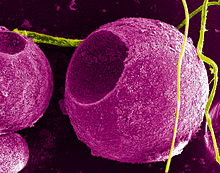
Colloidal
quantum dots irradiated with a UV light. Different sized quantum dots
emit different color light due to quantum confinement.
Quantum dots (QD) are very small semiconductor particles, only several nanometres
in size, so small that their optical and electronic properties differ
from those of larger LED particles. They are a central theme in
nanotechnology. Many types of quantum dot will emit light
of specific frequencies if electricity or light is applied to them, and
these frequencies can be precisely tuned by changing the dots' size, shape and material, giving rise to many applications.
In the language of materials science, nanoscale semiconductor materials tightly confine either electrons or electron holes. Quantum dots are also sometimes referred to as artificial atoms, a term that emphasizes that a quantum dot is a single object with bound, discrete electronic states, as is the case with naturally occurring atoms or molecules.
Quantum dots exhibit properties that are intermediate between
those of bulk semiconductors and those of discrete atoms or molecules.
Their optoelectronic properties change as a function of both size and
shape.
Larger QDs (diameter of 5–6 nm, for example) emit longer wavelengths
resulting in emission colors such as orange or red. Smaller QDs
(diameter of 2–3 nm, for example) emit shorter wavelengths resulting in
colors like blue and green, although the specific colors and sizes vary
depending on the exact composition of the QD.
Because of their highly tunable properties, QDs are of wide interest. Potential applications include transistors, solar cells, LEDs, diode lasers and second-harmonic generation, quantum computing, and medical imaging. Additionally, their small size allows for QDs to be suspended in solution which leads to possible uses in inkjet printing and spin-coating. They have also been used in Langmuir-Blodgett thin-films. These processing techniques result in less expensive and less time-consuming methods of semiconductor fabrication.
Production
Quantum Dots with gradually stepping emission from violet to deep red
There are several ways to prepare quantum dots, the principal ones involving colloids.
Colloidal synthesis
Colloidal semiconductor nanocrystals are synthesized from solutions, much like traditional chemical processes. The main difference is the product neither precipitates as a bulk solid nor remains dissolved. Heating the solution at high temperature, the precursors
decompose forming monomers which then nucleate and generate
nanocrystals. Temperature is a critical factor in determining optimal
conditions for the nanocrystal growth. It must be high enough to allow
for rearrangement and annealing of atoms during the synthesis process while being low enough to promote crystal growth. The concentration of monomers
is another critical factor that has to be stringently controlled during
nanocrystal growth. The growth process of nanocrystals can occur in two
different regimes, "focusing" and "defocusing". At high monomer
concentrations, the critical size (the size where nanocrystals neither
grow nor shrink) is relatively small, resulting in growth of nearly all
particles. In this regime, smaller particles grow faster than large ones
(since larger crystals need more atoms to grow than small crystals)
resulting in "focusing" of the size distribution to yield nearly
monodisperse particles. The size focusing is optimal when the monomer
concentration is kept such that the average nanocrystal size present is
always slightly larger than the critical size. Over time, the monomer
concentration diminishes, the critical size becomes larger than the
average size present, and the distribution "defocuses".
Cadmium sulfide quantum dots on cells
There are colloidal methods to produce many different semiconductors. Typical dots are made of binary compounds such as lead sulfide, lead selenide, cadmium selenide, cadmium sulfide, cadmium telluride, indium arsenide, and indium phosphide.
Dots may also be made from ternary compounds such as cadmium selenide
sulfide.
These quantum dots can contain as few as 100 to 100,000 atoms within the
quantum dot volume, with a diameter of ≈10 to 50 atoms. This
corresponds to about 2 to 10 nanometers,
and at 10 nm in diameter, nearly 3 million quantum dots could be lined
up end to end and fit within the width of a human thumb.
Ideallized
image of colloidal nanoparticle of lead sulfide (selenide) with
complete passivation by oleic acid, oleyl amine and hydroxyl ligands
(size ≈5nm)
Large batches of quantum dots may be synthesized via colloidal synthesis. Due to this scalability and the convenience of benchtop conditions, colloidal synthetic methods are promising for commercial applications. It is acknowledged[citation needed] to be the least toxic of all the different forms of synthesis.
Plasma synthesis
Plasma
synthesis has evolved to be one of the most popular gas-phase
approaches for the production of quantum dots, especially those with
covalent bonds.
