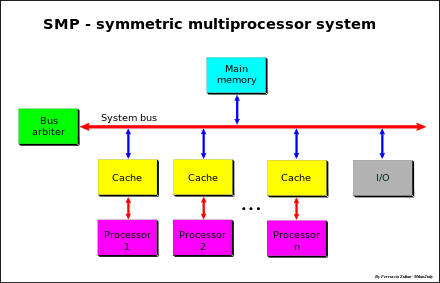
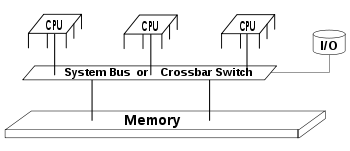
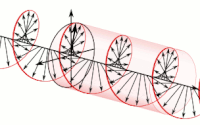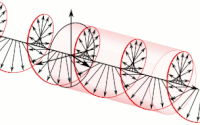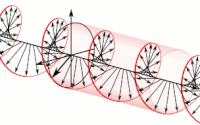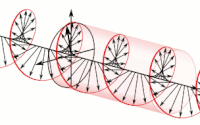
Diagram of a symmetric multiprocessing system
Symmetric multiprocessing (SMP) involves a multiprocessor computer hardware and software architecture where two or more identical processors are connected to a single, shared main memory,
have full access to all input and output devices, and are controlled by
a single operating system instance that treats all processors equally,
reserving none for special purposes. Most multiprocessor systems today
use an SMP architecture. In the case of multi-core processors, the SMP architecture applies to the cores, treating them as separate processors.
Professor John D. Kubiatowicz considers traditionally SMP systems to contain processors without caches.
Culler and Pal-Singh in their 1998 book "Parallel Computer
Architecture: A Hardware/Software Approach" mention: "The term SMP is
widely used but causes a bit of confusion. [...] The more precise
description of what is intended by SMP is a shared memory multiprocessor
where the cost of accessing a memory location is the same for all
processors; that is, it has uniform access costs when the access
actually is to memory. If the location is cached, the access will be
faster, but cache access times and memory access times are the same on
all processors."
SMP systems are tightly coupled multiprocessor systems
with a pool of homogeneous processors running independently of each
other. Each processor, executing different programs and working on
different sets of data, has the capability of sharing common resources
(memory, I/O device, interrupt system and so on) that are connected
using a system bus or a crossbar.
Design
SMP systems have centralized shared memory called main memory (MM) operating under a single operating system with two or more homogeneous processors. Usually each processor has an associated private high-speed memory known as cache memory (or cache) to speed up the main memory data access and to reduce the system bus traffic.
Processors may be interconnected using buses, crossbar switches
or on-chip mesh networks. The bottleneck in the scalability of SMP
using buses or crossbar switches is the bandwidth and power consumption
of the interconnect among the various processors, the memory, and the
disk arrays. Mesh architectures avoid these bottlenecks, and provide
nearly linear scalability to much higher processor counts at the
sacrifice of programmability:
Serious programming challenges remain with this kind of architecture because it requires two distinct modes of programming; one for the CPUs themselves and one for the interconnect between the CPUs. A single programming language would have to be able to not only partition the workload, but also comprehend the memory locality, which is severe in a mesh-based architecture.
SMP systems allow any processor to work on any task no matter where
the data for that task is located in memory, provided that each task in
the system is not in execution on two or more processors at the same
time. With proper operating system support, SMP systems can easily move tasks between processors to balance the workload efficiently.
History
The earliest production system with multiple identical processors was the Burroughs B5000, which was functional around 1961. However at run-time this was asymmetric,
with one processor restricted to application programs while the other
processor mainly handled the operating system and hardware interrupts.
The Burroughs D825 first implemented SMP in 1962.
