From Wikipedia, the free encyclopedia
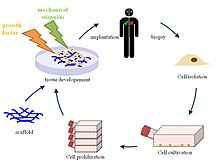
Tissue engineering is the use of a combination of cells, engineering and materials methods, and suitable biochemical and physicochemical factors to improve or replace biological functions. While it was once categorized as a sub-field of biomaterials, having grown in scope and importance it can be considered as a field in its own right.
While most definitions of tissue engineering cover a broad range of applications, in practice the term is closely associated with applications that repair or replace portions of or whole tissues (i.e., bone, cartilage, blood vessels, bladder, skin, muscle etc.). Often, the tissues involved require certain mechanical and structural properties for proper functioning. The term has also been applied to efforts to perform specific biochemical functions using cells within an artificially-created support system (e.g. an artificial pancreas, or a bio artificial liver). The term regenerative medicine is often used synonymously with tissue engineering, although those involved in regenerative medicine place more emphasis on the use of stem cells or progenitor cells to produce tissues.
Overview
A commonly applied definition of tissue engineering, as stated by Langer[1] and Vacanti,[2] is "an interdisciplinary field that applies the principles of engineering and life sciences toward the development of biological substitutes that restore, maintain, or improve tissue function or a whole organ".[3] Tissue engineering has also been defined as "understanding the principles of tissue growth, and applying this to produce functional replacement tissue for clinical use."[4] A further description goes on to say that an "underlying supposition of tissue engineering is that the employment of natural biology of the system will allow for greater success in developing therapeutic strategies aimed at the replacement, repair, maintenance, and/or enhancement of tissue function."[4]
Powerful developments in the multidisciplinary field of tissue engineering have yielded a novel set of tissue replacement parts and implementation strategies. Scientific advances in biomaterials, stem cells, growth and differentiation factors, and biomimetic environments have created unique opportunities to fabricate tissues in the laboratory from combinations of engineered extracellular matrices ("scaffolds"), cells, and biologically active molecules. Among the major challenges now facing tissue engineering is the need for more complex functionality, as well as both functional and biomechanical stability in laboratory-grown tissues destined for transplantation. The continued success of tissue engineering, and the eventual development of true human replacement parts, will grow from the convergence of engineering and basic research advances in tissue, matrix, growth factor, stem cell, and developmental biology, as well as materials science and bio informatics.
In 2003, the NSF published a report entitled "The Emergence of Tissue Engineering as a Research Field", which gives a thorough description of the history of this field.[5]
Examples
- Bioartificial windpipe : The first procedure of regenerative medicine of an implantation of a "bioartificial" organ.
- In vitro meat: Edible artificial animal muscle tissue cultured in vitro.
- Bioartificial liver device: several research efforts have produced hepatic assist devices utilizing living hepatocytes.
- Artificial pancreas: research involves using islet cells to produce and regulate insulin, particularly in cases of diabetes.
- Artificial bladders: Anthony Atala[6] (Wake Forest University) has successfully implanted artificially grown bladders into seven out of approximately 20 human test subjects as part of a long-term experiment.[7]
- Cartilage: lab-grown tissue was successfully used to repair knee cartilage.[8]
- Scaffold-free cartilage: Cartilage generated without the use of exogenous scaffold material. In this methodology, all material in the construct is cellular or material produced directly by the cells themselves.[9]
- Doris Taylor's heart in a jar
- Tissue-engineered airway[10]
- Tissue-engineered vessels[11]
- Artificial skin constructed from human skin cells embedded in a hydrogel, such as in the case of bioprinted constructs for battlefield burn repairs.[12]
- Artificial bone marrow[13]
- Artificial bone
- Artificial penis[14]
- Oral mucosa tissue engineering
- Foreskin[15]
Cells as building blocks
Tissue engineering utilizes living cells as engineering materials. Examples include using living fibroblasts in skin replacement or repair, cartilage repaired with living chondrocytes, or other types of cells used in other ways.