For example, silicon (Si) and germanium (Ge) quantum dots have been
synthesized by using nonthermal plasma. The size, shape, surface and
composition of quantum dots can all be controlled in nonthermal plasma. Doping that seems quite challenging for quantum dots has also been realized in plasma synthesis.
Quantum dots synthesized by plasma are usually in the form of powder,
for which surface modification may be carried out. This can lead to
excellent dispersion of quantum dots in either organic solvents or water (i. e., colloidal quantum dots).
Fabrication
- Self-assembled quantum dots are typically between 5 and 50 nm in size. Quantum dots defined by lithographically patterned gate electrodes, or by etching on two-dimensional electron gasses in semiconductor heterostructures can have lateral dimensions between 20 and 100 nm.
- Some quantum dots are small regions of one material buried in another with a larger band gap. These can be so-called core–shell structures, e.g., with CdSe in the core and ZnS in the shell, or from special forms of silica called ormosil. Sub-monolayer shells can also be effective ways of passivating the quantum dots, such as PbS cores with sub-monolayer CdS shells.
- Quantum dots sometimes occur spontaneously in quantum well structures due to monolayer fluctuations in the well's thickness.
- Self-assembled quantum dots nucleate spontaneously under certain conditions during molecular beam epitaxy (MBE) and metallorganic vapor phase epitaxy (MOVPE), when a material is grown on a substrate to which it is not lattice matched. The resulting strain produces coherently strained islands on top of a two-dimensional wetting layer. This growth mode is known as Stranski–Krastanov growth. The islands can be subsequently buried to form the quantum dot. This fabrication method has potential for applications in quantum cryptography (i.e. single photon sources) and quantum computation. The main limitations of this method are the cost of fabrication and the lack of control over positioning of individual dots.
- Individual quantum dots can be created from two-dimensional electron or hole gases present in remotely doped quantum wells or semiconductor heterostructures called lateral quantum dots. The sample surface is coated with a thin layer of resist. A lateral pattern is then defined in the resist by electron beam lithography. This pattern can then be transferred to the electron or hole gas by etching, or by depositing metal electrodes (lift-off process) that allow the application of external voltages between the electron gas and the electrodes. Such quantum dots are mainly of interest for experiments and applications involving electron or hole transport, i.e., an electrical current.
- The energy spectrum of a quantum dot can be engineered by controlling the geometrical size, shape, and the strength of the confinement potential. Also, in contrast to atoms, it is relatively easy to connect quantum dots by tunnel barriers to conducting leads, which allows the application of the techniques of tunneling spectroscopy for their investigation.
The quantum dot absorption features correspond to transitions between discrete, three-dimensional particle in a box states of the electron and the hole, both confined to the same nanometer-size box.These discrete transitions are reminiscent of atomic spectra and have resulted in quantum dots also being called artificial atoms.
- Confinement in quantum dots can also arise from electrostatic potentials (generated by external electrodes, doping, strain, or impurities).
- Complementary metal-oxide-semiconductor (CMOS) technology can be employed to fabricate silicon quantum dots. Ultra small (L=20 nm, W=20 nm) CMOS transistors behave as single electron quantum dots when operated at cryogenic temperature over a range of −269 °C (4 K) to about −258 °C (15 K). The transistor displays Coulomb blockade due to progressive charging of electrons one by one. The number of electrons confined in the channel is driven by the gate voltage, starting from an occupation of zero electrons, and it can be set to 1 or many.
Viral assembly
Genetically engineered M13 bacteriophage viruses allow preparation of quantum dot biocomposite structures. It had previously been shown that genetically engineered viruses can recognize specific semiconductor surfaces through the method of selection by combinatorial phage display. Additionally, it is known that liquid crystalline structures of wild-type viruses (Fd, M13, and TMV) are adjustable by controlling the solution concentrations, solution ionic strength, and the external magnetic field applied to the solutions. Consequently, the specific recognition properties of the virus can be used to organize inorganic nanocrystals,
forming ordered arrays over the length scale defined by liquid crystal
formation. Using this information, Lee et al. (2000) were able to create
self-assembled, highly oriented, self-supporting films from a phage and
ZnS
precursor solution. This system allowed them to vary both the length of
bacteriophage and the type of inorganic material through genetic
modification and selection.
