Sunlight shining through clouds, giving rise to crepuscular rays
Photograph called Sunlight (1930s)
Sunlight is a portion of the electromagnetic radiation given off by the Sun, in particular infrared, visible, and ultraviolet light. On Earth, sunlight is filtered through Earth's atmosphere, and is obvious as daylight when the Sun is above the horizon. When the direct solar radiation is not blocked by clouds, it is experienced as sunshine, a combination of bright light and radiant heat. When it is blocked by clouds or reflects off other objects, it is experienced as diffused light. The World Meteorological Organization uses the term "sunshine duration" to mean the cumulative time during which an area receives direct irradiance from the Sun of at least 120 watts per square meter.[1] Other sources indicate an "Average over the entire earth" of "164 Watts per square meter over a 24 hour day".[2]
The ultraviolet radiation in sunlight has both positive and negative health effects, as it is both a principal source of vitamin D3 and a mutagen.
Sunlight takes about 8.3 minutes to reach Earth from the surface of the Sun. A photon starting at the center of the Sun and changing direction every time it encounters a charged particle would take between 10,000 and 170,000 years to get to the surface.[3]
Sunlight is a key factor in photosynthesis, the process used by plants and other autotrophic organisms to convert light energy, normally from the Sun, into chemical energy that can be used to fuel the organisms' activities.
Measurement
Researchers can measure the intensity of sunlight using a sunshine recorder, pyranometer, or pyrheliometer. To calculate the amount of sunlight reaching the ground, both the eccentricity of Earth's elliptic orbit and the attenuation by Earth's atmosphere have to be taken into account. The extraterrestrial solar illuminance (Eext), corrected for the elliptic orbit by using the day number of the year (dn), is given to a good approximation by[4]The solar illuminance constant (Esc), is equal to 128×103 lx. The direct normal illuminance (Edn), corrected for the attenuating effects of the atmosphere is given by:
The total amount of energy received at ground level from the Sun at the zenith depends on the distance to the Sun and thus on the time of year. It is about 3.3% higher than average in January and 3.3% lower in July (see below). If the extraterrestrial solar radiation is 1367 watts per square meter (the value when the Earth–Sun distance is 1 astronomical unit), then the direct sunlight at Earth's surface when the Sun is at the zenith is about 1050 W/m2, but the total amount (direct and indirect from the atmosphere) hitting the ground is around 1120 W/m2.[5] In terms of energy, sunlight at Earth's surface is around 52 to 55 percent infrared (above 700 nm), 42 to 43 percent visible (400 to 700 nm), and 3 to 5 percent ultraviolet (below 400 nm).[6] At the top of the atmosphere, sunlight is about 30% more intense, having about 8% ultraviolet (UV),[7] with most of the extra UV consisting of biologically damaging short-wave ultraviolet.[8]
Direct sunlight has a luminous efficacy of about 93 lumens per watt of radiant flux. This is higher than the efficacy (of source) of most artificial lighting (including fluorescent), which means using sunlight for illumination heats up a room less than using most forms of artificial lighting.
Multiplying the figure of 1050 watts per square metre by 93 lumens per watt indicates that bright sunlight provides an illuminance of approximately 98 000 lux (lumens per square meter) on a perpendicular surface at sea level. The illumination of a horizontal surface will be considerably less than this if the Sun is not very high in the sky. Averaged over a day, the highest amount of sunlight on a horizontal surface occurs in January at the South Pole (see insolation).
Dividing the irradiance of 1050 W/m2 by the size of the sun's disk in steradians gives an average radiance of 15.4 MW per square metre per steradian. (However, the radiance at the centre of the sun's disk is somewhat higher than the average over the whole disk due to limb darkening.) Multiplying this by π gives an upper limit to the irradiance which can be focused on a surface using mirrors: 48.5 MW/m2.
Composition and power
Solar irradiance spectrum above atmosphere and at surface. Extreme UV
and X-rays are produced (at left of wavelength range shown) but comprise
very small amounts of the Sun's total output power.
The spectrum of the Sun's solar radiation is close to that of a black body[9][10] with a temperature of about 5,800 K.[11] The Sun emits EM radiation across most of the electromagnetic spectrum. Although the Sun produces gamma rays as a result of the nuclear-fusion process, internal absorption and thermalization convert these super-high-energy photons to lower-energy photons before they reach the Sun's surface and are emitted out into space. As a result, the Sun does not emit gamma rays from this process, but it does emit gamma rays from solar flares.[12] The Sun also emits X-rays, ultraviolet, visible light, infrared, and even radio waves;[13] the only direct signature of the nuclear process is the emission of neutrinos.
