| Radon | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ˈreɪdɒn/ | |||||||||||||||||||||||||||
| Appearance | colorless gas | |||||||||||||||||||||||||||
| Mass number | 222 (most stable isotope) | |||||||||||||||||||||||||||
| Radon in the periodic table | ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
| Atomic number (Z) | 86 | |||||||||||||||||||||||||||
| Group | group 18 (noble gases) | |||||||||||||||||||||||||||
| Period | period 6 | |||||||||||||||||||||||||||
| Block | p-block | |||||||||||||||||||||||||||
| Element category | noble gas | |||||||||||||||||||||||||||
| Electron configuration | [Xe] 4f14 5d10 6s2 6p6 | |||||||||||||||||||||||||||
Electrons per shell
| 2, 8, 18, 32, 18, 8 | |||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||
| Phase at STP | gas | |||||||||||||||||||||||||||
| Melting point | 202 K (−71 °C, −96 °F) | |||||||||||||||||||||||||||
| Boiling point | 211.5 K (−61.7 °C, −79.1 °F) | |||||||||||||||||||||||||||
| Density (at STP) | 9.73 g/L | |||||||||||||||||||||||||||
| when liquid (at b.p.) | 4.4 g/cm3 | |||||||||||||||||||||||||||
| Critical point | 377 K, 6.28 MPa | |||||||||||||||||||||||||||
| Heat of fusion | 3.247 kJ/mol | |||||||||||||||||||||||||||
| Heat of vaporization | 18.10 kJ/mol | |||||||||||||||||||||||||||
| Molar heat capacity | 5R/2 = 20.786 J/(mol·K) | |||||||||||||||||||||||||||
Vapor pressure
| ||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||
| Oxidation states | 0, +2, +6 | |||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 2.2 | |||||||||||||||||||||||||||
| Ionization energies |
| |||||||||||||||||||||||||||
| Covalent radius | 150 pm | |||||||||||||||||||||||||||
| Van der Waals radius | 220 pm | |||||||||||||||||||||||||||
| Spectral lines of radon | ||||||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||||
| Natural occurrence | from decay | |||||||||||||||||||||||||||
| Crystal structure | face-centered cubic (fcc) | |||||||||||||||||||||||||||
| Thermal conductivity | 3.61×10−3 W/(m·K) | |||||||||||||||||||||||||||
| Magnetic ordering | non-magnetic | |||||||||||||||||||||||||||
| CAS Number | 10043-92-2 | |||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||
| Discovery | Ernest Rutherford and Robert B. Owens (1899) | |||||||||||||||||||||||||||
| First isolation | William Ramsay and Robert Whytlaw-Gray (1910) | |||||||||||||||||||||||||||
| Main isotopes of radon | ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
Radon is a chemical element with the symbol Rn and atomic number 86. It is a radioactive, colorless, odorless, tasteless noble gas. It occurs naturally in minute quantities as an intermediate step in the normal radioactive decay chains through which thorium and uranium slowly decay into lead and various other short-lived radioactive elements; radon itself is the immediate decay product of radium. Its most stable isotope, 222Rn, has a half-life of only 3.8 days, making radon one of the rarest elements since it decays away so quickly. However, since thorium and uranium are two of the most common radioactive elements on Earth, and they have three isotopes with very long half-lives, on the order of several billions of years, radon will be present on Earth long into the future in spite of its short half-life as it is continually being generated. The decay of radon produces many other short-lived nuclides known as radon daughters, ending at stable isotopes of lead.
Unlike all the other intermediate elements in the aforementioned decay chains, radon is, under normal conditions, gaseous and easily inhaled. Radon gas is considered a health hazard. It is often the single largest contributor to an individual's background radiation dose, but due to local differences in geology, the level of the radon-gas hazard differs from location to location. Despite its short lifetime, radon gas from natural sources, such as uranium-containing minerals, can accumulate in buildings, especially, due to its high density, in low areas such as basements and crawl spaces. Radon can also occur in ground water – for example, in some spring waters and hot springs.
Epidemiological studies have shown a clear link between breathing high concentrations of radon and incidence of lung cancer. Radon is a contaminant that affects indoor air quality worldwide. According to the United States Environmental Protection Agency, radon is the second most frequent cause of lung cancer, after cigarette smoking, causing 21,000 lung cancer deaths per year in the United States. About 2,900 of these deaths occur among people who have never smoked. While radon is the second most frequent cause of lung cancer, it is the number one cause among non-smokers, according to EPA estimates. As radon itself decays, it produces decay products, which are other radioactive elements called radon daughters (also known as radon progeny). Unlike the gaseous radon itself, radon daughters are solids and stick to surfaces, such as dust particles in the air. If such contaminated dust is inhaled, these particles can also cause lung cancer.
Characteristics
Emission spectrum of radon, photographed by Ernest Rutherford
in 1908. Numbers at the side of the spectrum are wavelengths. The
middle spectrum is of Radium emanation (radon), while the outer two are
of helium (added to calibrate the wavelengths).
Physical properties
Radon is a colorless, odorless, and tasteless gas and therefore is not detectable by human senses alone. At standard temperature and pressure, radon forms a monatomic gas with a density of 9.73 kg/m3, about 8 times the density of the Earth's atmosphere at sea level, 1.217 kg/m3.
Radon is one of the densest gases at room temperature and is the
densest of the noble gases. Although colorless at standard temperature
and pressure, when cooled below its freezing point of 202 K (−71 °C; −96 °F), radon emits a brilliant radioluminescence that turns from yellow to orange-red as the temperature lowers. Upon condensation, radon glows because of the intense radiation it produces. Radon is sparingly soluble in water, but more soluble than lighter noble gases. Radon is appreciably more soluble in organic liquids than in water.
Chemical properties
Being
a noble gas, radon is chemically not very reactive. However, the
3.8-day half-life of radon-222 makes it useful in physical sciences as a
natural tracer. Because radon is a gas at standard conditions, unlike its parents, it can readily be extracted from them for research.