Electrochemical assembly
Highly ordered arrays of quantum dots may also be self-assembled by electrochemical
techniques. A template is created by causing an ionic reaction at an
electrolyte-metal interface which results in the spontaneous assembly of
nanostructures, including quantum dots, onto the metal which is then
used as a mask for mesa-etching these nanostructures on a chosen
substrate.
Bulk-manufacture
Quantum
dot manufacturing relies on a process called "high temperature dual
injection" which has been scaled by multiple companies for commercial
applications that require large quantities (hundreds of kilograms to
tonnes) of quantum dots. This reproducible production method can be
applied to a wide range of quantum dot sizes and compositions.
The bonding in certain cadmium-free quantum dots, such as
III-V-based quantum dots, is more covalent than that in II-VI materials,
therefore it is more difficult to separate nanoparticle nucleation and
growth via a high temperature dual injection synthesis. An alternative
method of quantum dot synthesis, the “molecular seeding” process,
provides a reproducible route to the production of high quality quantum
dots in large volumes. The process utilises identical molecules of a
molecular cluster compound as the nucleation sites for nanoparticle
growth, thus avoiding the need for a high temperature injection step.
Particle growth is maintained by the periodic addition of precursors at
moderate temperatures until the desired particle size is reached.
The molecular seeding process is not limited to the production of
cadmium-free quantum dots; for example, the process can be used to
synthesise kilogram batches of high quality II-VI quantum dots in just a
few hours.
Another approach for the mass production of colloidal quantum
dots can be seen in the transfer of the well-known hot-injection
methodology for the synthesis to a technical continuous flow system. The
batch-to-batch variations arising from the needs during the mentioned
methodology can be overcome by utilizing technical components for mixing
and growth as well as transport and temperature adjustments. For the
production of CdSe based semiconductor nanoparticles this method has
been investigated and tuned to production amounts of kg per month. Since
the use of technical components allows for easy interchange in regards
of maximum through-put and size, it can be further enhanced to tens or
even hundreds of kilograms.
In 2011 a consortium of U.S. and Dutch companies reported a
"milestone" in high volume quantum dot manufacturing by applying the
traditional high temperature dual injection method to a flow system.
On January 23, 2013 Dow entered into an exclusive licensing agreement with UK-based Nanoco
for the use of their low-temperature molecular seeding method for bulk
manufacture of cadmium-free quantum dots for electronic displays, and on
September 24, 2014 Dow commenced work on the production facility in
South Korea capable of producing sufficient quantum dots for "millions
of cadmium-free televisions and other devices, such as tablets". Mass
production is due to commence in mid-2015.
On 24 March 2015 Dow announced a partnership deal with LG Electronics
to develop the use of cadmium free quantum dots in displays.
Heavy-metal-free quantum dots
In many regions of the world there is now a restriction or ban on the use of heavy metals in many household goods, which means that most cadmium-based quantum dots are unusable for consumer-goods applications.
For commercial viability, a range of restricted, heavy-metal-free
quantum dots has been developed showing bright emissions in the visible
and near infra-red region of the spectrum and have similar optical
properties to those of CdSe quantum dots. Among these systems are
InP/ZnS and CuInS/ZnS, for example.
Peptides are being researched as potential quantum dot material.
Since peptides occur naturally in all organisms, such dots would likely be nontoxic and easily biodegraded.
Health and safety
Some quantum dots pose risks to human health and the environment under certain conditions. Notably, the studies on quantum dot toxicity are focused on cadmium containing particles and has yet to be demonstrated in animal models after physiologically relevant dosing. In vitro
studies, based on cell cultures, on quantum dots (QD) toxicity suggests
that their toxicity may derive from multiple factors including its physicochemical
characteristics (size, shape, composition, surface functional groups,
and surface charges) and environment. Assessing their potential toxicity
is complex as these factors include properties such as QD size, charge,
concentration, chemical composition, capping ligands, and also on their
oxidative, mechanical and photolytic stability.
Many studies have focused on the mechanism of QD cytotoxicity using model cell cultures. It has been demonstrated that after exposure to ultraviolet radiation or oxidation by air, CdSe QDs release free cadmium ions causing cell death. Group II-VI QDs also have been reported to induce the formation of reactive oxygen species after exposure to light, which in turn can damage cellular components such as proteins, lipids and DNA.