Although the solar corona is a source of extreme ultraviolet and X-ray radiation, these rays make up only a very small amount of the power output of the Sun (see spectrum at right). The spectrum of nearly all solar electromagnetic radiation striking the Earth's atmosphere spans a range of 100 nm to about 1 mm (1,000,000 nm).[citation needed] This band of significant radiation power can be divided into five regions in increasing order of wavelengths:[14]
- Ultraviolet C or (UVC) range, which spans a range of 100 to 280 nm. The term ultraviolet refers to the fact that the radiation is at higher frequency than violet light (and, hence, also invisible to the human eye). Due to absorption by the atmosphere very little reaches Earth's surface. This spectrum of radiation has germicidal properties, as used in germicidal lamps.
- Ultraviolet B or (UVB) range spans 280 to 315 nm. It is also greatly absorbed by the Earth's atmosphere, and along with UVC causes the photochemical reaction leading to the production of the ozone layer. It directly damages DNA and causes sunburn, but is also required for vitamin D synthesis in the skin and fur of mammals.[15]
- Ultraviolet A or (UVA) spans 315 to 400 nm. This band was once[when?] held to be less damaging to DNA, and hence is used in cosmetic artificial sun tanning (tanning booths and tanning beds) and PUVA therapy for psoriasis. However, UVA is now known to cause significant damage to DNA via indirect routes (formation of free radicals and reactive oxygen species), and can cause cancer.[16]
- Visible range or light spans 380 to 780 nm. As the name suggests, this range is visible to the naked eye. It is also the strongest output range of the Sun's total irradiance spectrum.
- Infrared range that spans 700 nm to 1,000,000 nm (1 mm).
It comprises an important part of the electromagnetic radiation that
reaches Earth. Scientists divide the infrared range into three types on
the basis of wavelength:
- Infrared-A: 700 nm to 1,400 nm
- Infrared-B: 1,400 nm to 3,000 nm
- Infrared-C: 3,000 nm to 1 mm.
Published tables
Tables of direct solar radiation on various slopes from 0 to 60 degrees north latitude, in calories per square centimetre, issued in 1972 and published by Pacific Northwest Forest and Range Experiment Station, Forest Service, U.S. Department of Agriculture, Portland, Oregon, USA, appear on the web.[17]Solar constant

Solar irradiance spectrum at top of atmosphere, on a linear scale and plotted against wavenumber
The solar constant, a measure of flux density, is the amount of incoming solar electromagnetic radiation per unit area that would be incident on a plane perpendicular to the rays, at a distance of one astronomical unit (AU) (roughly the mean distance from the Sun to Earth). The "solar constant" includes all types of solar radiation, not just the visible light. Its average value was thought to be approximately 1366 W/m²,[18] varying slightly with solar activity, but recent recalibrations of the relevant satellite observations indicate a value closer to 1361 W/m² is more realistic.[19]
Total solar irradiance (TSI) and spectral solar irradiance (SSI) upon Earth
Total solar irradiance (TSI) – the amount of solar radiation received at the top of Earth's atmosphere – has been measured since 1978 by a series of overlapping NASA and ESA satellite experiments to be 1.361 kilowatts per square meter (kW/m²).[18][20][21][22] TSI observations are continuing today with the ACRIMSAT/ACRIM3, SOHO/VIRGO and SORCE/TIM satellite experiments.[23] Variation of TSI has been discovered on many timescales including the solar magnetic cycle [24] and many shorter periodic cycles.[25] TSI provides the energy that drives Earth's climate, so continuation of the TSI time series database is critical to understanding the role of solar variability in climate change.Spectral solar irradiance (SSI) – the spectral distribution of the TSI – has been monitored since 2003 by the SORCE Spectral Irradiance Monitor (SIM). It has been found that SSI at UV (ultraviolet) wavelength corresponds in a less clear, and probably more complicated fashion, with Earth's climate responses than earlier assumed, fueling broad avenues of new research in “the connection of the Sun and stratosphere, troposphere, biosphere, ocean, and Earth’s climate”.[26]
Intensity in the Solar System
Sunlight on Mars is dimmer than on Earth. This photo of a Martian sunset was imaged by Mars Pathfinder.