IBM offered dual-processor computer systems based on its System/360 Model 65 and the closely related Model 67 and 67-2. The operating systems that ran on these machines were OS/360 M65MP and TSS/360. Other software developed at universities, notably the Michigan Terminal System
(MTS), used both CPUs. Both processors could access data channels and
initiate I/O. In OS/360 M65MP, peripherals could generally be attached
to either processor since the operating system kernel ran on both
processors (though with a "big lock" around the I/O handler).
The MTS supervisor (UMMPS) has the ability to run on both CPUs of the
IBM System/360 model 67-2. Supervisor locks were small and used to
protect individual common data structures that might be accessed
simultaneously from either CPU.
Other mainframes that supported SMP included the UNIVAC 1108 II, released in 1965, which supported up to three CPUs, and the GE-635 and GE-645, although GECOS on multiprocessor GE-635 systems ran in a master-slave asymmetric fashion, unlike Multics on multiprocessor GE-645 systems, which ran in a symmetric fashion.
Starting with its version 7.0 (1972), Digital Equipment Corporation's operating system TOPS-10 implemented the SMP feature, the earliest system running SMP was the DECSystem 1077 dual KI10 processor system. Later KL10 system could aggregate up to 8 CPUs in a SMP manner. In contrast, DECs first multi-processor VAX system, the VAX-11/782, was asymmetric, but later VAX multiprocessor systems were SMP.
Early commercial Unix SMP implementations included the Sequent Computer Systems Balance 8000 (released in 1984) and Balance 21000 (released in 1986). Both models were based on 10 MHz National Semiconductor NS32032 processors, each with a small write-through cache connected to a common memory to form a shared memory
system. Another early commercial Unix SMP implementation was the NUMA
based Honeywell Information Systems Italy XPS-100 designed by Dan Gielan
of VAST Corporation in 1985. Its design supported up to 14 processors,
but due to electrical limitations, the largest marketed version was a
dual processor system. The operating system was derived and ported by
VAST Corporation from AT&T 3B20 Unix SysVr3 code used internally
within AT&T.
Earlier non-commercial multiprocessing UNIX ports existed, including a port named MUNIX created at the Naval Postgraduate School by 1975.
Uses
Time-sharing and server systems can often use SMP without changes to applications, as they may have multiple processes running in parallel, and a system with more than one process running can run different processes on different processors.
On personal computers,
SMP is less useful for applications that have not been modified. If
the system rarely runs more than one process at a time, SMP is useful
only for applications that have been modified for multithreaded (multitasked) processing. Custom-programmed software can be written or modified to use multiple threads, so that it can make use of multiple processors.
Multithreaded programs can also be used in time-sharing and
server systems that support multithreading, allowing them to make more
use of multiple processors.
Advantages/Disadvantages
In
current SMP systems, all of the processors are tightly coupled inside
the same box with a bus or switch; on earlier SMP systems, a single CPU
took an entire cabinet. Some of the components that are shared are
global memory, disks, and I/O devices. Only one copy of an OS runs on
all the processors, and the OS must be designed to take advantage of
this architecture. Some of the basic advantages involves cost-effective
ways to increase throughput. To solve different problems and tasks,
SMP applies multiple processors to that one problem, known as parallel programming.
However, there are a few limits on the scalability of SMP due to cache coherence and shared objects.
Programming
Uniprocessor
and SMP systems require different programming methods to achieve
maximum performance. Programs running on SMP systems may experience an
increase in performance even when they have been written for
uniprocessor systems. This is because hardware interrupts usually suspends program execution while the kernel
that handles them can execute on an idle processor instead. The effect
in most applications (e.g. games) is not so much a performance increase
as the appearance that the program is running much more smoothly. Some
applications, particularly building software and some distributed computing
projects, run faster by a factor of (nearly) the number of additional
processors. (Compilers by themselves are single threaded, but, when
building a software project with multiple compilation units, if each
compilation unit is handled independently, this creates an embarrassingly parallel
situation across the entire multi-compilation-unit project, allowing
near linear scaling of compilation time. Distributed computing projects
are inherently parallel by design.)