Some studies have also demonstrated that addition of a ZnS shell
inhibit the process of reactive oxygen species in CdSe QDs. Another
aspect of QD toxicity is the process of their size dependent
intracellular pathways that concentrate these particles in cellular
organelles that are inaccessible by metal ions, which may result in
unique patterns of cytotoxicity compared to their constituent metal
ions. The reports of QD localization in the cell nucleus
present additional modes of toxicity because they may induce DNA
mutation, which in turn will propagate through future generation of
cells causing diseases.
Although concentration of QDs in certain organelles have been reported in in vivo
studies using animal models, no alterations in animal behavior, weight,
hematological markers or organ damage has been found through either
histological or biochemical analysis.
These finding have led scientists to believe that intracellular dose is
the most important deterring factor for QD toxicity. Therefore, factors
determining the QD endocytosis that determine the effective
intracellular concentration, such as QD size, shape and surface
chemistry determine their toxicity. Excretion of QDs through urine in
animal models also have demonstrated via injecting radio-labeled ZnS
capped CdSe QDs where the ligand shell was labelled with 99mTc. Though multiple other studies have concluded retention of QDs in cellular levels, exocytosis of QDs is still poorly studied in the literature.
While significant research efforts have broadened the
understanding of toxicity of QDs, there are large discrepancies in the
literature and questions still remains to be answered. Diversity of this
class material as compared to normal chemical substances makes the
assessment of their toxicity very challenging. As their toxicity may
also be dynamic depending on the environmental factors such as pH level,
light exposure and cell type, traditional methods of assessing toxicity of chemicals such as LD50
are not applicable for QDs. Therefore, researchers are focusing on
introducing novel approaches and adapting existing methods to include
this unique class of materials.
Furthermore, novel strategies to engineer safer QDs are still under
exploration by the scientific community. A recent novelty in the field
is the discovery of carbon quantum dots,
a new generation of optically-active nanoparticles potentially capable
of replacing semiconductor QDs, but with the advantage of much lower
toxicity.
Optical properties
Fluorescence
spectra of CdTe quantum dots of various sizes. Different sized quantum
dots emit different color light due to quantum confinement.
In semiconductors, light absorption generally leads to an electron
being excited from the valence to the conduction band, leaving behind a hole.
The electron and the hole can bind to each other to form an exciton.
When this exciton recombines (i.e. the electron resumes its ground
state), the exciton's energy can be emitted as light. This is called fluorescence.
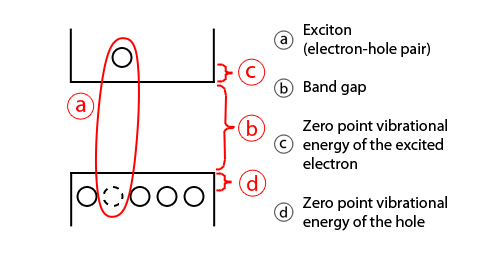
In a simplified model, the energy of the emitted photon can be
understood as the sum of the band gap energy between the highest
occupied level and the lowest unoccupied energy level, the confinement
energies of the hole and the excited electron, and the bound energy of
the exciton (the electron-hole pair):
As the confinement energy depends on the quantum dot's size, both absorption
onset and fluorescence emission can be tuned by changing the size of
the quantum dot during its synthesis. The larger the dot, the redder (lower energy) its absorption onset and fluorescence spectrum. Conversely, smaller dots absorb and emit bluer (higher energy) light. Recent articles in Nanotechnology
and in other journals have begun to suggest that the shape of the
quantum dot may be a factor in the coloration as well, but as yet not
enough information is available. Furthermore, it was shown that the lifetime of fluorescence is determined by the size of the
quantum dot. Larger dots have more closely spaced energy levels in which
the electron-hole pair can be trapped. Therefore, electron-hole pairs
in larger dots live longer causing larger dots to show a longer
lifetime.
To improve fluorescence quantum yield,
quantum dots can be made with "shells" of a larger bandgap
semiconductor material around them. The improvement is suggested to be
due to the reduced access of electron and hole to non-radiative surface
recombination pathways in some cases, but also due to reduced Auger recombination in others.
Potential applications
Quantum dots are particularly promising for optical applications due to their high extinction coefficient. They operate like a single electron transistor and show the Coulomb blockade effect. Quantum dots have also been suggested as implementations of qubits for quantum information processing.
Tuning the size of quantum dots is attractive for many potential
applications. For instance, larger quantum dots have a greater
spectrum-shift towards red compared to smaller dots, and exhibit less
pronounced quantum properties. Conversely, the smaller particles allow
one to take advantage of more subtle quantum effects.