Different bodies of the Solar System receive light of an intensity inversely proportional to the square of their distance from Sun. A rough table comparing the amount of solar radiation received by each planet in the Solar System follows (from data in [1]):
| Planet or dwarf planet | distance (AU) | Solar radiation (W/m²) | ||
|---|---|---|---|---|
| Perihelion | Aphelion | maximum | minimum | |
| Mercury | 0.3075 | 0.4667 | 14,446 | 6,272 |
| Venus | 0.7184 | 0.7282 | 2,647 | 2,576 |
| Earth | 0.9833 | 1.017 | 1,413 | 1,321 |
| Mars | 1.382 | 1.666 | 715 | 492 |
| Jupiter | 4.950 | 5.458 | 55.8 | 45.9 |
| Saturn | 9.048 | 10.12 | 16.7 | 13.4 |
| Uranus | 18.38 | 20.08 | 4.04 | 3.39 |
| Neptune | 29.77 | 30.44 | 1.54 | 1.47 |
| Pluto | 29.66 | 48.87 | 1.55 | 0.57 |
The actual brightness of sunlight that would be observed at the surface depends also on the presence and composition of an atmosphere. For example, Venus's thick atmosphere reflects more than 60% of the solar light it receives. The actual illumination of the surface is about 14,000 lux, comparable to that on Earth "in the daytime with overcast clouds".[27]
Sunlight on Mars would be more or less like daylight on Earth during a slightly overcast day, and, as can be seen in the pictures taken by the rovers, there is enough diffuse sky radiation that shadows would not seem particularly dark. Thus, it would give perceptions and "feel" very much like Earth daylight. The spectrum on the surface is slightly redder than that on Earth, due to scattering by reddish dust in the Martian atmosphere.
For comparison, sunlight on Saturn is slightly brighter than Earth sunlight at the average sunset or sunrise (see daylight for comparison table). Even on Pluto, the sunlight would still be bright enough to almost match the average living room. To see sunlight as dim as full moonlight on Earth, a distance of about 500 AU (~69 light-hours) is needed; there are only a handful of objects in the Solar System known to orbit farther than such a distance, among them 90377 Sedna and (87269) 2000 OO67.
Surface illumination
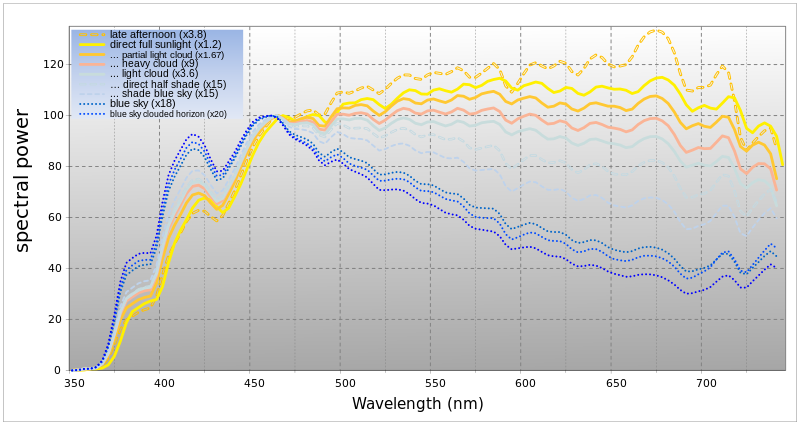
The spectrum of surface illumination depends upon solar elevation due to atmospheric effects, with the blue spectral component dominating during twilight before and after sunrise and sunset, respectively, and red dominating during sunrise and sunset. These effects are apparent in natural light photography where the principal source of illumination is sunlight as mediated by the atmosphere.While the color of the sky is usually determined by Rayleigh scattering, an exception occurs at sunset and twilight. "Preferential absorption of sunlight by ozone over long horizon paths gives the zenith sky its blueness when the sun is near the horizon".[28]
Spectral composition of sunlight at Earth's surface
The Sun's electromagnetic radiation which is received at the Earth's surface is predominantly light that falls within the range of wavelengths to which the visual systems of the animals that inhabit Earth's surface are sensitive. The Sun may therefore be said to illuminate, which is a measure of the light within a specific sensitivity range. Many animals (including humans) have a sensitivity range of approximately 400–700 nm,[29] and given optimal conditions the absorption and scattering by Earth's atmosphere produces illumination that approximates an equal-energy illuminant for most of this range.[30] The useful range for color vision in humans, for example, is approximately 450–650 nm. Aside from effects that arise at sunset and sunrise, the spectral composition changes primarily in respect to how directly sunlight is able to illuminate. When illumination is indirect, Rayleigh scattering in the upper atmosphere will lead blue wavelengths to dominate. Water vapour in the lower atmosphere produces further scattering and ozone, dust and water particles will also absorb selective wavelengths.[31][32]
Spectrum of the visible wavelengths at approximately sea level;
illumination by direct sunlight compared with direct sunlight scattered
by cloud cover and with indirect sunlight by varying degrees of cloud
cover. The yellow line shows the spectrum of direct illumination under
optimal conditions. The other illumination conditions are scaled to show
their relation to direct illumination. The units of spectral power are
simply raw sensor values (with a linear response at specific
wavelengths).
