From Wikipedia, the free encyclopedia
https://en.wikipedia.org/wiki/Radical_polymerization
In polymer chemistry, free-radical polymerization (FRP) is a method of polymerization by which a polymer forms by the successive addition of free-radical building blocks (repeat units). Free radicals can be formed by a number of different mechanisms, usually involving separate initiator molecules. Following its generation, the initiating free radical adds (nonradical) monomer units, thereby growing the polymer chain.
Free-radical polymerization is a key synthesis route for obtaining a wide variety of different polymers and materials composites. The relatively non-specific nature of free-radical chemical interactions makes this one of the most versatile forms of polymerization available and allows facile reactions of polymeric free-radical chain ends and other chemicals or substrates. In 2001, 40 billion of the 110 billion pounds of polymers produced in the United States were produced by free-radical polymerization.
Free-radical polymerization is a type of chain-growth polymerization, along with anionic, cationic and coordination polymerization.
A chain polymerization in which the kinetic-chain carriers are radicals.
Note: Usually, the growing chain end bears an unpaired electron.
Initiation
Initiation is the first step of the polymerization process. During initiation, an active center is created from which a polymer chain is generated. Not all monomers are susceptible to all types of initiators. Radical initiation works best on the carbon–carbon double bond of vinyl monomers and the carbon–oxygen double bond in aldehydes and ketones. Initiation has two steps. In the first step, one or two radicals are created from the initiating molecules. In the second step, radicals are transferred from the initiator molecules to the monomer units present. Several choices are available for these initiators.
Types of initiation and the initiators
- Photoinitiation can also occur by bi-molecular H abstraction when the radical is in its lowest triplet excited state. An acceptable photoinitiator system should fulfill the following requirements:Figure 2: Photolysis of azoisobutylnitrile (AIBN)
- High absorptivity in the 300–400 nm range.
- Efficient generation of radicals capable of attacking the alkene double bond of vinyl monomers.
- Adequate solubility in the binder system (prepolymer + monomer).
- Should not impart yellowing or unpleasant odors to the cured material.
- The photoinitiator and any byproducts resulting from its use should be non-toxic.
- Redox reactions
- Reduction of hydrogen peroxide or an alkyl hydrogen peroxide by iron (Figure 3). Other reductants such as Cr2+, V2+, Ti3+, Co2+, and Cu+ can be employed in place of ferrous ion in many instances.
Figure 3: Redox reaction of hydrogen peroxide and iron. |
- Persulfates
- The dissociation of a persulfate in the aqueous phase (Figure 4). This method is useful in emulsion polymerizations, in which the radical diffuses into a hydrophobic monomer-containing droplet.
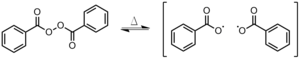
- This type of initiating system contains a metallocene, an initiator, and a heteroaromatic diketo carboxylic acid. Metallocenes in combination with initiators accelerate polymerization of poly(methyl methacrylate) and produce a polymer with a narrower molecular weight distribution. The example shown here consists of indenylzirconium (a metallocene) and benzoyl peroxide (an initiator). Also, initiating systems containing heteroaromatic diketo carboxylic acids, such as 3,6-bis(o-carboxybenzoyl)-N-isopropylcarbazole in this example, are known to catalyze the decomposition of benzoyl peroxide. Initiating systems with this particular heteroaromatic diket carboxylic acid are also known to have effects on the microstructure of the polymer. The combination of all of these components—a metallocene, an initiator, and a heteroaromatic diketo carboxylic acid—yields a ternary initiating system that was shown to accelerate the polymerization and produce polymers with enhanced heat resistance and regular microstructure.Figure 7: benzoyl peroxide-3,6-bis(o-carboxybenzoyl)-N-isopropylcarbazole-di-η5-indenylzicronium dichloride
Initiator efficiency
Due to side reactions and inefficient synthesis of the radical species, chain initiation is not 100%. The efficiency factor f is used to describe the effective radical concentration. The maximal value of f is 1, but typical values range from 0.3 to 0.8. The following is a list of reactions that decrease the efficiency of the initiator.