A device that produces visible light, through energy transfer from thin layers of quantum wells to crystals above the layers.
Being zero-dimensional, quantum dots have a sharper density of states
than higher-dimensional structures. As a result, they have superior
transport and optical properties. They have potential uses in diode lasers,
amplifiers, and biological sensors. Quantum dots may be excited within a
locally enhanced electromagnetic field produced by gold nanoparticles,
which can then be observed from the surface plasmon resonance
in the photoluminescent excitation spectrum of (CdSe)ZnS nanocrystals.
High-quality quantum dots are well suited for optical encoding and
multiplexing applications due to their broad excitation profiles and
narrow/symmetric emission spectra. The new generations of quantum dots
have far-reaching potential for the study of intracellular processes at
the single-molecule level, high-resolution cellular imaging, long-term
in vivo observation of cell trafficking, tumor targeting, and
diagnostics.
CdSe nanocrystals are efficient triplet photosensitizers.
Laser excitation of small CdSe nanoparticles enables the extraction of
the excited state energy from the Quantum Dots into bulk solution, thus
opening the door to a wide range of potential applications such as
photodynamic therapy, photovoltaic devices, molecular electronics, and
catalysis.
Biology
In modern biological analysis, various kinds of organic dyes are used. However, as technology advances, greater flexibility in these dyes is sought.
To this end, quantum dots have quickly filled in the role, being found
to be superior to traditional organic dyes on several counts, one of the
most immediately obvious being brightness (owing to the high extinction
coefficient combined with a comparable quantum yield to fluorescent
dyes) as well as their stability (allowing much less photobleaching). It has been estimated that quantum dots are 20 times brighter and 100 times more stable than traditional fluorescent reporters. For single-particle tracking, the irregular blinking of quantum dots
is a minor drawback. However, there have been groups which have
developed quantum dots which are essentially nonblinking and
demonstrated their utility in single molecule tracking experiments.
The use of quantum dots for highly sensitive cellular imaging has seen major advances.
The improved photostability of quantum dots, for example, allows the
acquisition of many consecutive focal-plane images that can be
reconstructed into a high-resolution three-dimensional image.
Another application that takes advantage of the extraordinary
photostability of quantum dot probes is the real-time tracking of
molecules and cells over extended periods of time. Antibodies, streptavidin, peptides, DNA, nucleic acid aptamers, or small-molecule ligands
can be used to target quantum dots to specific proteins on cells.
Researchers were able to observe quantum dots in lymph nodes of mice for
more than 4 months.
Quantum dots can have antibacterial properties similar to nanoparticles and can kill bacteria in a dose-dependent manner.
One mechanism by which quantum dots can kill bacteria is through
impairing the functions of antioxidative system in the cells and down
regulating the antioxidative genes. In addition, quantum dots can
directly damage the cell wall. Quantum dots have been shown to be
effective against both gram- positive and gram-negative bacteria.
Semiconductor quantum dots have also been employed for in vitro
imaging of pre-labeled cells. The ability to image single-cell
migration in real time is expected to be important to several research
areas such as embryogenesis, cancer metastasis, stem cell therapeutics, and lymphocyte immunology.
One application of quantum dots in biology is as donor fluorophores in Förster resonance energy transfer, where the large extinction coefficient and spectral purity of these fluorophores make them superior to molecular fluorophores
It is also worth noting that the broad absorbance of QDs allows
selective excitation of the QD donor and a minimum excitation of a dye
acceptor in FRET-based studies.
The applicability of the FRET model, which assumes that the Quantum Dot
can be approximated as a point dipole, has recently been demonstrated.
The use of quantum dots for tumor targeting under in vivo
conditions employ two targeting schemes: active targeting and passive
targeting. In the case of active targeting, quantum dots are
functionalized with tumor-specific binding sites to selectively bind to
tumor cells. Passive targeting uses the enhanced permeation and
retention of tumor cells for the delivery of quantum dot probes.
Fast-growing tumor cells typically have more permeable membranes than
healthy cells, allowing the leakage of small nanoparticles into the cell
body. Moreover, tumor cells lack an effective lymphatic drainage
system, which leads to subsequent nanoparticle-accumulation.