Cells became available as engineering materials when scientists at Geron Corp. discovered how to extend telomeres in 1998, producing immortalized cell lines.[citation needed] Before this, laboratory cultures of healthy, noncancerous mammalian cells would only divide a fixed number of times, up to the Hayflick limit.
Extraction
From fluid tissues such as blood, cells are extracted by bulk methods, usually centrifugation or apheresis. From solid tissues, extraction is more difficult. Usually the tissue is minced, and then digested with the enzymes trypsin or collagenase to remove the extracellular matrix (ECM) that holds the cells. After that, the cells are free floating, and extracted using centrifugation or apheresis.Digestion with trypsin is very dependent on temperature. Higher temperatures digest the matrix faster, but create more damage. Collagenase is less temperature dependent, and damages fewer cells, but takes longer and is a more expensive reagent.
Types of cells
Cells are often categorized by their source:
- Autologous cells are obtained from the same individual to which they will be reimplanted. Autologous cells have the fewest problems with rejection and pathogen transmission, however in some cases might not be available. For example in genetic disease suitable autologous cells are not available. Also very ill or elderly persons, as well as patients suffering from severe burns, may not have sufficient quantities of autologous cells to establish useful cell lines. Moreover since this category of cells needs to be harvested from the patient, there are also some concerns related to the necessity of performing such surgical operations that might lead to donor site infection or chronic pain. Autologous cells also must be cultured from samples before they can be used: this takes time, so autologous solutions may not be very quick. Recently there has been a trend towards the use of mesenchymal stem cells from bone marrow and fat. These cells can differentiate into a variety of tissue types, including bone, cartilage, fat, and nerve. A large number of cells can be easily and quickly isolated from fat, thus opening the potential for large numbers of cells to be quickly and easily obtained.
- Allogeneic cells come from the body of a donor of the same species. While there are some ethical constraints to the use of human cells for in vitro studies, the employment of dermal fibroblasts from human foreskin has been demonstrated to be immunologically safe and thus a viable choice for tissue engineering of skin.
- Xenogenic cells are these isolated from individuals of another species. In particular animal cells have been used quite extensively in experiments aimed at the construction of cardiovascular implants.
- Syngenic or isogenic cells are isolated from genetically identical organisms, such as twins, clones, or highly inbred research animal models.
- Primary cells are from an organism.
- Secondary cells are from a cell bank.
- Stem cells are undifferentiated cells with the ability to divide in culture and give rise to different forms of specialized cells. According to their source stem cells are divided into "adult" and "embryonic" stem cells, the first class being multipotent and the latter mostly pluripotent; some cells are totipotent, in the earliest stages of the embryo. While there is still a large ethical debate related with the use of embryonic stem cells, it is thought that another alternative source - induced stem cells may be useful for the repair of diseased or damaged tissues, or may be used to grow new organs.
Scaffolds
Cells are often implanted or 'seeded' into an artificial structure capable of supporting three-dimensional tissue formation. These structures, typically called scaffolds, are often critical, both ex vivo as well as in vivo, to recapitulating the in vivo milieu and allowing cells to influence their own microenvironments. Scaffolds usually serve at least one of the following purposes:- Allow cell attachment and migration
- Deliver and retain cells and biochemical factors
- Enable diffusion of vital cell nutrients and expressed products
- Exert certain mechanical and biological influences to modify the behaviour of the cell phase

This animation of a rotating Carbon nanotube shows its 3D structure. Carbon nanotubes are among the numerous candidates for tissue engineering scaffolds since they are biocompatible, resistant to biodegradation and can be functionalized with biomolecules. However, the possibility of toxicity with non-biodegradable nano-materials is not fully understood.[16]
To achieve the goal of tissue reconstruction, scaffolds must meet some specific requirements. A high porosity and an adequate pore size are necessary to facilitate cell seeding and diffusion throughout the whole structure of both cells and nutrients. Biodegradability is often an essential factor since scaffolds should preferably be absorbed by the surrounding tissues without the necessity of a surgical removal. The rate at which degradation occurs has to coincide as much as possible with the rate of tissue formation: this means that while cells are fabricating their own natural matrix structure around themselves, the scaffold is able to provide structural integrity within the body and eventually it will break down leaving the neotissue, newly formed tissue which will take over the mechanical load. Injectability is also important for clinical uses. Recent research on organ printing is showing how crucial a good control of the 3D environment is to ensure reproducibility of experiments and offer better results.