- Primary recombination
- Two radicals recombine before initiating a chain (Figure 8). This occurs within the solvent cage, meaning that no solvent has yet come between the new radicals.
- Other recombination pathways
- Two radical initiators recombine before initiating a chain, but not in the solvent cage (Figure 9).
- Side reactions
- One radical is produced instead of the three radicals that could be produced (Figure 10).
Figure 10: Reaction of polymer chain R with other species in reaction |
Propagation
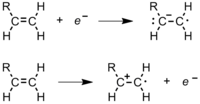
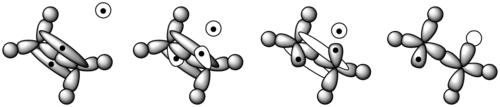
During polymerization, a polymer spends most of its time in increasing its chain length, or propagating. After the radical initiator is formed, it attacks a monomer (Figure 11). In an ethene monomer, one electron pair is held securely between the two carbons in a sigma bond. The other is more loosely held in a pi bond. The free radical uses one electron from the pi bond to form a more stable bond with the carbon atom. The other electron returns to the second carbon atom, turning the whole molecule into another radical. This begins the polymer chain. Figure 12 shows how the orbitals of an ethylene monomer interact with a radical initiator.
Once a chain has been initiated, the chain propagates (Figure 13) until there are no more monomers (living polymerization) or until termination occurs. There may be anywhere from a few to thousands of propagation steps depending on several factors such as radical and chain reactivity, the solvent, and temperature. The mechanism of chain propagation is as follows:
Termination
Chain termination is inevitable in radical polymerization due to the high reactivity of radicals. Termination can occur by several different mechanisms. If longer chains are desired, the initiator concentration should be kept low; otherwise, many shorter chains will result.
- Combination of two active chain ends: one or both of the following processes may occur.
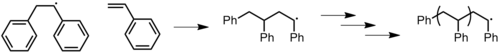
- Combination: two chain ends simply couple together to form one long chain (Figure 14). One can determine if this mode of termination is occurring by monitoring the molecular weight of the propagating species: combination will result in doubling of molecular weight. Also, combination will result in a polymer that is C2 symmetric about the point of the combination.
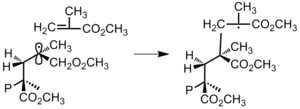
- Figure 15: Termination by disproportionation of poly(methyl methacrylate).
- Figure 18: Inhibition of polymer chain, R, by DPPH.
Chain transfer
Contrary to the other modes of termination, chain transfer results in the destruction of only one radical, but also the creation of another radical. Often, however, this newly created radical is not capable of further propagation. Similar to disproportionation, all chain-transfer mechanisms also involve the abstraction of a hydrogen or other atom. There are several types of chain-transfer mechanisms.
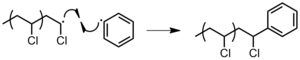
- To solvent: a hydrogen atom is abstracted from a solvent molecule, resulting in the formation of radical on the solvent molecules, which will not propagate further (Figure 19).
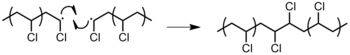
- Figure 22: Chain transfer from polypropylene to backbone of another polypropylene.
Effects of chain transfer: The most obvious effect of chain transfer is a decrease in the polymer chain length. If the rate of transfer is much larger than the rate of propagation, then very small polymers are formed with chain lengths of 2-5 repeating units (telomerization). The Mayo equation estimates the influence of chain transfer on chain length (xn): . Where ktr is the rate constant for chain transfer and kp is the rate constant for propagation. The Mayo equation assumes that transfer to solvent is the major termination pathway.
Methods
There are four industrial methods of radical polymerization:
- Bulk polymerization: reaction mixture contains only initiator and monomer, no solvent.
- Solution polymerization: reaction mixture contains solvent, initiator, and monomer.
- Suspension polymerization: reaction mixture contains an aqueous phase, water-insoluble monomer, and initiator soluble in the monomer droplets (both the monomer and the initiator are hydrophobic).