Quantum dot probes exhibit in vivo toxicity. For example, CdSe
nanocrystals are highly toxic to cultured cells under UV illumination,
because the particles dissolve, in a process known as photolysis,
to release toxic cadmium ions into the culture medium. In the absence
of UV irradiation, however, quantum dots with a stable polymer coating
have been found to be essentially nontoxic. Hydrogel encapsulation of quantum dots
allows for quantum dots to be introduced into a stable aqueous
solution, reducing the possibility of cadmium leakage.Then again, only
little is known about the excretion process of quantum dots from living
organisms.
In another potential application, quantum dots are being investigated as the inorganic fluorophore for intra-operative detection of tumors using fluorescence spectroscopy.
Delivery of undamaged quantum dots to the cell cytoplasm has been
a challenge with existing techniques. Vector-based methods have
resulted in aggregation and endosomal sequestration of quantum dots
while electroporation can damage the semi-conducting particles and
aggregate delivered dots in the cytosol. Via cell squeezing,
quantum dots can be efficiently delivered without inducing aggregation,
trapping material in endosomes, or significant loss of cell viability.
Moreover, it has shown that individual quantum dots delivered by this
approach are detectable in the cell cytosol, thus illustrating the
potential of this technique for single molecule tracking studies.
Photovoltaic devices
The tunable absorption spectrum and high extinction coefficients of
quantum dots make them attractive for light harvesting technologies such
as photovoltaics. Quantum dots may be able to increase the efficiency
and reduce the cost of today's typical silicon photovoltaic cells. According to an experimental proof from 2004, quantum dots of lead selenide can produce more than one exciton from one high energy photon via the process of carrier multiplication or multiple exciton generation
(MEG). This compares favorably to today's photovoltaic cells which can
only manage one exciton per high-energy photon, with high kinetic energy
carriers losing their energy as heat. Quantum dot photovoltaics would
theoretically be cheaper to manufacture, as they can be made "using
simple chemical reactions."
Quantum dot only solar cells
Aromatic self-assembled monolayers
(SAMs) (e.g. 4-nitrobenzoic acid) can be used to improve the band
alignment at electrodes for better efficiencies. This technique has
provided a record power conversion efficiency (PCE) of 10.7%.
The SAM is positioned between ZnO-PbS colloidal quantum dot (CQD) film
junction to modify band alignment via the dipole moment of the
constituent SAM molecule, and the band tuning may be modified via the
density, dipole and the orientation of the SAM molecule.
Quantum dot in hybrid solar cells
Colloidal quantum dots are also used in inorganic/organic hybrid solar cells. These solar cells are attractive because of the potential for low-cost fabrication and relatively high efficiency.
Incorporation of metal oxides, such as ZnO, TiO2, and Nb2O5
nanomaterials into organic photovoltaics have been commercialized using
full roll-to-roll processing. A 13.2% power conversion efficiency is claimed in Si nanowire/PEDOT:PSS hybrid solar cells.
Quantum dot with nanowire in solar cells
Another
potential use involves capped single-crystal ZnO nanowires with CdSe
quantum dots, immersed in mercaptopropionic acid as hole transport
medium in order to obtain a QD-sensitized solar cell. The morphology of
the nanowires allowed the electrons to have a direct pathway to the photoanode. This form of solar cell exhibits 50–60% internal quantum efficiencies.
Nanowires with quantum dot coatings on silicon nanowires (SiNW)
and carbon quantum dots. The use of SiNWs instead of planar silicon
enhances the antiflection properties of Si.
The SiNW exhibits a light-trapping effect due to light trapping in the
SiNW. This use of SiNWs in conjunction with carbon quantum dots resulted
in a solar cell that reached 9.10% PCE.
Graphene
quantum dots have also been blended with organic electronic materials
to improve efficiency and lower cost in photovoltaic devices and organic
light emitting diodes (OLEDs)
in compared to graphene sheets. These graphene quantum dots were
functionalized with organic ligands that experience photoluminescence
from UV-Vis absorption.
Light emitting diodes
Several methods are proposed for using quantum dots to improve existing light-emitting diode
(LED) design, including "Quantum Dot Light Emitting Diode" (QD-LED or
QLED) displays and "Quantum Dot White Light Emitting Diode" (QD-WLED)
displays. Because Quantum dots naturally produce monochromatic
light, they can be more efficient than light sources which must be
color filtered. QD-LEDs can be fabricated on a silicon substrate, which
allows them to be integrated onto standard silicon-based integrated circuits or microelectromechanical systems.