Radon is a member of the zero-valence elements that are called noble gases. It is inert to most common chemical reactions, such as combustion, because the outer valence shell contains eight electrons. This produces a stable, minimum energy configuration in which the outer electrons are tightly bound. 1037 kJ/mol is required to extract one electron from its shells (also known as the first ionization energy). In accordance with periodic trends, radon has a lower electronegativity than the element one period before it, xenon, and is therefore more reactive. Early studies concluded that the stability of radon hydrate should be of the same order as that of the hydrates of chlorine (Cl
2) or sulfur dioxide (SO
2), and significantly higher than the stability of the hydrate of hydrogen sulfide (H
2S).
2) or sulfur dioxide (SO
2), and significantly higher than the stability of the hydrate of hydrogen sulfide (H
2S).
Because of its cost and radioactivity, experimental chemical
research is seldom performed with radon, and as a result there are very
few reported compounds of radon, all either fluorides or oxides. Radon can be oxidized by powerful oxidizing agents such as fluorine, thus forming radon difluoride.
It decomposes back to its elements at a temperature of above 250 °C,
and is reduced by water to radon gas and hydrogen fluoride: it may also
be reduced back to its elements by hydrogen gas. It has a low volatility and was thought to be RnF
2. Because of the short half-life of radon and the radioactivity of its compounds, it has not been possible to study the compound in any detail. Theoretical studies on this molecule predict that it should have a Rn–F bond distance of 2.08 Å, and that the compound is thermodynamically more stable and less volatile than its lighter counterpart XeF2 . The octahedral molecule RnF
6 was predicted to have an even lower enthalpy of formation than the difluoride. The higher fluorides RnF4 and RnF6 have been claimed, and are calculated to be stable, but it is doubtful whether they have yet been synthesized. The [RnF]+ ion is believed to form by the following reaction:
2. Because of the short half-life of radon and the radioactivity of its compounds, it has not been possible to study the compound in any detail. Theoretical studies on this molecule predict that it should have a Rn–F bond distance of 2.08 Å, and that the compound is thermodynamically more stable and less volatile than its lighter counterpart XeF2 . The octahedral molecule RnF
6 was predicted to have an even lower enthalpy of formation than the difluoride. The higher fluorides RnF4 and RnF6 have been claimed, and are calculated to be stable, but it is doubtful whether they have yet been synthesized. The [RnF]+ ion is believed to form by the following reaction:
- Rn (g) + 2 [O
2]+[SbF
6]− (s) → [RnF]+[Sb
2F
11]− (s) + 2 O
2 (g)
For this reason, antimony pentafluoride together with chlorine trifluoride and N2F2Sb2F11 have been considered for radon gas removal in uranium mines due to the formation of radon–fluorine compounds. The existence of RnF2 allows for safer handling of radon's parent radium as the fluoride, as the alpha radiation from 226Ra is not strong enough to cause radiolysis of the strong Ra–F bond; thus 226RaF2 decays to form involatile 222RnF2. Additionally, salts of the [RnF]+ cation with the anions SbF−
6, TaF−
6, and BiF−
6 are known. Radon is also oxidised by dioxygen difluoride to RnF2 at −100 °C.
6, TaF−
6, and BiF−
6 are known. Radon is also oxidised by dioxygen difluoride to RnF2 at −100 °C.
Radon oxides are among the few other reported compounds of radon; only the trioxide (RnO3) has been confirmed. Higher fluorides may have been observed in experiments where unknown radon-containing products distilled together with xenon hexafluoride, and perhaps in the production of radon trioxide: these may have been RnF4, RnF6, or both. Extrapolation down the noble gas group would suggest also the possible existence of RnO, RnO2, and RnOF4, as well as the first chemically stable noble gas chlorides RnCl2 and RnCl4, but none of these have yet been found. Radon carbonyl RnCO has been predicted to be stable and to have a linear molecular geometry. The molecules Rn
2 and RnXe were found to be significantly stabilized by spin-orbit coupling. Radon caged inside a fullerene has been proposed as a drug for tumors. Despite the existence of Xe(VIII), no Rn(VIII) compounds have been claimed to exist; RnF8 should be highly unstable chemically (XeF8 is thermodynamically unstable). It is predicted that the most stable Rn(VIII) compound would be barium perradonate (Ba2RnO6), analogous to barium perxenate. The instability of Rn(VIII) is due to the relativistic stabilization of the 6s shell, also known as the inert pair effect.
2 and RnXe were found to be significantly stabilized by spin-orbit coupling. Radon caged inside a fullerene has been proposed as a drug for tumors. Despite the existence of Xe(VIII), no Rn(VIII) compounds have been claimed to exist; RnF8 should be highly unstable chemically (XeF8 is thermodynamically unstable). It is predicted that the most stable Rn(VIII) compound would be barium perradonate (Ba2RnO6), analogous to barium perxenate. The instability of Rn(VIII) is due to the relativistic stabilization of the 6s shell, also known as the inert pair effect.
Radon reacts with the liquid halogen fluorides ClF, ClF3, ClF5, BrF3, BrF5, and IF7 to form RnF2. In halogen fluoride solution, radon is involatile and exists as the RnF+ and Rn2+ cations; addition of fluoride anions results in the formation of the complexes RnF−
3 and RnF2−
4, paralleling the chemistry of beryllium(II) and aluminium(III). The standard electrode potential of the Rn2+/Rn couple has been estimated as +2.0 V, though there is no evidence for the formation of stable radon ions or compounds in aqueous solution.
3 and RnF2−
4, paralleling the chemistry of beryllium(II) and aluminium(III). The standard electrode potential of the Rn2+/Rn couple has been estimated as +2.0 V, though there is no evidence for the formation of stable radon ions or compounds in aqueous solution.
Isotopes
The radium or uranium series.