- Emulsion polymerization: similar to suspension polymerization except that the initiator is soluble in the aqueous phase rather than in the monomer droplets (the monomer is hydrophobic, and the initiator is hydrophilic). An emulsifying agent is also needed.
Other methods of radical polymerization include the following:
- Template polymerization: In this process, polymer chains are allowed to grow along template macromolecules for the greater part of their lifetime. A well-chosen template can affect the rate of polymerization as well as the molar mass and microstructure of the daughter polymer. The molar mass of a daughter polymer can be up to 70 times greater than those of polymers produced in the absence of the template and can be higher in molar mass than the templates themselves. This is because of retardation of the termination for template-associated radicals and by hopping of a radical to the neighboring template after reaching the end of a template polymer.
- Plasma polymerization: The polymerization is initiated with plasma. A variety of organic molecules including alkenes, alkynes, and alkanes undergo polymerization to high molecular weight products under these conditions. The propagation mechanisms appear to involve both ionic and radical species. Plasma polymerization offers a potentially unique method of forming thin polymer films for uses such as thin-film capacitors, antireflection coatings, and various types of thin membranes.
- Sonication: The polymerization is initiated by high-intensity ultrasound. Polymerization to high molecular weight polymer is observed but the conversions are low (<15%). The polymerization is self-limiting because of the high viscosity produced even at low conversion. High viscosity hinders cavitation and radical production.
Reversible deactivation radical polymerization
Also known as living radical polymerization, controlled radical polymerization, reversible deactivation radical polymerization (RDRP) relies on completely pure reactions, preventing termination caused by impurities. Because these polymerizations stop only when there is no more monomer, polymerization can continue upon the addition of more monomer. Block copolymers can be made this way. RDRP allows for control of molecular weight and dispersity. However, this is very difficult to achieve and instead a pseudo-living polymerization occurs with only partial control of molecular weight and dispersity. ATRP and RAFT are the main types of complete radical polymerization.
- Atom transfer radical polymerization (ATRP): based on the formation of a carbon-carbon bond by atom transfer radical addition. This method, independently discovered in 1995 by Mitsuo Sawamoto and by Jin-Shan Wang and Krzysztof Matyjaszewski, requires reversible activation of a dormant species (such as an alkyl halide) and a transition metal halide catalyst (to activate dormant species).
- Reversible Addition-Fragmentation Chain-Transfer Polymerization (RAFT): requires a compound that can act as a reversible chain-transfer agent, such as dithio compound.
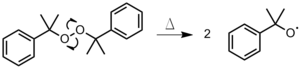
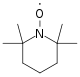
- Stable Free Radical Polymerization (SFRP): used to synthesize linear or branched polymers with narrow molecular weight distributions and reactive end groups on each polymer chain. The process has also been used to create block co-polymers with unique properties. Conversion rates are about 100% using this process but require temperatures of about 135 °C. This process is most commonly used with acrylates, styrenes, and dienes. The reaction scheme in Figure 23 illustrates the SFRP process.
- Because the chain end is functionalized with the TEMPO molecule (Figure 24), premature termination by coupling is reduced. As with all living polymerizations, the polymer chain grows until all of the monomer is consumed.Figure 24: TEMPO molecule used to functionalize the chain ends.
Kinetics
In typical chain growth polymerizations, the reaction rates for initiation, propagation and termination can be described as follows:
where f is the efficiency of the initiator and kd, kp, and kt are the constants for initiator dissociation, chain propagation and termination, respectively. [I] [M] and [M•] are the concentrations of the initiator, monomer and the active growing chain.
Under the steady-state approximation, the concentration of the active growing chains remains constant, i.e. the rates of initiation and of termination are equal. The concentration of active chain can be derived and expressed in terms of the other known species in the system.
In this case, the rate of chain propagation can be further described using a function of the initiator and monomer concentrations
The kinetic chain length v is a measure of the average number of monomer units reacting with an active center during its lifetime and is related to the molecular weight through the mechanism of the termination. Without chain transfer, the kinetic chain length is only a function of propagation rate and initiation rate.