Materials
Many different materials (natural and synthetic, biodegradable and permanent) have been investigated. Most of these materials have been known in the medical field before the advent of tissue engineering as a research topic, being already employed as bioresorbable sutures. Examples of these materials are collagen and some polyesters.New biomaterials have been engineered to have ideal properties and functional customization: injectability, synthetic manufacture, biocompatibility, non-immunogenicity, transparency, nano-scale fibers, low concentration, resorption rates, etc. PuraMatrix, originating from the MIT labs of Zhang, Rich, Grodzinsky and Langer is one of these new biomimetic scaffold families which has now been commercialized and is impacting clinical tissue engineering.
A commonly used synthetic material is PLA - polylactic acid. This is a polyester which degrades within the human body to form lactic acid, a naturally occurring chemical which is easily removed from the body. Similar materials are polyglycolic acid (PGA) and polycaprolactone (PCL): their degradation mechanism is similar to that of PLA, but they exhibit respectively a faster and a slower rate of degradation compared to PLA.
Scaffolds may also be constructed from natural materials: in particular different derivatives of the extracellular matrix have been studied to evaluate their ability to support cell growth. Proteic materials, such as collagen or fibrin, and polysaccharidic materials, like chitosan[17] or glycosaminoglycans (GAGs), have all proved suitable in terms of cell compatibility, but some issues with potential immunogenicity still remains. Among GAGs hyaluronic acid, possibly in combination with cross linking agents (e.g. glutaraldehyde, water soluble carbodiimide, etc...), is one of the possible choices as scaffold material. Functionalized groups of scaffolds may be useful in the delivery of small molecules (drugs) to specific tissues. Another form of scaffold under investigation is decellularised tissue extracts whereby the remaining cellular remnants/extracellular matrices act as the scaffold.
A 2009 study by Ratmir et al. aimed to improve in vivo-like conditions for 3D tissue via "stacking and de-stacking layers of paper impregnated with suspensions of cells in extracellular matrix hydrogel, making it possible to control oxygen and nutrient gradients in 3D, and to analyze molecular and genetic responses".[18] It is possible to manipulate gradients of soluble molecules, and to characterize cells in these complex gradients more effectively than conventional 3D cultures based on hydrogels, cell spheroids, or 3D perfusion reactors.[19] Different thicknesses of paper and types of medium can support a variety of experimental environments. Upon deconstruction, these sheets can be useful in cell-based high-throughput screening and drug discovery.[19]
Synthesis
A number of different methods have been described in literature for preparing porous structures to be employed as tissue engineering scaffolds. Each of these techniques presents its own advantages, but none are free of drawbacks.
- Nanofiber Self-Assembly
- Molecular self-assembly is one of the few methods for creating biomaterials with properties similar in scale and chemistry to that of the natural in vivo extracellular matrix (ECM), a crucial step toward tissue engineering of complex tissues.[20] Moreover, these hydrogel scaffolds have shown superiority in in vivo toxicology and biocompatibility compared to traditional macroscaffolds and animal-derived materials.
- Textile technologies
- These techniques include all the approaches that have been successfully employed for the preparation of non-woven meshes of different polymers. In particular, non-woven polyglycolide structures have been tested for tissue engineering applications: such fibrous structures have been found useful to grow different types of cells. The principal drawbacks are related to the difficulties in obtaining high porosity and regular pore size.
