From Wikipedia, the free encyclopedia
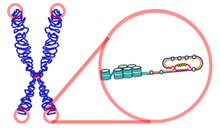
A telomere is a region of repetitive nucleotide sequences at each end of a chromatid, which protects the end of the chromosome from deterioration or from fusion with neighbouring chromosomes. Its name is derived from the Greek nouns telos (τέλος) 'end' and merοs (μέρος, root: μερ-) 'part.' For vertebrates, the sequence of nucleotides in telomeres is TTAGGG.
During chromosome replication, the enzymes that duplicate DNA cannot continue their duplication all the way to the end of a chromosome, so in each duplication the end of the chromosome is shortened[1] (this is because the synthesis of Okazaki fragments requires RNA primers attaching ahead on the lagging strand). The telomeres are disposable buffers at the ends of chromosomes which are truncated during cell division; their presence protects the genes before them on the chromosome from being truncated instead.
Over time, due to each cell division, the telomere ends become shorter.[2] They are replenished by an enzyme, telomerase reverse transcriptase.
Discovery
In the early 1970s, Russian theorist Alexei Olovnikov first recognized that chromosomes could not completely replicate their ends. Building on this, and to accommodate Leonard Hayflick's idea of limited somatic cell division, Olovnikov suggested that DNA sequences are lost every time a cell/DNA replicates until the loss reaches a critical level, at which point cell division ends.[3][4] However, Olovnikov's prediction was not widely known except by a handful of researchers studying cellular aging and immortalization.[5]In 1975–1977, Elizabeth Blackburn, working as a postdoctoral fellow at Yale University with Joseph Gall, discovered the unusual nature of telomeres, with their simple repeated DNA sequences composing chromosome ends. Their work was published in 1978.[6] Elizabeth Blackburn, Carol Greider, and Jack Szostak were awarded the 2009 Nobel Prize in Physiology or Medicine for the discovery of how chromosomes are protected by telomeres and the enzyme telomerase.[7]
Nevertheless, in the 1970s there was no recognition that the telomere-shortening mechanism normally limits cells to a fixed number of divisions, or animal studies suggesting that this is responsible for aging on the cellular level and sets a limit on lifespans.[8][9]
It remained for a privately funded collaboration from biotechnology company Geron to isolate the genes for the RNA and protein component of human telomerase in order to establish the causal role of telomere shortening in cellular aging and telomerase reactivation in cell immortalization.[10]
Nature and function
Structure, function and evolutionary biology
Telomeres are repetitive nucleotide sequences located at the termini of linear chromosomes of most eukaryotic organisms. For vertebrates, the sequence of nucleotides in telomeres is TTAGGG. Most prokaryotes, lacking this linear arrangement, do not have telomeres. Telomeres compensate for incomplete semi-conservative DNA replication at chromosomal ends. A protein complex known as shelterin serves as protection against double-strand break (DSB) repair by homologous recombination (HR) and non-homologous end joining (NHEJ).[11][12]In most prokaryotes, chromosomes are circular and, thus, do not have ends to suffer premature replication termination. A small fraction of bacterial chromosomes (such as those in Streptomyces and Borrelia) are linear and possess telomeres, which are very different from those of the eukaryotic chromosomes in structure and functions. The known structures of bacterial telomeres take the form of proteins bound to the ends of linear chromosomes, or hairpin loops of single-stranded DNA at the ends of the linear chromosomes.[13]
While replicating DNA, the eukaryotic DNA replication enzymes (the DNA polymerase protein complex) cannot replicate the sequences present at the ends of the chromosomes (or more precisely the chromatid fibres). Hence, these sequences and the information they carry may get lost. This is the reason telomeres are so important in context of successful cell division: They "cap" the end-sequences and themselves get lost in the process of DNA replication. But the cell has an enzyme called telomerase, which carries out the task of adding repetitive nucleotide sequences to the ends of the DNA. Telomerase, thus, "replenishes" the telomere "cap" of the DNA. In most multicellular eukaryotic organisms, telomerase is active only in germ cells, some types of stem cells such as embryonic stem cells, and certain white blood cells. Telomerase can be re activated and telomeres reset back to an embryonic state by somatic cell nuclear transfer.[14] There are theories that claim that the steady shortening of telomeres with each replication in somatic (body) cells may have a role in senescence and in the prevention of cancer. This is because the telomeres act as a sort of time-delay "fuse", eventually running out after a certain number of cell divisions and resulting in the eventual loss of vital genetic information from the cell's chromosome with future divisions.