Radon has no stable isotopes. Thirty-nine radioactive isotopes have been characterized, with atomic masses ranging from 193 to 231. The most stable isotope is 222Rn, which is a decay product of 226Ra, a decay product of 238U. A trace amount of the (highly unstable) isotope 218Rn is also among the daughters of 222Rn.
Three other radon isotopes have a half-life of over an hour: 211Rn, 210Rn and 224Rn. The 220Rn isotope is a natural decay product of the most stable thorium isotope (232Th), and is commonly referred to as thoron. It has a half-life of 55.6 seconds and also emits alpha radiation. Similarly, 219Rn is derived from the most stable isotope of actinium (227Ac)—named "actinon"—and is an alpha emitter with a half-life of 3.96 seconds. No radon isotopes occur significantly in the neptunium (237Np) decay series, though a trace amount of the (extremely unstable) isotope 217Rn is produced.
Daughters
222Rn belongs to the radium and uranium-238 decay chain,
and has a half-life of 3.8235 days. Its four first products (excluding
marginal decay schemes) are very short-lived, meaning that the
corresponding disintegrations are indicative of the initial radon
distribution. Its decay goes through the following sequence:
- 222Rn, 3.82 days, alpha decaying to...
- 218Po, 3.10 minutes, alpha decaying to...
- 214Pb, 26.8 minutes, beta decaying to...
- 214Bi, 19.9 minutes, beta decaying to...
- 214Po, 0.1643 ms, alpha decaying to...
- 210Pb, which has a much longer half-life of 22.3 years, beta decaying to...
- 210Bi, 5.013 days, beta decaying to...
- 210Po, 138.376 days, alpha decaying to...
- 206Pb, stable.
The radon equilibrium factor
is the ratio between the activity of all short-period radon progenies
(which are responsible for most of radon's biological effects), and the
activity that would be at equilibrium with the radon parent.
If a closed volume is constantly supplied with radon, the
concentration of short-lived isotopes will increase until an equilibrium
is reached where the rate of decay of each decay product will equal
that of the radon itself. The equilibrium factor is 1 when both
activities are equal, meaning that the decay products have stayed close
to the radon parent long enough for the equilibrium to be reached,
within a couple of hours. Under these conditions each additional pCi/L
of radon will increase exposure, by 0.01 WL (Working Level
-a measure of radioactivity commonly used in mining. A detailed
explanation of WL is given in Concentration Units). These conditions are
not always met; in many homes, the equilibrium fraction is typically
40%; that is, there will be 0.004 WL of daughters for each pCi/L of
radon in air. 210Pb
takes much longer (decades) to come in equilibrium with radon, but, if
the environment permits accumulation of dust over extended periods of
time, 210Pb and its decay products may contribute to overall radiation levels as well.
Because of their electrostatic charge, radon progenies adhere to
surfaces or dust particles, whereas gaseous radon does not. Attachment
removes them from the air, usually causing the equilibrium factor in the
atmosphere to be less than one. The equilibrium factor is also lowered
by air circulation or air filtration devices, and is increased by
airborne dust particles, including cigarette smoke. In high
concentrations, airborne radon isotopes contribute significantly to
human health risk. The equilibrium factor found in epidemiological
studies is 0.4.
History and etymology
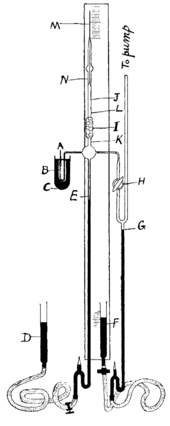
Apparatus used by Ramsay and Whytlaw-Gray to isolate radon. M is a capillary tube where approximately 0.1 mm3 were isolated. Radon mixed with hydrogen entered the evacuated system through siphon A; mercury is shown in black.
Radon was the fifth radioactive element to be discovered, in 1899 by Ernest Rutherford and Robert B. Owens, after uranium, thorium, radium and polonium. In 1900, Friedrich Ernst Dorn
reported some experiments in which he noticed that radium compounds
emanate a radioactive gas he named 'Radium Emanation' ('Ra Em'). Before that, in 1899, Pierre and Marie Curie observed that the gas emitted by radium remained radioactive for a month. Later that year, Robert B. Owens and Ernest Rutherford, at McGill University in Montreal, noticed variations when trying to measure radiation from thorium oxide.
Rutherford noticed that the compounds of thorium continuously emit a
radioactive gas that retains the radioactive powers for several minutes,
and called this gas 'emanation' (from Latin emanare—to elapse and emanatio—expiration), and later Thorium Emanation (Th Em). In 1901, Rutherford and Harriet Brooks demonstrated that the emanations are radioactive, but credited the Curies for the discovery of the element. In 1903, similar emanations were observed from actinium by André-Louis Debierne and were called 'Actinium Emanation' ('Ac Em').
Several shortened names were soon suggested for the three emanations: exradio, exthorio, and exactinio in 1904; radon (Ro), thoron (To), and akton or acton (Ao) in 1918; radeon, thoreon, and actineon in 1919, and eventually radon, thoron, and actinon in 1920. (The name radon is not related to that of the Austrian mathematician Johann Radon.)
The likeness of the spectra of these three gases with those of argon,
krypton, and xenon, and their observed chemical inertia led Sir William Ramsay to suggest in 1904 that the "emanations" might contain a new element of the noble gas family.
In the early part of the 20th century in the US, gold contaminated with the radon daughter 210Pb entered the jewelry industry. This was from gold seeds that had held 222Rn that had been melted down after the radon had decayed.