- Solvent Casting & Particulate Leaching (SCPL)
- This approach allows for the preparation of structures with regular porosity, but with limited thickness. First, the polymer is dissolved into a suitable organic solvent (e.g. polylactic acid could be dissolved into dichloromethane), then the solution is cast into a mold filled with porogen particles. Such porogen can be an inorganic salt like sodium chloride, crystals of saccharose, gelatin spheres or paraffin spheres. The size of the porogen particles will affect the size of the scaffold pores, while the polymer to porogen ratio is directly correlated to the amount of porosity of the final structure. After the polymer solution has been cast the solvent is allowed to fully evaporate, then the composite structure in the mold is immersed in a bath of a liquid suitable for dissolving the porogen: water in the case of sodium chloride, saccharose and gelatin or an aliphatic solvent like hexane for use with paraffin. Once the porogen has been fully dissolved, a porous structure is obtained. Other than the small thickness range that can be obtained, another drawback of SCPL lies in its use of organic solvents which must be fully removed to avoid any possible damage to the cells seeded on the scaffold.
- Gas Foaming
- To overcome the need to use organic solvents and solid porogens, a technique using gas as a porogen has been developed. First, disc-shaped structures made of the desired polymer are prepared by means of compression molding using a heated mold. The discs are then placed in a chamber where they are exposed to high pressure CO2 for several days. The pressure inside the chamber is gradually restored to atmospheric levels. During this procedure the pores are formed by the carbon dioxide molecules that abandon the polymer, resulting in a sponge-like structure. The main problems resulting from such a technique are caused by the excessive heat used during compression molding (which prohibits the incorporation of any temperature labile material into the polymer matrix) and by the fact that the pores do not form an interconnected structure.
- Emulsification/Freeze-drying
- This technique does not require the use of a solid porogen like SCPL. First, a synthetic polymer is dissolved into a suitable solvent (e.g. polylactic acid in dichloromethane) then water is added to the polymeric solution and the two liquids are mixed in order to obtain an emulsion. Before the two phases can separate, the emulsion is cast into a mold and quickly frozen by means of immersion into liquid nitrogen. The frozen emulsion is subsequently freeze-dried to remove the dispersed water and the solvent, thus leaving a solidified, porous polymeric structure. While emulsification and freeze-drying allow for a faster preparation when compared to SCPL (since it does not require a time consuming leaching step), it still requires the use of solvents. Moreover, pore size is relatively small and porosity is often irregular. Freeze-drying by itself is also a commonly employed technique for the fabrication of scaffolds. In particular, it is used to prepare collagen sponges: collagen is dissolved into acidic solutions of acetic acid or hydrochloric acid that are cast into a mold, frozen with liquid nitrogen and then lyophilized.
- Thermally Induced Phase Separation (TIPS)
- Similar to the previous technique, this phase separation procedure requires the use of a solvent with a low melting point that is easy to sublime. For example dioxane could be used to dissolve polylactic acid, then phase separation is induced through the addition of a small quantity of water: a polymer-rich and a polymer-poor phase are formed. Following cooling below the solvent melting point and some days of vacuum-drying to sublime the solvent, a porous scaffold is obtained. Liquid-liquid phase separation presents the same drawbacks of emulsification/freeze-drying.
- Electrospinning
- A highly versatile technique that can be used to produce continuous fibers from submicrometer to nanometer diameters. In a typical electrospinning set-up, a solution is fed through a spinneret and a high voltage is applied to the tip. The buildup of electrostatic repulsion within the charged solution, causes it to eject a thin fibrous stream. A mounted collector plate or rod with an opposite or grounded charge draws in the continuous fibers, which arrive to form a highly porous network. The primary advantages of this technique are its simplicity and ease of variation. At a laboratory level, a typical electrospinning set-up only requires a high voltage power supply (up to 30 kV), a syringe, a flat tip needle and a conducting collector. For these reasons, electrospinning has become a common method of scaffold manufacture in many labs. By modifying variables such as the distance to collector, magnitude of applied voltage, or solution flow rate—researchers can dramatically change the overall scaffold architecture.