Quantum dot displays
Quantum dots are valued for displays because they emit light in very specific gaussian distributions. This can result in a display with visibly more accurate colors.
A conventional color liquid crystal display (LCD) is usually backlit by fluorescent lamps (CCFLs) or conventional white LEDs
that are color filtered to produce red, green, and blue pixels. Quantum
dot displays use blue-emitting LEDs rather than white LEDs as the light
sources. The converting part of the emitted light is converted into
pure green and red light by the corresponding color quantum dots placed
in front of the blue LED or using a quantum dot infused diffuser sheet
in the backlight optical stack. Blank pixels are also used to allow the
blue LED light to still generate blue hues. This type of white light as
the backlight of an LCD panel allows for the best color gamut at lower
cost than an RGB LED combination using three LEDs.
Another method by which quantum dot displays can be achieved is
the electroluminescent (EL) or electro-emissive method. This involves
embedding quantum dots in each individual pixel. These are then
activated and controlled via an electric current application. Since this is often light emitting itself, the achievable colors may be limited in this method. Electro-emissive QD-LED TVs exist in laboratories only.
The ability of QDs to precisely convert and tune a spectrum makes them attractive for LCD
displays. Previous LCD displays can waste energy converting red-green
poor, blue-yellow rich white light into a more balanced lighting. By
using QDs, only the necessary colors for ideal images are contained in
the screen. The result is a screen that is brighter, clearer, and more
energy-efficient. The first commercial application of quantum dots was
the Sony XBR X900A series of flat panel televisions released in 2013.
In June 2006, QD Vision announced technical success in making a proof-of-concept quantum dot display
and show a bright emission in the visible and near infra-red region of
the spectrum. A QD-LED integrated at a scanning microscopy tip was used
to demonstrate fluorescence near-field scanning optical microscopy (NSOM) imaging.
Photodetector devices
Quantum dot photodetectors (QDPs) can be fabricated either via solution-processing, or from conventional single-crystalline semiconductors.
Conventional single-crystalline semiconductor QDPs are precluded from
integration with flexible organic electronics due to the incompatibility
of their growth conditions with the process windows required by organic
semiconductors. On the other hand, solution-processed QDPs can be
readily integrated with an almost infinite variety of substrates, and
also postprocessed atop other integrated circuits. Such colloidal QDPs have potential applications in surveillance, machine vision, industrial inspection, spectroscopy, and fluorescent biomedical imaging.
Photocatalysts
Quantum dots also function as photocatalysts for the light driven chemical conversion of water into hydrogen as a pathway to solar fuel. In photocatalysis, electron hole pairs formed in the dot under band gap excitation drive redox reactions
in the surrounding liquid. Generally, the photocatalytic activity of
the dots is related to the particle size and its degree of quantum confinement. This is because the band gap determines the chemical energy that is stored in the dot in the excited state. An obstacle for the use of quantum dots in photocatalysis is the presence of surfactants on the surface of the dots. These surfactants (or ligands) interfere with the chemical reactivity of the dots by slowing down mass transfer and electron transfer processes. Also, quantum dots made of metal chalcogenides are chemically unstable under oxidizing conditions and undergo photo corrosion reactions.
Theory
Quantum
dots are theoretically described as a point like, or a zero dimensional
(0D) entity. Most of their properties depend on the dimensions, shape
and materials of which QDs are made. Generally QDs present different thermodynamic properties from the bulk materials of which they are made. One of these effects is the Melting-point depression. Optical properties of spherical metallic QDs are well described by the Mie scattering theory.
Quantum confinement in semiconductors
3D
confined electron wave functions in a quantum dot. Here, rectangular
and triangular-shaped quantum dots are shown. Energy states in
rectangular dots are more s-type and p-type. However, in a triangular dot the wave functions are mixed due to confinement symmetry. (Click for animation)
In a semiconductor crystallite whose size is smaller than twice the size of its exciton Bohr radius, the excitons are squeezed, leading to quantum confinement. The energy levels can then be predicted using the particle in a box
model in which the energies of states depend on the length of the box.
Comparing the quantum dots size to the Bohr radius of the electron and
hole wave functions, 3 regimes can be defined. A 'strong confinement
regime' is defined as the quantum dots radius being smaller than both
electron and hole Bohr radius, 'weak confinement' is given when the
quantum dot is larger than both. For semiconductors in which electron
and hole radii are markedly different, an 'intermediate confinement
regime' exists, where the quantum dot's radius is larger than the Bohr
radius of one charge carrier (typically the hole), but not the other
charge carrier.