Telomere length varies greatly between species, from approximately 300 base pairs in yeast[15] to many kilobases in humans, and usually is composed of arrays of guanine-rich, six- to eight-base-pair-long repeats. Eukaryotic telomeres normally terminate with 3′ single-stranded-DNA overhang, which is essential for telomere maintenance and capping. Multiple proteins binding single- and double-stranded telomere DNA have been identified.[16] These function in both telomere maintenance and capping. Telomeres form large loop structures called telomere loops, or T-loops. Here, the single-stranded DNA curls around in a long circle stabilized by telomere-binding proteins.[17] At the very end of the T-loop, the single-stranded telomere DNA is held onto a region of double-stranded DNA by the telomere strand disrupting the double-helical DNA and base pairing to one of the two strands. This triple-stranded structure is called a displacement loop or D-loop.[18]
Telomere shortening in humans can induce replicative senescence, which blocks cell division. This mechanism appears to prevent genomic instability and development of cancer in human aged cells by limiting the number of cell divisions. However, shortened telomeres impair immune function that might also increase cancer susceptibility.[19] If telomeres become too short, they have the potential to unfold from their presumed closed structure. The cell may detect this uncapping as DNA damage and then either stop growing, enter cellular old age (senescence), or begin programmed cell self-destruction (apoptosis) depending on the cell's genetic background (p53 status). Uncapped telomeres also result in chromosomal fusions. Since this damage cannot be repaired in normal somatic cells, the cell may even go into apoptosis. Many aging-related diseases are linked to shortened telomeres. Organs deteriorate as more and more of their cells die off or enter cellular senescence.
At the very distal end of the telomere is a 300 bp single-stranded portion, which forms the T-Loop. This loop is analogous to a knot, which stabilizes the telomere, preventing the telomere ends from being recognized as break points by the DNA repair machinery. Should non-homologous end joining occur at the telomeric ends, chromosomal fusion will result. The T-loop is held together by several proteins, the most notable ones being TRF1, TRF2, POT1, TIN1, and TIN2, collectively referred to as the shelterin complex. In humans, the shelterin complex consists of six proteins identified as TRF1, TRF2, TIN2, POT1, TPP1, and RAP1.[11]
Cancer, telomerase and ALT (alternative lengthening of telomeres)
Malignant cells that bypass this arrest become immortalized by telomere extension due mostly to the activation of telomerase (the reverse transcriptase enzyme responsible for synthesis of telomeres). Telomerase is a "ribonucleoprotein complex" composed of a protein component and an RNA primer sequence that acts to protect the terminal ends of chromosomes from being broken down by enzymes. The telomeres (and the actions of telomerase) are necessary because, during replication, DNA polymerase can synthesize DNA in only a 5' to 3' direction (each DNA strand having a polarity that is determined by the precise manner in which sugar molecules of the strand's "backbone" are linked together) and can do so only by adding nucleotides to RNA primers (that have already been placed at various points along the length of the DNA). The RNA strands are replaced with newly synthesized DNA, but DNA polymerase can only "backfill" deoxyribonucleotides if there is already DNA "upstream" from (i.e., located 5' to) the RNA primer. At the chromosome terminal, however, there is no nucleotide sequence in the 5' direction (and therefore no upstream RNA primer or DNA), so DNA polymerase cannot function and genetic sequence might be lost through chromosomal fraying.Chromosomal ends might also be processed as breaks in double-strand DNA with chromosome-to-chromosome telomere fusions resulting.