In 1909, Ramsay and Robert Whytlaw-Gray isolated radon, determined its melting temperature and approximate density. In 1910 they determined that it was the heaviest known gas. and wrote that "L'expression l'émanation du radium est fort incommode",
(the expression 'radium emanation' is very awkward) and suggested the
new name niton (Nt) (from the Latin "nitens" meaning "shining") to
emphasize the radioluminescence property, and in 1912 it was accepted by the International Commission for Atomic Weights. In 1923, the International Committee for Chemical Elements and International Union of Pure and Applied Chemistry
(IUPAC) chose among the names radon (Rn), thoron (Tn), and actinon
(An). Later, when isotopes were numbered instead of named, the element
took the name of the most stable isotope, radon, while Tn was renamed 220Rn and An was renamed 219Rn,
which caused some confusion in the literature regarding the element's
discovery as while Dorn had discovered radon the isotope, he had not
been the first to discover radon the element. As late as the 1960s, the
element was also referred to simply as emanation. The first synthesized compound of radon, radon fluoride, was obtained in 1962. Even today, the word radon may refer to either the element or its isotope 222Rn, with thoron remaining in use as a short name for 220Rn to stem this ambiguity.
The danger of high exposure to radon in mines, where exposures can reach 1,000,000 Bq/m3, has long been known. In 1530, Paracelsus described a wasting disease of miners, the mala metallorum, and Georg Agricola recommended ventilation in mines to avoid this mountain sickness (Bergsucht).
In 1879, this condition was identified as lung cancer by Harting and
Hesse in their investigation of miners from Schneeberg, Germany. The
first major studies with radon and health occurred in the context of uranium mining in the Joachimsthal region of Bohemia. In the US, studies and mitigation only followed decades of health effects on uranium miners of the Southwestern United States employed during the early Cold War; standards were not implemented until 1971.
The presence of radon in indoor air was documented as early as
1950. Beginning in the 1970s research was initiated to address sources
of indoor radon, determinants of concentration, health effects, and
mitigation approaches. In the United States, the problem of indoor radon
received widespread publicity and intensified investigation after a
widely publicized incident in 1984. During routine monitoring at a
Pennsylvania nuclear power plant, a worker was found to be contaminated
with radioactivity. A high concentration of radon in his home was
subsequently identified as responsible.
Occurrence
Concentration units
210Pb is formed from the decay of 222Rn. Here is a typical deposition rate of 210Pb as observed in Japan as a function of time, due to variations in radon concentration.
All discussions of radon concentrations in the environment refer to 222Rn. While the average rate of production of 220Rn (from the thorium decay series) is about the same as that of 222Rn, the amount of 220Rn in the environment is much less than that of 222Rn because of the short half-life of 220Rn (55 seconds, versus 3.8 days respectively).
Radon concentration in the atmosphere is usually measured in becquerel per cubic meter (Bq/m3), the SI derived unit. Another unit of measurement common in the US is picocuries per liter (pCi/L); 1 pCi/L=37 Bq/m3. Typical domestic exposures average about 48 Bq/m3 indoors, though this varies widely, and 15 Bq/m3 outdoors.
In the mining industry, the exposure is traditionally measured in working level (WL), and the cumulative exposure in working level month (WLM); 1 WL equals any combination of short-lived 222Rn daughters (218Po, 214Pb, 214Bi, and 214Po) in 1 liter of air that releases 1.3 × 105 MeV of potential alpha energy; one WL is equivalent to 2.08 × 10−5 joules per cubic meter of air (J/m3). The SI unit of cumulative exposure is expressed in joule-hours per cubic meter (J·h/m3). One WLM is equivalent to 3.6 × 10−3 J·h/m3.
An exposure to 1 WL for 1 working month (170 hours) equals 1 WLM
cumulative exposure. A cumulative exposure of 1 WLM is roughly
equivalent to living one year in an atmosphere with a radon
concentration of 230 Bq/m3.
222Rn decays to 210Pb and other radioisotopes. The levels of 210Pb can be measured. The rate of deposition of this radioisotope is weather-dependent.
Radon concentrations found in natural environments are much too low to be detected by chemical means. A 1000 Bq/m3 (relatively high) concentration corresponds to 0.17 picogram per cubic meter. The average concentration of radon in the atmosphere is about 6×10−18 molar percent, or about 150 atoms in each ml of air.
The radon activity of the entire Earth's atmosphere originates from
only a few tens of grams of radon, consistently replaced by decay of
larger amounts of radium, thorium, and uranium.
Natural
Radon concentration next to a uranium mine
Radon is produced by the radioactive decay of radium-226, which is
found in uranium ores, phosphate rock, shales, igneous and metamorphic
rocks such as granite, gneiss, and schist, and to a lesser degree, in
common rocks such as limestone. Every square mile of surface soil, to a depth of 6 inches (2.6 km2 to a depth of 15 cm), contains approximately 1 gram of radium, which releases radon in small amounts to the atmosphere. On a global scale, it is estimated that 2,400 million curies (90 EBq) of radon are released from soil annually.
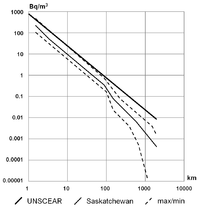
Radon concentration can differ widely from place to place. In the open air, it ranges from 1 to 100 Bq/m3, even less (0.1 Bq/m3) above the ocean. In caves or aerated mines, or ill-aerated houses, its concentration climbs to 20–2,000 Bq/m3.
Radon concentration can be much higher in mining contexts.
Ventilation regulations instruct to maintain radon concentration in
uranium mines under the "working level", with 95th percentile levels
ranging up to nearly 3 WL (546 pCi 222Rn per liter of air; 20.2 kBq/m3, measured from 1976 to 1985).
The concentration in the air at the (unventilated) Gastein Healing Gallery averages 43 kBq/m3 (1.2 nCi/L) with maximal value of 160 kBq/m3 (4.3 nCi/L).
Radon mostly appears with the decay chain of the radium and uranium series (222Rn), and marginally with the thorium series (220Rn).