Assuming no chain-transfer effect occurs in the reaction, the number average degree of polymerization Pn can be correlated with the kinetic chain length. In the case of termination by disproportionation, one polymer molecule is produced per every kinetic chain:
Termination by combination leads to one polymer molecule per two kinetic chains:
Any mixture of both these mechanisms can be described by using the value δ, the contribution of disproportionation to the overall termination process:
If chain transfer is considered, the kinetic chain length is not affected by the transfer process because the growing free-radical center generated by the initiation step stays alive after any chain-transfer event, although multiple polymer chains are produced. However, the number average degree of polymerization decreases as the chain transfers, since the growing chains are terminated by the chain-transfer events. Taking into account the chain-transfer reaction towards solvent S, initiator I, polymer P, and added chain-transfer agent T. The equation of Pn will be modified as follows:
It is usual to define chain-transfer constants C for the different molecules
- , , , ,
Thermodynamics
In chain growth polymerization, the position of the equilibrium between polymer and monomers can be determined by the thermodynamics of the polymerization. The Gibbs free energy (ΔGp) of the polymerization is commonly used to quantify the tendency of a polymeric reaction. The polymerization will be favored if ΔGp < 0; if ΔGp > 0, the polymer will undergo depolymerization. According to the thermodynamic equation ΔG = ΔH – TΔS, a negative enthalpy and an increasing entropy will shift the equilibrium towards polymerization.
In general, the polymerization is an exothermic process, i.e. negative enthalpy change, since addition of a monomer to the growing polymer chain involves the conversion of π bonds into σ bonds, or a ring–opening reaction that releases the ring tension in a cyclic monomer. Meanwhile, during polymerization, a large amount of small molecules are associated, losing rotation and translational degrees of freedom. As a result, the entropy decreases in the system, ΔSp < 0 for nearly all polymerization processes. Since depolymerization is almost always entropically favored, the ΔHp must then be sufficiently negative to compensate for the unfavorable entropic term. Only then will polymerization be thermodynamically favored by the resulting negative ΔGp.
In practice, polymerization is favored at low temperatures: TΔSp is small. Depolymerization is favored at high temperatures: TΔSp is large. As the temperature increases, ΔGp become less negative. At a certain temperature, the polymerization reaches equilibrium (rate of polymerization = rate of depolymerization). This temperature is called the ceiling temperature (Tc). ΔGp = 0.
Stereochemistry
The stereochemistry of polymerization is concerned with the difference in atom connectivity and spatial orientation in polymers that has the same chemical composition.
Hermann Staudinger studied the stereoisomerism in chain polymerization of vinyl monomers in the late 1920s, and it took another two decades for people to fully appreciate the idea that each of the propagation steps in the polymer growth could give rise to stereoisomerism. The major milestone in the stereochemistry was established by Ziegler and Natta and their coworkers in 1950s, as they developed metal based catalyst to synthesize stereoregular polymers. The reason why the stereochemistry of the polymer is of particular interest is because the physical behavior of a polymer depends not only on the general chemical composition but also on the more subtle differences in microstructure. Atactic polymers consist of a random arrangement of stereochemistry and are amorphous (noncrystalline), soft materials with lower physical strength. The corresponding isotactic (like substituents all on the same side) and syndiotactic (like substituents of alternate repeating units on the same side) polymers are usually obtained as highly crystalline materials. It is easier for the stereoregular polymers to pack into a crystal lattice since they are more ordered and the resulting crystallinity leads to higher physical strength and increased solvent and chemical resistance as well as differences in other properties that depend on crystallinity. The prime example of the industrial utility of stereoregular polymers is polypropene. Isotactic polypropene is a high-melting (165 °C), strong, crystalline polymer, which is used as both a plastic and fiber. Atactic polypropene is an amorphous material with an oily to waxy soft appearance that finds use in asphalt blends and formulations for lubricants, sealants, and adhesives, but the volumes are minuscule compared to that of isotactic polypropene.