Telomeres at the end of DNA prevent the chromosome from growing shorter during replications (with loss of genetic information) by employing "telomerases" to synthesize DNA at the chromosome terminal. These include a protein subgroup of specialized reverse transcriptase enzymes known as TERT (TElomerase Reverse Transcriptases) and are involved in synthesis of telomeres in humans and many other, but not all, organisms. Because DNA replication mechanisms are affected by oxidative stress and because TERT expression is very low in most types of human cell, telomeres shrink a little bit every time a cell divides. Among cell types characterized by extensive cell division (such as stem cells and certain white blood cells), however, TERT is expressed at higher levels and telomere shortening is partially or fully prevented.

Structure of parallel quadruplexes that can be formed by human telomeric DNA. Image created from NDB UD0017.
In addition to its TERT protein component, telomerase also contains a piece of template RNA known as the TERC (TElomerase RNA Component) or TR (Telomerase RNA). In humans, this TERC telomere sequence is a repeating string of TTAGGG, between 3 and 20 kilobases in length. There are an additional 100-300 kilobases of telomere-associated repeats between the telomere and the rest of the chromosome. Telomere sequences vary from species to species, but, in general, one strand is rich in G with fewer Cs. These G-rich sequences can form four-stranded structures (G-quadruplexes), with sets of four bases held in plane and then stacked on top of each other, with either a sodium or a potassium ion between the planar quadruplexes.
Mammalian (and other) somatic cells without telomerase gradually lose telomeric sequences as a result of incomplete replication (Counter et al., 1992). As mammalian telomeres shorten, eventually cells reach their replicative limit and progress into senescence or old age. Senescence involves p53 and pRb pathways and leads to the halting of cell proliferation (Campisi, 2005). Senescence may play an important role in suppression of cancer emergence, although inheriting shorter telomeres probably does not protect against cancer.[19] With critically shortened telomeres, further cell proliferation can be achieved by inactivation of p53 and pRb pathways. Cells entering proliferation after inactivation of p53 and pRb pathways undergo crisis. Crisis is characterized by gross chromosomal rearrangements and genome instability, and almost all cells die.
However, 5–10% of human cancers activate the Alternative Lengthening of Telomeres (ALT) pathway, which relies on recombination-mediated elongation.[20] Rarely, cells emerge from crisis immortalized through telomere lengthening by either activated telomerase or ALT (Colgina and Reddel, 1999; Reddel and Bryan, 2003). The first description of an ALT cell line demonstrated that their telomeres are highly heterogeneous in length and predicted a mechanism involving recombination (Murnane et al., 1994). Subsequent studies have confirmed a role for recombination in telomere maintenance by ALT (Dunham et al., 2000), however the exact mechanism of this pathway is yet to be determined. ALT cells produce abundant t-circles, possible products of intratelomeric recombination and t-loop resolution (Tomaska et al., 2000; 2009; Cesare and Griffith, 2004; Wang et al., 2004).
Since shorter telomeres are thought to be a cause of poorer health and aging, this raises the question of why longer telomeres are not selected for to ameliorate these effects. A prominent explanation suggests that inheriting longer telomeres would cause increased cancer rates (e.g. Weinstein and Ciszek, 2002). However, a recent literature review and analysis [19] suggests this is unlikely, because shorter telomeres and telomerase inactivation is more often associated with increased cancer rates, and the mortality from cancer occurs late in life when the force of natural selection is very low. An alternative explanation to the hypothesis that long telomeres are selected against due to their cancer promoting effects is the "thrifty telomere" hypothesis, which suggests that the cellular proliferation effects of longer telomeres causes increased energy expenditures.[19] In environments of energetic limitation, shorter telomeres might be an energy sparing mechanism.
In healthy female breast, a proportion of cells called luminal progenitors that line the milk ducts have proliferative and differentiation potential and most of them contain critically short telomeres with DNA damage foci. These cells are believed to be the possible common cellular loci where cancers of the breast involving telomere dysregulation may arise.[21] The telomere shortening in these progenitors is not age dependent but is speculated to be basal to luminal epithelial differentiation program-dependent. Also, the telomerase activity are unusually high in these cells when isolated from younger women but decline with age.[22]
Shortening
Telomeres shorten in part because of the end replication problem that is exhibited during DNA replication in eukaryotes only. Because DNA replication does not begin at either end of the DNA strand, but starts in the center, and considering that all known DNA polymerases move in the 5' to 3' direction, one finds a leading and a lagging strand on the DNA molecule being replicated.