- CAD/CAM Technologies
- Because most of the above techniques are limited when it comes to the control of porosity and pore size, computer assisted design and manufacturing techniques have been introduced to tissue engineering. First, a three-dimensional structure is designed using CAD software. The porosity can be tailored using algorithms within the software.[21] The scaffold is then realized by using ink-jet printing of polymer powders or through Fused Deposition Modeling of a polymer melt.[22]
- Laser-assisted BioPrinting (LaBP)
- In a 2012 study,[24] Koch et al. focused on whether Laser-assisted BioPrinting (LaBP) can be used to build multicellular 3D patterns in natural matrix, and whether the generated constructs are functioning and forming tissue. LaBP arranges small volumes of living cell suspensions in set high-resolution patterns.[24] The investigation was successful, the researchers foresee that "generated tissue constructs might be used for in vivo testing by implanting them into animal models" (14). As of this study, only human skin tissue has been synthesized, though researchers project that "by integrating further cell types (e.g. melanocytes, Schwann cells, hair follicle cells) into the printed cell construct, the behavior of these cells in a 3D in vitro microenvironment similar to their natural one can be analyzed", useful for drug discovery and toxicology studies.[24]
Assembly methods
One of the continuing, persistent problems with tissue engineering is mass transport limitations. Engineered tissues generally lack an initial blood supply, thus making it difficult for any implanted cells to obtain sufficient oxygen and nutrients to survive, and/or function properly.Self-assembly
Self-assembly may play an important role here, both from the perspective of encapsulating cells and proteins, as well as creating scaffolds on the right physical scale for engineered tissue constructs and cellular ingrowth. The micromasonry is a prime technology to get cells grown in a lab to assemble into three-dimensional shapes. To break down tissue into single-cell building blocks, researchers have to dissolve the extracellular mortar that normally binds them together. But once that glue is removed, it's quite difficult to get cells to reassemble into the complex structures that make up our natural tissues. While cells aren't easily stackable, building blocks are. So the micromasonry starts with the encapsulation of living cells in polymer cubes. From there, the blocks self-assemble in any shape using templates.Liquid-based template assembly
The air-liquid surface established by Faraday waves is explored as a template to assemble biological entities for bottom-up tissue engineering. This liquid-based template can be dynamically reconfigured in a few seconds, and the assembly on the template can be achieved in a scalable and parallel manner. Assembly of microscale hydrogels, cells, neuron-seeded micro-carrier beads, cell spheroids into various symmetrical and periodic structures was demonstrated with good cell viability. Formation of 3D neural network was achieved after 14-day tissue culture.[25]Additive manufacturing
It might be possible to print organs, or possibly entire organisms using additive manufacturing techniques. A recent innovative method of construction uses an ink-jet mechanism to print precise layers of cells in a matrix of thermoreversible gel. Endothelial cells, the cells that line blood vessels, have been printed in a set of stacked rings. When incubated, these fused into a tube.[22][26]The field of three-dimensional and highly accurate models of biological systems is pioneered by multiple projects and technologies including a rapid method for creating tissues and even whole organs involves a 3D printer that can print the scaffolding and cells layer by layer into a working tissue sample or organ. The device is presented in a TED talk by Dr. Anthony Atala, M.D. the Director of the Wake Forest Institute for Regenerative Medicine, and the W.H. Boyce Professor and Chair of the Department of Urology at Wake Forest University, in which a full bladder is printed on stage during the seminar and then presented to the crowd.[27][28] This specific technology has been used to print a bladder for a young man that would have otherwise died without the availability of a transplant, and is anticipated to be able to print kidneys and perhaps livers in the future for transplantation and theoretically for toxicology and other biological studies as well.
