the tubules, water and ion
permeability in the descending limb of the loop, water impermeability in
the ascending loop, and active ion transport out of most of the
ascending limb. In addition, passive
countercurrent exchange by the vessels carrying the blood supply to the nephron is essential for enabling this function.
Acid-base balance
Two organ systems, the kidneys and lungs, maintain acid-base homeostasis, which is the maintenance of
pH around a relatively stable value. The lungs contribute to acid-base homeostasis by regulating
carbon dioxide (CO
2)
concentration. The kidneys have two very important roles in
maintaining the acid-base balance: to reabsorb and regenerate
bicarbonate from urine, and to excrete
hydrogen ions and fixed acids (anions of acids) into urine.
Regulation of osmolality
Maintaining water and salt level of the body. Any significant rise in
plasma osmolality is detected by the
hypothalamus, which communicates directly with the
posterior pituitary gland. An increase in osmolality causes the gland to secrete
antidiuretic hormone
(ADH), resulting in water reabsorption by the kidney and an increase in
urine concentration. The two factors work together to return the plasma
osmolality to its normal levels.
ADH binds to principal cells in the collecting duct that
translocate aquaporins to the membrane, allowing water to leave the
normally impermeable membrane and be reabsorbed into the body by the
vasa recta, thus increasing the plasma volume of the body.
There are two systems that create a hyperosmotic medulla and thus
increase the body plasma volume: Urea recycling and the 'single
effect.'
Urea is usually excreted as a waste product from the kidneys.
However, when plasma blood volume is low and ADH is released the
aquaporins that are opened are also permeable to urea. This allows urea
to leave the collecting duct into the medulla, creating a hyperosmotic
solution that "attracts" water. Urea can then re-enter the nephron and
be excreted or recycled again depending on whether ADH is still present
or not.
The 'single effect' describes the fact that the ascending thick limb of the
loop of Henle is not permeable to water but is permeable to
sodium chloride. This allows for a
countercurrent exchange
system whereby the medulla becomes increasingly concentrated, but at
the same time setting up an osmotic gradient for water to follow should
the aquaporins of the collecting duct be opened by ADH.
Hormone secretion
The kidneys secrete a variety of
hormones, including
erythropoietin,
calcitriol, and
renin.
Erythropoietin is released in response to
hypoxia (low levels of oxygen at tissue level) in the renal circulation. It stimulates
erythropoiesis (production of red blood cells) in the
bone marrow.
Calcitriol, the activated form of
vitamin D, promotes intestinal absorption of
calcium and the renal
reabsorption of
phosphate. Renin is an
enzyme which regulates
angiotensin and
aldosterone levels.
Blood pressure regulation
Although the kidney cannot directly sense blood, long-term regulation of
blood pressure predominantly depends upon the kidney. This primarily occurs through maintenance of the
extracellular fluid compartment, the size of which depends on the plasma
sodium concentration. Renin is the first in a series of important chemical messengers that make up the
renin–angiotensin system. Changes in renin ultimately alter the output of this system, principally the hormones
angiotensin II and
aldosterone. Each hormone acts via multiple mechanisms, but both increase the kidney's absorption of
sodium chloride,
thereby expanding the extracellular fluid compartment and raising blood
pressure. When renin levels are elevated, the concentrations of
angiotensin II and aldosterone increase, leading to increased sodium
chloride reabsorption, expansion of the extracellular fluid compartment,
and an increase in blood pressure. Conversely, when renin levels are
low, angiotensin II and aldosterone levels decrease, contracting the
extracellular fluid compartment, and decreasing blood pressure.
Calculations of function
Calculations of kidney performance are an important part of physiology and can be estimated using the calculations below.
Filtration fraction
The
filtration fraction is the amount of plasma that is actually filtered
through the kidney. This can be defined using the equation:
FF=GFR/RPF
Normal human FF is 20%.
Renal clearance
Renal clearance is the volume of plasma from which the substance is completely cleared from the blood per unit time.
Cx=(Ux)V/Px
- Cx is the clearance of X (normally in units of mL/min).
- Ux is the urine concentration of X.
- Px is the plasma concentration of X.
- V is the urine flow rate.
Mathematical modelling of function
The kidney is a very complex organ and
mathematical modelling has been used to better understand kidney function at several scales, including fluid uptake and secretion.
Clinical significance
Kidney disease
is an abnormal structure, function or process in the kidney(s).
Nephrosis is non-inflammatory nephropathy and nephritis is inflammatory
kidney disease.
Nephrology is the speciality that deals with kidney function and disease. Medical terms related to the kidneys commonly use terms such as
renal and the prefix
nephro-. The
adjective renal, meaning related to the kidney, is from the
Latin rēnēs, meaning kidneys; the prefix
nephro- is from the
Ancient Greek word for kidney,
nephros (νεφρός). For example, surgical removal of the kidney is a
nephrectomy, while a reduction in kidney function is called
renal dysfunction.