On the leading strand, DNA polymerase can make a complementary DNA strand without any difficulty because it goes from 5' to 3'. However, there is a problem going in the other direction on the lagging strand. To counter this, short sequences of RNA acting as primers attach to the lagging strand a short distance ahead of where the initiation site was. The DNA polymerase can start replication at that point and go to the end of the initiation site. This causes the formation of Okazaki fragments. More RNA primers attach further on the DNA strand and DNA polymerase comes along and continues to make a new DNA strand.
Eventually, the last RNA primer attaches, and DNA polymerase, RNA nuclease, and DNA ligase come along to convert the RNA (of the primers) to DNA and to seal the gaps in between the Okazaki fragments. But, in order to change RNA to DNA, there must be another DNA strand in front of the RNA primer. This happens at all the sites of the lagging strand, but it does not happen at the end where the last RNA primer is attached. Ultimately, that RNA is destroyed by enzymes that degrade any RNA left on the DNA. Thus, a section of the telomere is lost during each cycle of replication at the 5' end of the lagging strand's daughter.
However, in vitro studies have shown that telomeres are highly susceptible to oxidative stress, and Richter and Zglinicki presented evidence that oxidative stress-mediated DNA damage is an important determinant of telomere shortening.[23] Telomere shortening due to free radicals explains the difference between the estimated loss per division because of the end-replication problem (ca. 20 bp) and actual telomere shortening rates (50–100 bp), and has a greater absolute impact on telomere length than shortening caused by the end-replication problem. Population-based studies have also indicated an interaction between anti-oxidant intake and telomere length. In the Long Island Breast Cancer Study Project (LIBCSP), authors found a moderate increase in breast cancer risk among women with the shortest telomeres and lower dietary intake of beta carotene, vitamin C or E.[24] These results suggest that cancer risk due to telomere shortening may interact with other mechanisms of DNA damage, specifically oxidative stress.
Telomere shortening is associated with ageing, mortality and ageing-related diseases. In 2003, Richard Cawthon discovered that those with longer telomeres lead longer lives than those with short telomeres.[25] However, it is not known whether short telomeres are just a sign of cellular age or actually contribute to the aging process.[citation needed]
Lengthening
The phenomenon of limited cellular division was first observed by Leonard Hayflick, and is now referred to as the Hayflick limit.[26][27] Significant discoveries were subsequently made by a group of scientists organized at Geron Corporation by Geron's founder Michael D. West that tied telomere shortening with the Hayflick limit. This team included Calvin Harley, Bryant Villeponteau, Gregg Morin, William Andrews, Karen Chapman, as well as collaborators at the University of Colorado and the University of Texas Southwestern Medical Center at Dallas.[28] The cloning of the catalytic component of telomerase enabled experiments to test whether the expression of telomerase at levels sufficient to prevent telomere shortening was capable of immortalizing human cells. Telomerase was demonstrated in a 1998 publication in Science to be capable of extending cell lifespan, and now is well-recognized as capable of immortalizing human somatic cells.[29]It is becoming apparent that reversing shortening of telomeres through temporary activation of telomerase may be a potent means to slow aging. They reason that this would extend human life because it would extend the Hayflick limit. Three routes have been proposed to reverse telomere shortening: drugs, gene therapy, or metabolic suppression, so-called, torpor/hibernation. So far these ideas have not been proven in humans, but it has been demonstrated that telomere shortening is reversed in hibernation and aging is slowed (Turbill, et al. 2012 & 2013) and that hibernation prolongs life-span (Lyman et al. 1981). It has also been demonstrated that telomere extension has successfully reversed some signs of aging in laboratory mice [30][31] and the nematode worm species Caenorhabditis elegans.[32] It has been hypothesized that longer telomeres and especially telomerase activation might cause increased cancer (e.g. Weinstein and Ciszek, 2002). However, longer telomeres might also protect against cancer, because short telomeres are associated with cancer. It has also been suggested that longer telomeres might cause increased energy consumption.[19]
Techniques to extend telomeres could be useful for tissue engineering, because they might permit healthy, noncancerous mammalian cells to be cultured in amounts large enough to be engineering materials for biomedical repairs.