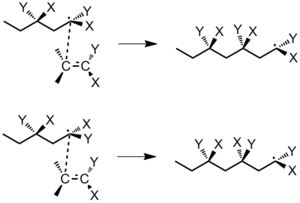
When a monomer adds to a radical chain end, there are two factors to consider regarding its stereochemistry: 1) the interaction between the terminal chain carbon and the approaching monomer molecule and 2) the configuration of the penultimate repeating unit in the polymer chain. The terminal carbon atom has sp2 hybridization and is planar. Consider the polymerization of the monomer CH2=CXY. There are two ways that a monomer molecule can approach the terminal carbon: the mirror approach (with like substituents on the same side) or the non-mirror approach (like substituents on opposite sides). If free rotation does not occur before the next monomer adds, the mirror approach will always lead to an isotactic polymer and the non-mirror approach will always lead to a syndiotactic polymer (Figure 25).
However, if interactions between the substituents of the penultimate repeating unit and the terminal carbon atom are significant, then conformational factors could cause the monomer to add to the polymer in a way that minimizes steric or electrostatic interaction (Figure 26).
Reactivity
Traditionally, the reactivity of monomers and radicals are assessed by the means of copolymerization data. The Q–e scheme, the most widely used tool for the semi-quantitative prediction of monomer reactivity ratios, was first proposed by Alfrey and Price in 1947. The scheme takes into account the intrinsic thermodynamic stability and polar effects in the transition state. A given radical and a monomer are considered to have intrinsic reactivities Pi and Qj, respectively. The polar effects in the transition state, the supposed permanent electric charge carried by that entity (radical or molecule), is quantified by the factor e, which is a constant for a given monomer, and has the same value for the radical derived from that specific monomer. For addition of monomer 2 to a growing polymer chain whose active end is the radical of monomer 1, the rate constant, k12, is postulated to be related to the four relevant reactivity parameters by
The monomer reactivity ratio for the addition of monomers 1 and 2 to this chain is given by
For the copolymerization of a given pair of monomers, the two experimental reactivity ratios r1 and r2 permit the evaluation of (Q1/Q2) and (e1 – e2). Values for each monomer can then be assigned relative to a reference monomer, usually chosen as styrene with the arbitrary values Q = 1.0 and e = –0.8.
Applications
Free radical polymerization has found applications including the manufacture of polystyrene, thermoplastic block copolymer elastomers, cardiovascular stents, chemical surfactants and lubricants. Block copolymers are used for a wide variety of applications including adhesives, footwear and toys.
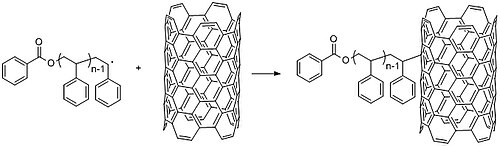
Free radical polymerization has uses in research as well, such as in the functionalization of carbon nanotubes. CNTs intrinsic electronic properties lead them to form large aggregates in solution, precluding useful applications. Adding small chemical groups to the walls of CNT can eliminate this propensity and tune the response to the surrounding environment. The use of polymers instead of smaller molecules can modify CNT properties (and conversely, nanotubes can modify polymer mechanical and electronic properties). For example, researchers coated carbon nanotubes with polystyrene by first polymerizing polystyrene via chain radical polymerization and subsequently mixing it at 130 °C with carbon nanotubes to generate radicals and graft them onto the walls of carbon nanotubes (Figure 27). Chain growth polymerization ("grafting to") synthesizes a polymer with predetermined properties. Purification of the polymer can be used to obtain a more uniform length distribution before grafting. Conversely, “grafting from”, with radical polymerization techniques such as atom transfer radical polymerization (ATRP) or nitroxide-mediated polymerization (NMP), allows rapid growth of high molecular weight polymers.