The element emanates naturally from the ground, and some building
materials, all over the world, wherever traces of uranium or thorium can be found, and particularly in regions with soils containing granite or shale,
which have a higher concentration of uranium. Not all granitic regions
are prone to high emissions of radon. Being a rare gas, it usually
migrates freely through faults and fragmented soils, and may accumulate
in caves or water. Owing to its very short half-life (four days for 222Rn),
radon concentration decreases very quickly when the distance from the
production area increases. Radon concentration varies greatly with
season and atmospheric conditions. For instance, it has been shown to
accumulate in the air if there is a meteorological inversion and little wind.
High concentrations of radon can be found in some spring waters and hot springs. The towns of Boulder, Montana; Misasa; Bad Kreuznach,
Germany; and the country of Japan have radium-rich springs that emit
radon. To be classified as a radon mineral water, radon concentration
must be above 2 nCi/L (74 kBq/m3). The activity of radon mineral water reaches 2,000 kBq/m3 in Merano and 4,000 kBq/m3 in Lurisia (Italy).
Natural radon concentrations in the Earth's atmosphere are so low that radon-rich water in contact with the atmosphere will continually lose radon by volatilization. Hence, ground water has a higher concentration of 222Rn than surface water, because radon is continuously produced by radioactive decay of 226Ra present in rocks. Likewise, the saturated zone of a soil frequently has a higher radon content than the unsaturated zone because of diffusional losses to the atmosphere.
In 1971, Apollo 15 passed 110 km (68 mi) above the Aristarchus plateau on the Moon, and detected a significant rise in alpha particles thought to be caused by the decay of 222Rn. The presence of 222Rn has been inferred later from data obtained from the Lunar Prospector alpha particle spectrometer.
Radon is found in some petroleum. Because radon has a similar pressure and temperature curve to propane, and oil refineries
separate petrochemicals based on their boiling points, the piping
carrying freshly separated propane in oil refineries can become
radioactive because of decaying radon and its products.
Residues from the petroleum and natural gas industry often contain radium and its daughters. The sulfate scale from an oil well
can be radium rich, while the water, oil, and gas from a well often
contains radon. Radon decays to form solid radioisotopes that form
coatings on the inside of pipework.
Accumulation in buildings
Typical log-normal radon distribution in dwellings.
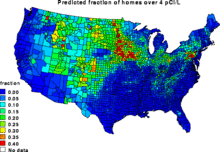
Predicted fraction of U.S. homes having concentrations of radon exceeding the EPA's recommended action level of 4 pCi/L
High concentrations of radon in homes were discovered by chance in
1985 after the stringent radiation testing conducted at a nuclear power
plant entrance revealed that Stanley Watras, an engineer at the plant, was contaminated by radioactive substances. Typical domestic exposures are of approximately 100 Bq/m3
(2.7 pCi/L) indoors. Some level of radon will be found in all
buildings. Radon mostly enters a building directly from the soil through
the lowest level in the building that is in contact with the ground.
High levels of radon in the water supply can also increase indoor radon
air levels. Typical entry points of radon into buildings are cracks in
solid foundations, construction joints, cracks in walls, gaps in
suspended floors, gaps around service pipes, cavities inside walls, and
the water supply.
Radon concentrations in the same location may differ by a factor of two
over a period of 1 hour. Also, the concentration in one room of a
building may be significantly different from the concentration in an
adjoining room.
The soil characteristics of the dwellings are the most important source
of radon for the ground floor and higher concentration of indoor radon
observed on lower floors. Most of the high radon concentrations have
been reported from places near fault zones; hence the existence of a
relation between the exhalation rate from faults and indoor radon
concentrations is obvious.
The distribution of radon concentrations will generally differ
from room to room, and the readings are averaged according to regulatory
protocols. Indoor radon concentration is usually assumed to follow a lognormal distribution on a given territory. Thus, the geometric mean is generally used for estimating the "average" radon concentration in an area.
The mean concentration ranges from less than 10 Bq/m3 to over 100 Bq/m3 in some European countries. Typical geometric standard deviations found in studies range between 2 and 3, meaning (given the 68–95–99.7 rule) that the radon concentration is expected to be more than a hundred times the mean concentration for 2 to 3% of the cases.
Some of the highest radon hazard in the United States is found in Iowa and in the Appalachian Mountain areas in southeastern Pennsylvania. The second highest readings in Ireland were found in office buildings in the Irish town of Mallow, County Cork, prompting local fears regarding lung cancer. Iowa has the highest average radon concentrations in the United States due to significant glaciation that ground the granitic rocks from the Canadian Shield and deposited it as soils making up the rich Iowa farmland. Many cities within the state, such as Iowa City, have passed requirements for radon-resistant construction in new homes.
In a few locations, uranium tailings have been used for landfills and were subsequently built on, resulting in possible increased exposure to radon.
Since radon is a colorless, odorless gas the only way to know how
much is present in the air or water is to perform tests. In the United
States radon test kits are available to the public at retail stores,
such as hardware stores, for home use and testing is available through
licensed professionals, who are often home inspectors. Efforts to reduce indoor radon levels are called radon mitigation. In the U.S. the Environmental Protection Agency recommends all houses be tested for radon.
Industrial production
Radon is obtained as a by-product of uraniferous ores processing after transferring into 1% solutions of hydrochloric or hydrobromic acids. The gas mixture extracted from the solutions contains H
2, O
2, He, Rn, CO
2, H
2O and hydrocarbons. The mixture is purified by passing it over copper at 720 °C to remove the H
2 and the O
2, and then KOH and P
2O
5 are used to remove the acids and moisture by sorption. Radon is condensed by liquid nitrogen and purified from residue gases by sublimation.
2, O
2, He, Rn, CO
2, H
2O and hydrocarbons. The mixture is purified by passing it over copper at 720 °C to remove the H
2 and the O
2, and then KOH and P
2O
5 are used to remove the acids and moisture by sorption. Radon is condensed by liquid nitrogen and purified from residue gases by sublimation.