Two recent studies on long-lived seabirds demonstrate that the role of telomeres is far from being understood . In 2003, scientists observed that the telomeres of Leach's Storm-petrel (Oceanodroma leucorhoa) seem to lengthen with chronological age, the first observed instance of such behaviour of telomeres.[33] In 2006, Juola et al.[34] reported that in another unrelated, long-lived seabird species, the Great Frigatebird (Fregata minor), telomere length did decrease until at least c.40 years of age (i.e. probably over the entire lifespan), but the speed of decrease slowed down massively with increasing ages, and that rates of telomere length decrease varied strongly between individual birds. They concluded that in this species (and probably in frigatebirds and their relatives in general), telomere length could not be used to determine a bird's age sufficiently well. Thus, it seems that there is much more variation in the behavior of telomere length than initially believed.
Furthermore, Gomes et al. found, in a study of the comparative biology of mammalian telomeres, that telomere length of different mammalian species correlates inversely, rather than directly, with lifespan, and they concluded that the contribution of telomere length to lifespan remains controversial.[35] Harris et al. found little evidence that, in humans, telomere length is a significant biomarker of normal aging with respect to important cognitive and physical abilities.[36] Gilley and Blackburn tested whether cellular senescence in paramecium is caused by telomere shortening, and found that telomeres were not shortened during senescence.[37]
Sequences
Known, up-to-date telomere nucleotide sequences are listed in Telomerase Database website.| Group | Organism | Telomeric repeat (5' to 3' toward the end) |
|---|---|---|
| Vertebrates | Human, mouse, Xenopus | TTAGGG |
| Filamentous fungi | Neurospora crassa | TTAGGG |
| Slime moulds | Physarum, Didymium | TTAGGG |
| Dictyostelium | AG(1-8) | |
| Kinetoplastid protozoa | Trypanosoma, Crithidia | TTAGGG |
| Ciliate protozoa | Tetrahymena, Glaucoma | TTGGGG |
| Paramecium | TTGGG(T/G) | |
| Oxytricha, Stylonychia, Euplotes | TTTTGGGG | |
| Apicomplexan protozoa | Plasmodium | TTAGGG(T/C) |
| Higher plants | Arabidopsis thaliana | TTTAGGG |
| Green algae | Chlamydomonas | TTTTAGGG |
| Insects | Bombyx mori | TTAGG |
| Roundworms | Ascaris lumbricoides | TTAGGC |
| Fission yeasts | Schizosaccharomyces pombe | TTAC(A)(C)G(1-8) |
| Budding yeasts | Saccharomyces cerevisiae | TGTGGGTGTGGTG (from RNA template) or G(2-3)(TG)(1-6)T (consensus) |
| Saccharomyces castellii | TCTGGGTG | |
| Candida glabrata | GGGGTCTGGGTGCTG | |
| Candida albicans | GGTGTACGGATGTCTAACTTCTT | |
| Candida tropicalis | GGTGTA[C/A]GGATGTCACGATCATT | |
| Candida maltosa | GGTGTACGGATGCAGACTCGCTT | |
| Candida guillermondii | GGTGTAC | |
| Candida pseudotropicalis | GGTGTACGGATTTGATTAGTTATGT | |
| Kluyveromyces lactis | GGTGTACGGATTTGATTAGGTATGT |
Cancer
Telomeres are critical for maintaining genomic integrity and studies show that telomere dysfunction or shortening is commonly acquired during the process of tumor development.[38] Short telomeres can lead to genomic instability, chromosome loss and the formation of non-reciprocal translocations; and telomeres in tumor cells and their precursor lesions are significantly shorter than surrounding normal tissue.[39][40]Observational studies have found shortened telomeres in many cancers: including pancreatic, bone, prostate, bladder, lung, kidney, and head and neck. In addition, people with many types of cancer have been found to possess shorter leukocyte telomeres than healthy controls.[41] Recent meta-analyses suggest 1.4 to 3.0 fold increased risk of cancer for those with the shortest vs. longest telomeres.[42][43] However the increase risk varies by age, sex, tumor type and differences in lifestyle factors.