Scaffolding
In 2013, using a 3-d scaffolding of Matrigel in various configurations, substantial pancreatic organoids was produced in vitro. Clusters of small numbers of cells proliferated into 40,000 cells within one week. The clusters transform into cells that make either digestive enzymes or hormones like insulin, self-organizing into branched pancreatic organoids that resemble the pancreas.[29]The cells are sensitive to the environment, such as gel stiffness and contact with other cells.
Individual cells do not thrive; a minimum of four proximate cells was required for subsequent organoid development. Modifications to the medium composition produced either hollow spheres mainly composed of pancreatic progenitors, or complex organoids that spontaneously undergo pancreatic morphogenesis and differentiation. Maintenance and expansion of pancreatic progenitors require active Notch and FGF signaling, recapitulating in vivo niche signaling interactions.[30]
The organoids were seen as potentially offering mini-organs for drug testing and for spare insulin-producing cells.[29]
Tissue culture
In many cases, creation of functional tissues and biological structures in vitro requires extensive culturing to promote survival, growth and inducement of functionality. In general, the basic requirements of cells must be maintained in culture, which include oxygen, pH, humidity, temperature, nutrients and osmotic pressure maintenance.Tissue engineered cultures also present additional problems in maintaining culture conditions. In standard cell culture, diffusion is often the sole means of nutrient and metabolite transport. However, as a culture becomes larger and more complex, such as the case with engineered organs and whole tissues, other mechanisms must be employed to maintain the culture, such as the creation of capillary networks within the tissue.
Another issue with tissue culture is introducing the proper factors or stimuli required to induce functionality. In many cases, simple maintenance culture is not sufficient. Growth factors, hormones, specific metabolites or nutrients, chemical and physical stimuli are sometimes required. For example, certain cells respond to changes in oxygen tension as part of their normal development, such as chondrocytes, which must adapt to low oxygen conditions or hypoxia during skeletal development. Others, such as endothelial cells, respond to shear stress from fluid flow, which is encountered in blood vessels. Mechanical stimuli, such as pressure pulses seem to be beneficial to all kind of cardiovascular tissue such as heart valves, blood vessels or pericardium.
Bioreactors
A bioreactor in tissue engineering, as opposed to industrial bioreactors, is a device that attempts to simulate a physiological environment in order to promote cell or tissue growth in vivo. A physiological environment can consist of many different parameters such as temperature and oxygen or carbon dioxide concentration, but can extend to all kinds of biological, chemical or mechanical stimuli. Therefore, there are systems that may include the application of forces or stresses to the tissue or even of electrical current in two- or three-dimensional setups.In academic and industry research facilities, it is typical for bioreactors to be developed to replicate the specific physiological environment of the tissue being grown (e.g., flex and fluid shearing for heart tissue growth).[31] Several general-use and application-specific bioreactors are also commercially available, and may provide static chemical stimulation or combination of chemical and mechanical stimulation.
The Bioreactors used for 3D cell cultures are small plastic cylindrical chambers with regulated internal humidity and moisture specifically engineered for the purpose of growing cells in three dimensions.[32] The bioreactor uses bioactive synthetic materials such as polyethylene terephthalate membranes to surround the spheroid cells in an environment that maintains high levels of nutrients.[19][33] They are easy to open and close, so that cell spheroids can be removed for testing, yet the chamber is able to maintain 100% humidity throughout.[34] This humidity is important to achieve maximum cell growth and function. The bioreactor chamber is part of a larger device that rotates to ensure equal cell growth in each direction across three dimensions.[34] MC2 Biotek has developed a bioreactor known as ProtoTissue[32] that uses gas exchange to maintain high oxygen levels within the cell chamber; improving upon previous bioreactors, because the higher oxygen levels help the cell grow and undergo normal cell respiration.[35]