Splitting
of energy levels for small quantum dots due to the quantum confinement
effect. The horizontal axis is the radius, or the size, of the quantum
dots and ab* is the Exciton Bohr radius.
- Band gap energy
- The band gap can become smaller in the strong confinement regime as
the energy levels split up. The Exciton Bohr radius can be expressed as:
- where ab is the Bohr radius=0.053 nm, m is the mass, μ is the reduced mass, and εr is the size-dependent dielectric constant (Relative permittivity). This results in the increase in the total emission energy (the sum of the energy levels in the smaller band gaps in the strong confinement regime is larger than the energy levels in the band gaps of the original levels in the weak confinement regime) and the emission at various wavelengths. If the size distribution of QDs is not enough peaked, the convolution of multiple emission wavelengths is observed as a continuous spectra.
- Confinement energy
- The exciton entity can be modeled using the particle in the box. The electron and the hole can be seen as hydrogen in the Bohr model with the hydrogen nucleus replaced by the hole of positive charge and negative electron mass. Then the energy levels of the exciton can be represented as the solution to the particle in a box at the ground level (n = 1) with the mass replaced by the reduced mass. Thus by varying the size of the quantum dot, the confinement energy of the exciton can be controlled.
- Bound exciton energy
- There is Coulomb attraction between the negatively charged electron and the positively charged hole. The negative energy involved in the attraction is proportional to Rydberg's energy and inversely proportional to square of the size-dependent dielectric constant of the semiconductor. When the size of the semiconductor crystal is smaller than the Exciton Bohr radius, the Coulomb interaction must be modified to fit the situation.
Therefore, the sum of these energies can be represented as:
where μ is the reduced mass, a is the radius of the quantum dot, me is the free electron mass, mh is the hole mass, and εr is the size-dependent dielectric constant.
Although the above equations were derived using simplifying
assumptions, they imply that the electronic transitions of the quantum
dots will depend on their size. These quantum confinement effects are
apparent only below the critical size. Larger particles do not exhibit
this effect. This effect of quantum confinement on the quantum dots has
been repeatedly verified experimentally and is a key feature of many emerging electronic structures.
The Coulomb
interaction between confined carriers can also be studied by numerical
means when results unconstrained by asymptotic approximations are
pursued.
Besides confinement in all three dimensions (i.e., a quantum dot), other quantum confined semiconductors include:
- Quantum wires, which confine electrons or holes in two spatial dimensions and allow free propagation in the third.
- Quantum wells, which confine electrons or holes in one dimension and allow free propagation in two dimensions.
Models
A variety
of theoretical frameworks exist to model optical, electronic, and
structural properties of quantum dots. These may be broadly divided into
quantum mechanical, semiclassical, and classical.
Quantum mechanics
Quantum mechanical models and simulations of quantum dots often involve the interaction of electrons with a pseudopotential or random matrix.
Semiclassical
Semiclassical models of quantum dots frequently incorporate a chemical potential. For example, the thermodynamic chemical potential of an N-particle system is given by
whose energy terms may be obtained as solutions of the Schrödinger equation. The definition of capacitance,
- ,
with the potential difference
may be applied to a quantum dot with the addition or removal of individual electrons,
- and .
Then
is the "quantum capacitance" of a quantum dot, where we denoted by I(N) the ionization potential and by A(N) the electron affinity of the N-particle system.
Classical mechanics
Classical models of electrostatic properties of electrons in quantum dots are similar in nature to the Thomson problem of optimally distributing electrons on a unit sphere.
The classical electrostatic treatment of electrons confined to
spherical quantum dots is similar to their treatment in the Thomson, or plum pudding model, of the atom.
The classical treatment of both two-dimensional and three-dimensional quantum dots exhibit electron shell-filling behavior. A "periodic table of classical artificial atoms" has been described for two-dimensional quantum dots.
As well, several connections have been reported between the
three-dimensional Thomson problem and electron shell-filling patterns
found in naturally-occurring atoms found throughout the periodic table.
This latter work originated in classical electrostatic modeling of
electrons in a spherical quantum dot represented by an ideal dielectric
sphere.
History
The term “quantum dot” was coined in 1986. They were first discovered in a glass matrix and in colloidal solutions by Alexey Ekimov and Louis Brus.