Variations in solar irradiance
Seasonal and orbital variation
On Earth, the solar radiation varies with the angle of the sun above the horizon, with longer sunlight duration at high latitudes during summer, varying to no sunlight at all in winter near the pertinent pole. When the direct radiation is not blocked by clouds, it is experienced as sunshine. The warming of the ground (and other objects) depends on the absorption of the electromagnetic radiation in the form of heat.The amount of radiation intercepted by a planetary body varies inversely with the square of the distance between the star and the planet. Earth's orbit and obliquity change with time (over thousands of years), sometimes forming a nearly perfect circle, and at other times stretching out to an orbital eccentricity of 5% (currently 1.67%). As the orbital eccentricity changes, the average distance from the sun (the semimajor axis does not significantly vary, and so the total insolation over a year remains almost constant due to Kepler's second law,
 is the "areal velocity" invariant. That is, the integration over the orbital period (also invariant) is a constant.
is the "areal velocity" invariant. That is, the integration over the orbital period (also invariant) is a constant.But the seasonal and latitudinal distribution and intensity of solar radiation received at Earth's surface does vary.[33] The effect of sun angle on climate results in the change in solar energy in summer and winter. For example, at latitudes of 65 degrees, this can vary by more than 25% as a result of Earth's orbital variation. Because changes in winter and summer tend to offset, the change in the annual average insolation at any given location is near zero, but the redistribution of energy between summer and winter does strongly affect the intensity of seasonal cycles. Such changes associated with the redistribution of solar energy are considered a likely cause for the coming and going of recent ice ages (see: Milankovitch cycles).
Solar intensity variation
Space-based observations of solar irradiance started in 1978. These measurements show that the solar constant is not constant. It varies on many time scales, including the 11-year sunspot solar cycle.[24] When going further back in time, one has to rely on irradiance reconstructions, using sunspots for the past 400 years or cosmogenic radionuclides for going back 10,000 years. Such reconstructions have been done.[34][35][36][37] These studies show that in addition to the solar irradiance variation with the solar cycle (the (Schwabe) cycle), the solar activitiy varies with longer cycles, such as the proposed 88 year (Gleisberg cycle), 208 year (DeVries cycle) and 1,000 year (Eddy cycle).Life on Earth
The existence of nearly all life on Earth is fueled by light from the Sun. Most autotrophs, such as plants, use the energy of sunlight, combined with carbon dioxide and water, to produce simple sugars—a process known as photosynthesis. These sugars are then used as building-blocks and in other synthetic pathways that allow the organism to grow.Heterotrophs, such as animals, use light from the Sun indirectly by consuming the products of autotrophs, either by consuming autotrophs, by consuming their products, or by consuming other heterotrophs. The sugars and other molecular components produced by the autotrophs are then broken down, releasing stored solar energy, and giving the heterotroph the energy required for survival. This process is known as cellular respiration.
In prehistory, humans began to further extend this process by putting plant and animal materials to other uses. They used animal skins for warmth, for example, or wooden weapons to hunt. These skills allowed humans to harvest more of the sunlight than was possible through glycolysis alone, and human population began to grow.