Acquired
Kidney injury and failure
Generally, humans can live normally with just one kidney, as one has
more functioning renal tissue than is needed to survive. Only when the
amount of functioning kidney tissue is greatly diminished does one
develop
chronic kidney disease.
Renal replacement therapy, in the form of
dialysis or
kidney transplantation, is indicated when the
glomerular filtration rate has fallen very low or if the renal dysfunction leads to severe symptoms.
Dialysis
Dialysis is a treatment that takes over jobs that healthy kidneys
normally do. Kidneys are in need of dialysis when approximately 85%-90%
of kidney function is lost, in addition to a Glomerular Filtration Rate
(GFR) of less than 15. Dialysis maintains homeostasis by removing excess
water and other salts, regulating blood pressure, and maintaining
chemical levels within the body. Dialysis is a treatment that does not
cure kidney disease, a kidney transplant will cure kidney disease. While
a costly procedure, Dialysis has a life expectancy of 5–10 years with
patients having lived up to 30 years while receiving treatment. However,
patients receiving the dialysis treatments are able to lead normal
lives, despite the regular appointments.
Congenital disease
Congenital hydronephrosis
Congenital obstruction of urinary tract
Duplex kidneys, or double kidneys, occur in approximately 1% of the
population. This occurrence normally causes no complications, but can
occasionally cause urinary tract infections.
Duplicated ureter occurs in approximately one in 100 live births
Horseshoe kidney occurs in approximately one in 400 live births
Nutcracker syndrome
Polycystic kidney disease
A depiction of Peritoneal dialysis in case of kidney failure.
- Renal agenesis.
Failure of one kidney to form occurs in approximately one in 750 live
births. Failure of both kidneys to form used to be fatal; however,
medical advances such as amnioinfusion therapy during pregnancy and
peritoneal dialysis have made it possible to stay alive until a
transplant can occur.
- Renal dysplasia
- Unilateral small kidney
- Multicystic dysplastic kidney occurs in approximately one in every 2400 live births
- Ureteropelvic Junction Obstruction or UPJO; although most cases appear congenital, some appear to be an acquired condition[24]
Diagnosis
Many renal diseases are diagnosed on the basis of a detailed
medical history, and
physical examination.
The medical history takes into account present and past symptoms,
especially those of kidney disease; recent infections; exposure to
substances toxic to the kidney; and family history of kidney disease.
Kidney function is tested for using
blood tests and
urine tests. A usual blood test is for
urea and
electrolytes, known as a
U and E.
Creatinine is also tested for. Urine tests such as
urinalysis can evaluate for pH, protein, glucose, and the presence of blood. Microscopic analysis can also identify the presence of
urinary casts and crystals.
[25] The
glomerular filtration rate (GFR) can be calculated.
Imaging
Renal ultrasonography is essential in the diagnosis and management of kidney-related diseases. Other modalities, such as
CT and
MRI, should always be considered as supplementary imaging modalities in the assessment of renal disease.
Biopsy
The role
of the renal biopsy is to diagnose renal disease in which the etiology
is not clear based upon noninvasive means (clinical history, past
medical history, medication history, physical exam, laboratory studies,
imaging studies). In general, a renal pathologist will perform a
detailed morphological evaluation and integrate the morphologic findings
with the clinical history and laboratory data, ultimately arriving at a
pathological diagnosis. A renal
pathologist
is a physician who has undergone general training in anatomic pathology
and additional specially training in the interpretation of renal biopsy
specimens.
Ideally, multiple core sections are obtained and evaluated for
adequacy (presence of glomeruli) intraoperatively. A
pathologist/pathology assistant divides the specimen(s) for submission
for light microscopy, immunofluorescence microscopy and electron
microscopy.
The pathologist will examine the specimen using light microscopy
with multiple staining techniques (hematoxylin and eosin/H&E, PAS,
trichrome, silver stain) on multiple level sections. Multiple
immunofluorescence stains are performed to evaluate for antibody,
protein and complement deposition. Finally, ultra-structural examination
is performed with electron microscopy and may reveal the presence of
electron-dense deposits or other characteristic abnormalities that may
suggest an etiology for the patient's renal disease.
Other animals
In the majority of vertebrates, the
mesonephros persists into the adult, albeit usually fused with the more advanced
metanephros; only in
amniotes is the mesonephros restricted to the embryo. The kidneys of
fish and
amphibians
are typically narrow, elongated organs, occupying a significant portion
of the trunk. The collecting ducts from each cluster of nephrons
usually drain into an
archinephric duct, which is
homologous with the
vas deferens of amniotes. However, the situation is not always so simple; in
cartilaginous fish
and some amphibians, there is also a shorter duct, similar to the
amniote ureter, which drains the posterior (metanephric) parts of the
kidney, and joins with the archinephric duct at the
bladder or
cloaca.