Radon commercialization is regulated, but it is available in small quantities for the calibration of 222Rn
measurement systems, at a price of almost $6,000 per milliliter of
radium solution (which only contains about 15 picograms of actual radon
at a given moment).
Radon is produced by a solution of radium-226
(half-life of 1600 years). Radium-226 decays by alpha-particle
emission, producing radon that collects over samples of radium-226 at a
rate of about 1 mm3/day per gram of radium; equilibrium is
quickly achieved and radon is produced in a steady flow, with an
activity equal to that of the radium (50 Bq). Gaseous 222Rn (half-life of about four days) escapes from the capsule through diffusion.
Concentration scale
| Bq/m3 | pCi/L | Occurrence example |
|---|---|---|
| 1 | ~0.027 | Radon concentration at the shores of large oceans is typically 1 Bq/m3.
Radon trace concentration above oceans or in Antarctica can be lower than 0.1 Bq/m3.
|
| 10 | 0.27 | Mean continental concentration in the open air: 10 to 30 Bq/m3.
Based on a series of surveys, the global mean indoor radon concentration is estimated to be 39 Bq/m3.
|
| 100 | 2.7 | Typical indoor domestic exposure. Most countries have adopted a radon concentration of 200–400 Bq/m3 for indoor air as an Action or Reference Level. If testing shows levels less than 4 picocuries radon per liter of air (150 Bq/m3), then no action is necessary. A cumulated exposure of 230 Bq/m3 of radon gas concentration during a period of 1 year corresponds to 1 WLM. |
| 1,000 | 27 | Very high radon concentrations (>1000 Bq/m3) have been found in houses built on soils with a high uranium content and/or high permeability of the ground. If levels are 20 picocuries radon per liter of air (800 Bq/m3) or higher, the home owner should consider some type of procedure to decrease indoor radon levels. Allowable concentrations in uranium mines are approximately 1,220 Bq/m3 (33 pCi/L) |
| 10,000 | 270 |
The concentration in the air at the (unventilated) Gastein Healing Gallery averages 43 kBq/m3 (about 1.2 nCi/L) with maximal value of 160 kBq/m3 (about 4.3 nCi/L).
|
| 100,000 | ~2700 |
About 100,000 Bq/m3 (2.7 nCi/L) was measured in Stanley Watras's basement.
|
| 1,000,000 | 27000 | Concentrations reaching 1,000,000 Bq/m3 can be found in unventilated uranium mines. |
| 5.54 × 1019 | ~1.5 × 1018 | Theoretical upper limit: Radon gas (222Rn) at 100% concentration (1 atmosphere, 0 °C); 1.538×105 curies/gram; 5.54×1019 Bq/m3. |
Applications
Medical
An early-20th-century form of quackery was the treatment of maladies in a radiotorium.
It was a small, sealed room for patients to be exposed to radon for its
"medicinal effects". The carcinogenic nature of radon due to its
ionizing radiation became apparent later on. Radon's molecule-damaging
radioactivity has been used to kill cancerous cells, but it does not increase the health of healthy cells. The ionizing radiation causes the formation of free radicals, which results in genetic and other cell damage, resulting in increased rates of illness, including cancer.
Exposure to radon, a process known as radiation hormesis, has been suggested to mitigate autoimmune diseases such as arthritis. As a result, in the late 20th century and early 21st century, "health mines" established in Basin, Montana
attracted people seeking relief from health problems such as arthritis
through limited exposure to radioactive mine water and radon. The
practice is discouraged because of the well-documented ill effects of
high-doses of radiation on the body.
Radioactive water baths have been applied since 1906 in Jáchymov, Czech Republic, but even before radon discovery they were used in Bad Gastein, Austria. Radium-rich springs are also used in traditional Japanese onsen in Misasa, Tottori Prefecture. Drinking therapy is applied in Bad Brambach, Germany. Inhalation therapy is carried out in Gasteiner-Heilstollen, Austria, in Świeradów-Zdrój, Czerniawa-Zdrój, Kowary, Lądek Zdrój, Poland, in Harghita Băi, Romania, and in Boulder, United States. In the US and Europe there are several "radon spas",
where people sit for minutes or hours in a high-radon atmosphere in the
belief that low doses of radiation will invigorate or energize them.
Radon has been produced commercially for use in radiation
therapy, but for the most part has been replaced by radionuclides made
in accelerators and nuclear reactors. Radon has been used in implantable seeds, made of gold or glass, primarily used to treat cancers.
The gold seeds were produced by filling a long tube with radon pumped
from a radium source, the tube being then divided into short sections by
crimping and cutting. The gold layer keeps the radon within, and
filters out the alpha and beta radiations, while allowing the gamma rays to escape (which kill the diseased tissue). The activities might range from 0.05 to 5 millicuries per seed (2 to 200 MBq). The gamma rays are produced by radon and the first short-lived elements of its decay chain (218Po, 214Pb, 214Bi, 214Po).
Radon and its first decay products being very short-lived, the
seed is left in place. After 12 half-lives (43 days), radon
radioactivity is at 1/2000 of its original level. At this stage, the
predominant residual activity originates from the radon decay product 210Pb,
whose half-life (22.3 years) is 2000 times that of radon (and whose
activity is thus 1/2000 of radon's), and its descendants 210Bi and 210Po.
Scientific
Radon
emanation from the soil varies with soil type and with surface uranium
content, so outdoor radon concentrations can be used to track air masses
to a limited degree. This fact has been put to use by some atmospheric
scientists. Because of radon's rapid loss to air and comparatively rapid
decay, radon is used in hydrologic research that studies the interaction between groundwater and streams. Any significant concentration of radon in a stream is a good indicator that there are local inputs of groundwater.
Radon soil-concentration has been used in an experimental way to map buried close-subsurface geological faults because concentrations are generally higher over the faults. Similarly, it has found some limited use in prospecting for geothermal gradients.
Some researchers have investigated changes in groundwater radon concentrations for earthquake prediction.