Some of the same lifestyle factors which increase risk of developing cancer have also been associated with shortened telomeres: including smoking, physical inactivity and diet high in refined sugars [44] Diet and physical activity influence inflammation and oxidative stress. These factors are known to influence telomere maintenance.[45] Psychologic stress has also been linked to accelerated cell aging, as reflected by decreased telomerase activity and short telomeres.[46] It has been suggested that a combination of lifestyle modifications, including healthy diet, exercise and stress reduction, have the potential to increase telomere length, reverse cellular aging, and reduce the risk for aging-related diseases. In a recent clinical trial for early-stage prostate cancer patients, comprehensive lifestyle changes resulted in a short-term increase in telomerase activity and long-term modification in telomere length.[47][48] Lifestyle modifications have the potential to naturally regulate telomere maintenance without promoting tumorgenesis, as traditional mechanisms of telomere lengthening involve the use of telomerase activating agents.
Cancer cells require a mechanism to maintain their telomeric DNA in order to continue dividing indefinitely (immortalization). A mechanism for telomere elongation or maintenance is one of the key steps in cellular immortalization and can be used as a diagnostic marker in the clinic. Telomerase, the enzyme complex responsible for elongating telomeres, is activated in approximately 90% of tumors. However, a sizeable fraction of cancerous cells employ alternative lengthening of telomeres (ALT),[49] a non-conservative telomere lengthening pathway involving the transfer of telomere tandem repeats between sister-chromatids.[50]
Telomerase is the natural enzyme that promotes telomere repair. It is active in stem cells, germ cells, hair follicles, and 90 percent of cancer cells, but its expression is low or absent in somatic cells. Telomerase functions by adding bases to the ends of the telomeres. Cells with sufficient telomerase activity are considered immortal in the sense that they can divide past the Hayflick limit without entering senescence or apoptosis. For this reason, telomerase is viewed as a potential target for anti-cancer drugs (such as Geron's Imetelstat currently in human clinical trials and telomestatin).[51]
Studies using knockout mice have demonstrated that the role of telomeres in cancer can both be limiting to tumor growth, as well as promote tumorigenesis, depending on the cell type and genomic context.[52][53]
Measurement
Several techniques are currently employed to assess average telomere length in eukaryotic cells. The most widely used method is the Terminal Restriction Fragment (TRF) southern blot,[54] which involves hybridization of a radioactive 32P-(TTAGGG)n oligonucleotide probe to Hinf / Rsa I digested genomic DNA embedded on a nylon membrane and subsequently exposed to autoradiographic film or phosphoimager screen. Another histochemical method, termed Q-FISH, involves fluorescent in situ hybridization (FISH).[55] Q-FISH, however, requires significant amounts of genomic DNA (2-20 micrograms) and labor that renders its use limited in large epidemiological studies. Some of these impediments have been overcome with a Real-Time PCR assay for telomere length and Flow-FISH. Real-time PCR assay involves determining the Telomere-to-Single Copy Gene (T/S)ratio,[56] which is demonstrated to be proportional to the average telomere length in a cell.Another technique, referred to as single telomere elongation length analysis (STELA), was developed in 2003 by Duncan Baird. This technique allows investigations that can target specific telomere ends, which is not possible with TRF analysis. However, due to this technique's being PCR-based, telomeres larger than 25Kb cannot be amplified and there is a bias towards shorter telomeres.
Telomere length is associated with the general health of an individual as well as certain diseases, beyond cancer.[57][58] While multiple companies offer telomere length measurement services,[59][60] the utility of these measurements for widespread clinical or personal use has been questioned by prominent scientists without financial interests in these companies.[61][62] Nobel Prize winner Elizabeth Blackburn, who was the co-founder of one of these companies and has prominently promoted the clinical utility of telomere length measures,[63] resigned from the company in June 2013 "owing to an impending change in the control of Telome Health".[64]




 years.
years. to
to  years from now
years from now