During the Neolithic Revolution, the domestication of plants and animals further increased human access to solar energy. Fields devoted to crops were enriched by inedible plant matter, providing sugars and nutrients for future harvests. Animals that had previously provided humans with only meat and tools once they were killed were now used for labour throughout their lives, fueled by grasses inedible to humans.
The more recent discoveries of coal, petroleum and natural gas are modern extensions of this trend. These fossil fuels are the remnants of ancient plant and animal matter, formed using energy from sunlight and then trapped within Earth for millions of years. Because the stored energy in these fossil fuels has accumulated over many millions of years, they have allowed modern humans to massively increase the production and consumption of primary energy. As the amount of fossil fuel is large but finite, this cannot continue indefinitely, and various theories exist as to what will follow this stage of human civilization (e.g., alternative fuels, Malthusian catastrophe, new urbanism, peak oil).
Cultural aspects
The effect of sunlight is relevant to painting, evidenced for instance in works of Claude Monet on outdoor scenes and landscapes.
Téli verőfény ("Winter Sunshine") by László Mednyánszky
Many people find direct sunlight to be too bright for comfort, especially when reading from white paper upon which the sun is directly shining. Indeed, looking directly at the sun can cause long-term vision damage. To compensate for the brightness of sunlight, many people wear sunglasses. Cars, many helmets and caps are equipped with visors to block the sun from direct vision when the sun is at a low angle. Sunshine is often blocked from entering buildings through the use of walls, window blinds, awnings, shutters, curtains, or nearby shade trees.
In colder countries, many people prefer sunnier days and often avoid the shade. In hotter countries, the converse is true; during the midday hours, many people prefer to stay inside to remain cool. If they do go outside, they seek shade that may be provided by trees, parasols, and so on.
In Hinduism, the sun is considered to be a god, as it is the source of life and energy on earth.
Sunbathing
Sunbathing is a popular leisure activity in which a person sits or lies in direct sunshine. People often sunbathe in comfortable places where there is ample sunlight. Some common places for sunbathing include beaches, open air swimming pools, parks, gardens, and sidewalk cafes. Sunbathers typically wear limited amounts of clothing or some simply go nude. For some, an alternative to sunbathing is the use of a sunbed that generates ultraviolet light and can be used indoors regardless of weather conditions. Tanning beds have been banned in a number of states in the world.For many people with light skin, one purpose for sunbathing is to darken one's skin color (get a sun tan), as this is considered in some cultures to be attractive, associated with outdoor activity, vacations/holidays, and health. Some people prefer naked sunbathing so that an "all-over" or "even" tan can be obtained, sometimes as part of a specific lifestyle.
For people suffering from psoriasis, sunbathing is an effective way of healing the symptoms.
Skin tanning is achieved by an increase in the dark pigment inside skin cells called melanocytes, and is an automatic response mechanism of the body to sufficient exposure to ultraviolet radiation from the sun or from artificial sunlamps. Thus, the tan gradually disappears with time, when one is no longer exposed to these sources.
Effects on human health
The ultraviolet radiation in sunlight has both positive and negative health effects, as it is both a principal source of vitamin D3 and a mutagen.[38] A dietary supplement can supply vitamin D without this mutagenic effect,[39] but bypasses natural mechanisms that would prevent overdoses of vitamin D generated internally from sunlight. Vitamin D has a wide range of positive health effects, which include strengthening bones[40] and possibly inhibiting the growth of some cancers.[41][42] Sun exposure has also been associated with the timing of melatonin synthesis, maintenance of normal circadian rhythms, and reduced risk of seasonal affective disorder.[43]Long-term sunlight exposure is known to be associated with the development of skin cancer, skin aging, immune suppression, and eye diseases such as cataracts and macular degeneration.[44] Short-term overexposure is the cause of sunburn, snow blindness, and solar retinopathy.
UV rays, and therefore sunlight and sunlamps, are the only listed carcinogens that are known to have health benefits,[45] and a number of public health organizations state that there needs to be a balance between the risks of having too much sunlight or too little.[46] There is a general consensus that sunburn should always be avoided.
Epidemiological data shows that people who have more exposure to the sun have less high blood pressure and cardiovascular-related mortality. While sunlight (and its UV rays) are a risk factor for skin cancer, "sun avoidance may carry more of a cost than benefit for over-all good health."[47] A study found that there is no evidence that UV reduces lifespan in contrast to other risk factors like smoking, alcohol and high blood pressure.[47]