Radon has a half-life of approximately 3.8 days, which means that it
can be found only shortly after it has been produced in the radioactive
decay chain. For this reason, it has been hypothesized that increases in
radon concentration is due to the generation of new cracks underground,
which would allow increased ground water circulation, flushing out
radon. The generation of new cracks might not unreasonably be assumed to
precede major earthquakes. In the 1970s and 1980s, scientific
measurements of radon emissions near faults found that earthquakes often
occurred with no radon signal, and radon was often detected with no
earthquake to follow. It was then dismissed by many as an unreliable
indicator. As of 2009, it was under investigation as a possible precursor by NASA.
Radon is a known pollutant emitted from geothermal power stations
because it is present in the material pumped from deep underground. It
disperses rapidly, and no radiological hazard has been demonstrated in
various investigations. In addition, typical systems re-inject the
material deep underground rather that releasing it at the surface, so
its environmental impact is minimal.
In the 1940s and '50s, radon was used for industrial radiography,
Other X-ray sources, which became available after World War II, quickly
replaced radon for this application, as they were lower in cost and had
less hazard of alpha radiation.
Health risks
In mines
Radon-222 decay products have been classified by the International Agency for Research on Cancer as being carcinogenic to humans,
and as a gas that can be inhaled, lung cancer is a particular concern
for people exposed to elevated levels of radon for sustained periods.
During the 1940s and '50s, when safety standards requiring expensive
ventilation in mines were not widely implemented,
radon exposure was linked to lung cancer among non-smoking miners of
uranium and other hard rock materials in what is now the Czech Republic,
and later among miners from the Southwestern United States and South Australia. Despite these hazards being known in the early 1950s,
this occupational hazard remained poorly managed in many mines until
the 1970s. During this period, several entrepreneurs opened former
uranium mines in the US to the general public and advertised alleged
health benefits from breathing radon gas underground. Health benefits
claimed included pain, sinus, asthma and arthritis relief but these were proven to be false and the government banned such ads in 1975.
Since that time, ventilation and other measures have been used to
reduce radon levels in most affected mines that continue to operate. In
recent years, the average annual exposure of uranium miners has fallen
to levels similar to the concentrations inhaled in some homes. This has
reduced the risk of occupationally induced cancer from radon, although
health issues may persist for those who are currently employed in
affected mines and for those who have been employed in them in the past. As the relative risk for miners has decreased, so has the ability to detect excess risks among that population.
Residues from processing of uranium ore can also be a source of
radon. Radon resulting from the high radium content in uncovered dumps
and tailing ponds can be easily released into the atmosphere and affect
people living in the vicinity.
In addition to lung cancer, researchers have theorized a possible increased risk of leukemia
due to radon exposure. Empirical support from studies of the general
population is inconsistent, and a study of uranium miners found a
correlation between radon exposure and chronic lymphocytic leukemia.
Miners (as well as milling and ore transportation workers) who
worked in the uranium industry in the United States between the 1940s
and 1971 may be eligible for compensation under the Radiation Exposure Compensation Act (RECA). Surviving relatives may also apply in cases where the formerly employed person is deceased.
Domestic-level exposure
Radon
exposure (mostly radon daughters) has been linked to lung cancer in
numerous case-control studies performed in the United States, Europe and
China. There are approximately 21,000 deaths per year in the US due to
radon-induced lung cancers. One of the most comprehensive radon studies performed in the United States by Dr. R. William Field
and colleagues found a 50% increased lung cancer risk even at the
protracted exposures at the EPA's action level of 4 pCi/L. North
American and European Pooled analyses further support these findings. However, the discussion about the opposite results is still continuing,
especially a recent retrospective case-control study of lung cancer
risk which showed substantial cancer rate reduction for radon
concentrations between 50 and 123 Bq per cubic meter.
Most models of residential radon exposure are based on studies of
miners, and direct estimates of the risks posed to homeowners would be
more desirable.
Because of the difficulties of measuring the risk of radon relative to
smoking, models of their effect have often made use of them.
Radon has been considered the second leading cause of lung cancer and leading environmental cause of cancer mortality by the United States Environmental Protection Agency. Others have reached similar conclusions for the United Kingdom and France.
Radon exposure in homes and offices may arise from certain subsurface
rock formations, and also from certain building materials (e.g., some
granites). The greatest risk of radon exposure arises in buildings that
are airtight, insufficiently ventilated, and have foundation leaks that
allow air from the soil into basements and dwelling rooms.
Action and reference level
WHO presented in 2009 a recommended reference level (the national reference level), 100 Bq/m3, for radon in dwellings. The recommendation also says that where this is not possible, 300 Bq/m3
should be selected as the highest level. A national reference level
should not be a limit, but should represent the maximum acceptable
annual average radon concentration in a dwelling.
The actionable concentration of radon in a home varies depending
on the organization doing the recommendation, for example, the United
States Environmental Protection Agency encourages that action be taken
at concentrations as low as 74 Bq/m3 (2 pCi/L), and the European Union recommends action be taken when concentrations reach 400 Bq/m3 (11 pCi/L) for old houses and 200 Bq/m3 (5 pCi/L) for new ones. On 8 July 2010 the UK's Health Protection Agency issued new advice setting a "Target Level" of 100 Bq/m3 whilst retaining an "Action Level" of 200 Bq/m3.
The same levels (as UK) apply to Norway from 2010; in all new housings
preventative measures should be taken against radon accumulation.
Relationship to smoking
Results
from epidemiological studies indicate that the risk of lung cancer
increases with exposure to residential radon. A well-known example of
source of error is smoking, the main risk factor for lung cancer. In the
West, tobacco smoke is estimated to cause about 90% of all lung
cancers.
According to the EPA, the risk of lung cancer for smokers is
significant due to synergistic effects of radon and smoking. For this
population about 62 people in a total of 1,000 will die of lung cancer
compared to 7 people in a total of 1,000 for people who have never
smoked.