Radical polymerization also aids synthesis of nanocomposite hydrogels. These gels are made of water-swellable nano-scale clay (especially those classed as smectites) enveloped by a network polymer. They are often biocompatible and have mechanical properties (such as flexibility and strength) that promise applications such as synthetic tissue. Synthesis involves free radical polymerization. The general synthesis procedure is depicted in Figure 28. Clay is dispersed in water, where it forms very small, porous plates. Next the initiator and a catalyst are added, followed by adding the organic monomer, generally an acrylamide or acrylamide derivative. The initiator is chosen to have stronger interaction with the clay than the organic monomer, so it preferentially adsorbs to the clay surface. The mixture and water solvent is heated to initiate polymerization. Polymers grow off the initiators that are in turn bound to the clay. Due to recombination and disproportionation reactions, growing polymer chains bind to one another, forming a strong, cross-linked network polymer, with clay particles acting as branching points for multiple polymer chain segments. Free radical polymerization used in this context allows the synthesis of polymers from a wide variety of substrates (the chemistries of suitable clays vary). Termination reactions unique to chain growth polymerization produce a material with flexibility, mechanical strength and biocompatibility.
Electronics
The radical polymer glass PTMA is about 10 times more electrically conductive than common semiconducting polymers. PTMA is in a class of electrically active polymers that could find use in transparent solar cells, antistatic and antiglare coatings for mobile phone displays, antistatic coverings for aircraft to protect against lightning strikes, flexible flash drives, and thermoelectric devices, which convert electricity into heat and the reverse. Widespread practical applications require increasing conductivity another 100 to 1,000 times.
The polymer was created using deprotection, which involves replacing a specific hydrogen atom in the pendant group with an oxygen atom. The resulting oxygen atom in PTMA has one unpaired electron in its outer shell, making it amenable to transporting charge. The deprotection step can lead to four distinct chemical functionalities, two of which are promising for increasing conductivity.
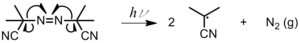

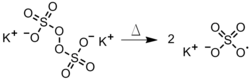







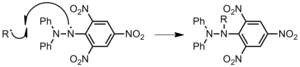

![{\frac {1}{x_{n}}}=\left({\frac {1}{x_{n}}}\right)_{o}+{\frac {k_{{tr}}[solvent]}{k_{p}[monomer]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/3af10ecbdc45b856a8cd76ecc9502f63fc6bdc03)

![v_{i}={\operatorname {d}[M\cdot ]/\operatorname {d}t}=2k_{d}f[I]](https://wikimedia.org/api/rest_v1/media/math/render/svg/f02573160d99ce28cd15dfe6c9f111e9c47c082d)
![v_{p}=k_{p}[M][M\cdot ]](https://wikimedia.org/api/rest_v1/media/math/render/svg/bc7bb155b647c8e9a58d7d300e71a52898b8f221)
![v_{t}={-\operatorname {d}[M\cdot ]/\operatorname {d}t}=2k_{t}[M\cdot ]^{2}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e451f440b60e6b1e9fbe627b99a0d6eb734aeea4)
![[M\cdot ]=\left({\frac {k_{d}[I]f}{k_{t}}}\right)^{{1/2}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/92af6e128be7fa6a566a3590c6f267e601e5e3e2)
![{\displaystyle v_{p}={k_{p}}\left({\frac {fk_{d}}{k_{t}}}\right)^{1/2}[I]^{1/2}[M]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/8fab2227059300c21a6173d5108ca221645da4cf)
![{\displaystyle \nu ={\frac {v_{p}}{v_{i}}}={\frac {k_{p}[M][M\cdot ]}{2fk_{d}[I]}}={\frac {k_{p}[M]}{2(fk_{d}k_{t}[I])^{1/2}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/60318ebbc0960f885949ea451e3692f7cebae026)



![{\displaystyle {\frac {1}{x_{n}}}={\frac {2k_{t,d}+k_{t,c}}{{k_{p}}^{2}[M]^{2}}}v_{p}+C_{M}+C_{S}{\frac {[S]}{[M]}}+C_{I}{\frac {[I]}{[M]}}+C_{P}{\frac {[P]}{[M]}}+C_{T}{\frac {[T]}{[M]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/f87872861546ef6fc87a203d04693d6898ca25d2)