Indeed, in many cartilaginous fish, the anterior portion of the kidney
may degenerate or cease to function altogether in the adult.
In the most primitive vertebrates, the
hagfish and
lampreys,
the kidney is unusually simple: it consists of a row of nephrons, each
emptying directly into the archinephric duct. Invertebrates may possess
excretory organs that are sometimes referred to as "kidneys", but, even
in
Amphioxus, these are never homologous with the kidneys of vertebrates, and are more accurately referred to by other names, such as
nephridia. In
amphibians, kidneys and the
urinary bladder harbour specialized
parasites,
monogeneans of the family Polystomatidae.
The kidneys of
reptiles
consist of a number of lobules arranged in a broadly linear pattern.
Each lobule contains a single branch of the ureter in its centre, into
which the collecting ducts empty. Reptiles have relatively few nephrons
compared with other amniotes of a similar size, possibly because of
their lower
metabolic rate.
Birds
have relatively large, elongated kidneys, each of which is divided into
three or more distinct lobes. The lobes consists of several small,
irregularly arranged, lobules, each centred on a branch of the ureter.
Birds have small glomeruli, but about twice as many nephrons as
similarly sized mammals.
The human kidney is fairly typical of that of
mammals.
Distinctive features of the mammalian kidney, in comparison with that
of other vertebrates, include the presence of the renal pelvis and renal
pyramids and a clearly distinguishable cortex and medulla. The latter
feature is due to the presence of elongated
loops of Henle; these are much shorter in birds, and not truly present in other vertebrates (although the nephron often has a short
intermediate segment
between the convoluted tubules). It is only in mammals that the kidney
takes on its classical "kidney" shape, although there are some
exceptions, such as the multilobed
reniculate kidneys of
pinnipeds and
cetaceans.
Evolutionary adaptation
Kidneys of various animals show evidence of evolutionary
adaptation and have long been studied in
ecophysiology and
comparative physiology. Kidney morphology, often indexed as the relative medullary thickness, is associated with habitat
aridity among species of mammals and diet (e.g., carnivores have only long loops of Henle).
Society and culture
Significance
Egyptian
In
ancient Egypt,
the kidneys, like the heart, were left inside the mummified bodies,
unlike other organs which were removed. Comparing this to the biblical
statements, and to drawings of human body with the heart and two kidneys
portraying a set of scales for weighing justice, it seems that the
Egyptian beliefs had also connected the kidneys with judgement and
perhaps with moral decisions.
Hebrew
According
to studies in modern and ancient Hebrew, various body organs in humans
and animals served also an emotional or logical role, today mostly
attributed to the
brain and the
endocrine system. The kidney is mentioned in several biblical verses in conjunction with the heart, much as the
bowels were understood to be the "seat" of emotion – grief, joy and pain. Similarly, the
Talmud (
Berakhoth 61.a) states that one of the two kidneys counsels what is good, and the other evil.
In the sacrifices offered at the biblical
Tabernacle and later on at the temple in
Jerusalem, the priests were instructed
to remove the kidneys and the adrenal gland covering the kidneys of the
sheep, goat and cattle offerings, and to burn them on the altar, as the
holy part of the "offering for God" never to be eaten.
India: Ayurvedic system
In ancient India, according to the
Ayurvedic medical systems, the kidneys were considered the beginning of the excursion channels system, the 'head' of the
Mutra Srotas,
receiving from all other systems, and therefore important in
determining a person's health balance and temperament by the balance and
mixture of the three 'Dosha's – the three health elements: Vatha (or
Vata) – air, Pitta –
bile, and Kapha –
mucus. The temperament and health of a person can then be seen in the resulting color of the urine.
Modern Ayurveda practitioners, a practice which is characterized as pseudoscience, have attempted to revive these methods in medical procedures as part of Ayurveda
Urine therapy. These procedures have been called "nonsensical" by skeptics.
Medieval Christianity
The Latin term
renes is related to the English word "reins", a synonym for the kidneys in
Shakespearean English (e.g.
Merry Wives of Windsor 3.5), which was also the time when the
King James Version of the
Bible was translated. Kidneys were once popularly regarded as the seat of the
conscience and reflection,
and a number of verses in the Bible (e.g. Ps. 7:9, Rev. 2:23) state
that God searches out and inspects the kidneys, or "reins", of humans,
together with the heart.
As food
Hökarpanna, Swedish pork and kidney stew
The kidneys, like other
offal, can be
cooked and eaten.
Kidneys are usually grilled or sautéed, but in more complex
dishes they are stewed with a sauce that will improve their flavor. In
many preparations, kidneys are combined with pieces of meat or liver, as
in
mixed grill. Dishes include the
British steak and kidney pie, the
Swedish hökarpanna (pork and kidney stew), the
French rognons de veau sauce moutarde (veal kidneys in
mustard sauce) and the
Spanish riñones al Jerez (kidneys stewed in
sherry sauce) .