It cannot be excluded that the risk of non-smokers should be primarily
explained by a combination effect of radon and passive smoking (see
below).
Radon, like other known or suspected external risk factors for
lung cancer, is a threat for smokers and former smokers. This was
demonstrated by the European pooling study. A commentary
to the pooling study stated: "it is not appropriate to talk simply of a
risk from radon in homes. The risk is from smoking, compounded by a
synergistic effect of radon for smokers. Without smoking, the effect
seems to be so small as to be insignificant."
According to the European pooling study, there is a difference in
risk from radon between histological types. Small cell lung carcinoma,
which practically only affects smokers have high risk from radon. For
other histological types such as adenocarcinoma, the type that primarily
affects never smokers, the risk from radon appears to be lower.
A study of radiation from post mastectomy radiotherapy
shows that the simple models previously used to assess the combined and
separate risks from radiation and smoking need to be developed. This is
also supported by new discussion about the calculation method, LNT, which routinely has been used.
An important, but unanswered question concerns the possibility
that the cancer risk from passive smoking can increase with exposure to
residential radon. The basic data for the European pooling study makes
it impossible to exclude that such synergy effect is an explanation for
the (very limited) increase in the risk from radon that was stated for
non-smokers.
A study
from 2001, which included 436 cases (never smokers who had lung
cancer), and a control group (1649 never smokers) showed that exposure
to radon increased the risk of lung cancer in never smokers. But the
group that had been exposed to passive smoking at home appeared to bear
the entire risk increase, while those who were not exposed to passive
smoking did not show any increased risk with increasing radon level.
In drinking water
The
effects of radon if ingested are similarly unknown, although studies
have found that its biological half-life ranges from 30–70 minutes, with
90 percent removal at 100 minutes. In 1999 National Research Council
investigated the issue of radon in drinking water. The risk associated
with ingestion was considered almost negligible.
Water from underground sources may contain significant amounts of radon
depending on the surrounding rock and soil conditions, whereas surface
sources generally do not.
As well as being ingested through drinking water, radon is also
released from water when temperature is increased, pressure is decreased
and when water is aerated. Optimum conditions for radon release and
exposure occur during showering. Water with a radon concentration of 104 pCi/L can increase the indoor airborne radon concentration by 1 pCi/L under normal conditions.
Testing and mitigation
A digital radon detector
A radon test kit
There are relatively simple tests for radon gas. In some countries
these tests are methodically done in areas of known systematic hazards.
Radon detection devices are commercially available. Digital radon
detectors provide ongoing measurements giving both daily, weekly,
short-term and long-term average readouts via a digital display.
Short-term radon test devices used for initial screening purposes are
inexpensive, in some cases free. There are important protocols for
taking short-term radon tests and it is imperative that they be strictly
followed. The kit includes a collector that the user hangs in the
lowest habitable floor of the house for 2 to 7 days. The user then sends
the collector to a laboratory for analysis. Long term kits, taking
collections for up to one year or more, are also available. An open-land
test kit can test radon emissions from the land before construction
begins.
Radon concentrations can vary daily, and accurate radon exposure
estimates require long-term average radon measurements in the spaces
where an individual spends a significant amount of time.
Radon levels fluctuate naturally, due to factors like transient
weather conditions, so an initial test might not be an accurate
assessment of a home's average radon level. Radon levels are at a
maximum during the coolest part of the day when pressure differentials
are greatest.
Therefore, a high result (over 4 pCi/L) justifies repeating the test
before undertaking more expensive abatement projects. Measurements
between 4 and 10 pCi/L warrant a long term radon test. Measurements over
10 pCi/L warrant only another short term test so that abatement
measures are not unduly delayed. Purchasers of real estate are advised
to delay or decline a purchase if the seller has not successfully abated
radon to 4 pCi/L or less.
Because the half-life of radon is only 3.8 days, removing or
isolating the source will greatly reduce the hazard within a few weeks.
Another method of reducing radon levels is to modify the building's
ventilation. Generally, the indoor radon concentrations increase as
ventilation rates decrease. In a well ventilated place, the radon concentration tends to align with outdoor values (typically 10 Bq/m3, ranging from 1 to 100 Bq/m3).
The four principal ways of reducing the amount of radon accumulating in a house are:
- Sub-slab depressurization (soil suction) by increasing under-floor ventilation;
- Improving the ventilation of the house and avoiding the transport of radon from the basement into living rooms;
- Installing a radon sump system in the basement;
- Installing a positive pressurization or positive supply ventilation system.
According to the EPA
the method to reduce radon "...primarily used is a vent pipe system and
fan, which pulls radon from beneath the house and vents it to the
outside", which is also called sub-slab depressurization, active soil
depressurization, or soil suction. Generally indoor radon can be
mitigated by sub-slab depressurization and exhausting such radon-laden
air to the outdoors, away from windows and other building openings. "EPA
generally recommends methods which prevent the entry of radon. Soil
suction, for example, prevents radon from entering your home by drawing
the radon from below the home and venting it through a pipe, or pipes,
to the air above the home where it is quickly diluted" and "EPA does not
recommend the use of sealing alone to reduce radon because, by itself,
sealing has not been shown to lower radon levels significantly or
consistently".
Positive-pressure ventilation systems can be combined with a heat
exchanger to recover energy in the process of exchanging air with the
outside, and simply exhausting basement air to the outside is not
necessarily a viable solution as this can actually draw radon gas into
a dwelling. Homes built on a crawl space may benefit from a radon
collector installed under a "radon barrier" (a sheet of plastic that
covers the crawl space).
For crawlspaces, the EPA states "An effective method to reduce radon
levels in crawlspace homes involves covering the earth floor with a
high-density plastic sheet. A vent pipe and fan are used to draw the
radon from under the sheet and vent it to the outdoors. This form of
soil suction is called submembrane suction, and when properly applied is
the most effective way to reduce radon levels in crawlspace homes."