Systems programmers must build support for SMP into the operating system, otherwise, the additional processors remain idle and the system functions as a uniprocessor system.
SMP systems can also lead to more complexity regarding
instruction sets. A homogeneous processor system typically requires
extra registers for "special instructions" such as SIMD (MMX, SSE,
etc.), while a heterogeneous system can implement different types of
hardware for different instructions/uses.
Performance
When
more than one program executes at the same time, an SMP system has
considerably better performance than a uni-processor, because different
programs can run on different CPUs simultaneously. Similarly, Asymmetric multiprocessing
(AMP) usually allows only one processor to run a program or task at a
time. For example, AMP can be used in assigning specific tasks to CPU
based to priority and importance of task completion. AMP was created
well before SMP in terms of handling multiple CPUs, which explains the
lack of performance based on the example provided.
In cases where an SMP environment processes many jobs,
administrators often experience a loss of hardware efficiency. Software
programs have been developed to schedule jobs and other functions of the
computer so that the processor utilization reaches its maximum
potential. Good software packages can achieve this maximum potential by
scheduling each CPU separately, as well as being able to integrate
multiple SMP machines and clusters.
Access to RAM is serialized; this and cache coherency issues causes performance to lag slightly behind the number of additional processors in the system.
Alternatives
Diagram of a typical SMP system. Three processors are connected to the same memory module through a system bus or crossbar switch
SMP uses a single shared system bus
that represents one of the earliest styles of multiprocessor machine
architectures, typically used for building smaller computers with up to 8
processors.
Larger computer systems might use newer architectures such as NUMA
(Non-Uniform Memory Access), which dedicates different memory banks to
different processors. In a NUMA architecture, processors may access
local memory quickly and remote memory more slowly. This can
dramatically improve memory throughput as long as the data are localized
to specific processes (and thus processors). On the downside, NUMA
makes the cost of moving data from one processor to another, as in
workload balancing, more expensive. The benefits of NUMA are limited to
particular workloads, notably on servers where the data are often associated strongly with certain tasks or users.
Finally, there is computer clustered multiprocessing (such as Beowulf),
in which not all memory is available to all processors. Clustering
techniques are used fairly extensively to build very large
supercomputers.
Variable SMP
Variable Symmetric Multiprocessing (vSMP) is a specific mobile use
case technology initiated by NVIDIA. This technology includes an extra
fifth core in a quad-core device, called the Companion core, built
specifically for executing tasks at a lower frequency during mobile
active standby mode, video playback, and music playback.
Project Kal-El (Tegra 3),
patented by NVIDIA, was the first SoC (System on Chip) to implement
this new vSMP technology. This technology not only reduces mobile power
consumption during active standby state, but also maximizes quad core
performance during active usage for intensive mobile applications.
Overall this technology addresses the need for increase in battery life
performance during active and standby usage by reducing the power
consumption in mobile processors.
Unlike current SMP architectures, the vSMP Companion core is OS
transparent meaning that the operating system and the running
applications are totally unaware of this extra core but are still able
to take advantage of it. Some of the advantages of the vSMP architecture
includes cache coherency, OS efficiency, and power optimization. The
advantages for this architecture are explained below:
- Cache Coherency: There are no consequences for synchronizing caches between cores running at different frequencies since vSMP does not allow the Companion core and the main cores to run simultaneously.
- OS Efficiency: It is inefficient when multiple CPU cores are run at different asynchronous frequencies because this could lead to possible scheduling issues. With vSMP, the active CPU cores will run at similar frequencies to optimize OS scheduling.
- Power Optimization: In asynchronous clocking based architecture, each core is on a different power plane to handle voltage adjustments for different operating frequencies. The result of this could impact performance. vSMP technology is able to dynamically enable and disable certain cores for active and standby usage, reducing overall power consumption.
These advantages lead the vSMP architecture to considerably benefit over other architectures using asynchronous clocking technologies.