From Wikipedia, the free encyclopedia
The
neutron is a
subatomic particle, symbol
n
or
n0, with no net
electric charge and a
mass slightly larger than that of a
proton. Protons and neutrons constitute the
nuclei of
atoms. Since protons and neutrons behave similarly within the nucleus, and each has a mass of approximately one
atomic mass unit, they are both referred to as
nucleons. Their properties and interactions are described by
nuclear physics.
The chemical and nuclear properties of the nucleus are determined by the number of protons, called the
atomic number, and the number of neutrons, called the
neutron number. The
atomic mass number is the total number of nucleons. For example,
carbon has atomic number 6, and its abundant
carbon-12 isotope has 6 neutrons, whereas its rare
carbon-13 isotope has 7 neutrons. Some elements occur in nature with only one
stable isotope, such as
fluorine. Other elements occur with many stable isotopes, such as
tin with ten stable isotopes.
Within the nucleus, protons and neutrons are bound together through the
nuclear force. Neutrons are required for the stability of nuclei, with the exception of the single-proton
hydrogen atom. Neutrons are produced copiously in
nuclear fission and
fusion. They are a primary contributor to the
nucleosynthesis of chemical elements within
stars through fission, fusion, and
neutron capture processes.
The neutron is essential to the production of nuclear power. In the decade after the neutron was discovered by
James Chadwick in 1932,
[6] neutrons were used to induce many different types of
nuclear transmutations. With the discovery of
nuclear fission in 1938,
[7]
it was quickly realized that, if a fission event produced neutrons,
each of these neutrons might cause further fission events, etc., in a
cascade known as a
nuclear chain reaction.
[8] These events and findings led to the first self-sustaining
nuclear reactor (
Chicago Pile-1, 1942) and the first
nuclear weapon (
Trinity, 1945).
Free neutrons, while not directly ionizing atoms, cause
ionizing radiation. As such they can be a biological hazard, depending upon dose.
[8] A small natural "neutron background" flux of free neutrons exists on Earth, caused by
cosmic ray showers, and by the natural radioactivity of spontaneously fissionable elements in the Earth's crust.
[9] Dedicated
neutron sources like
neutron generators,
research reactors and
spallation sources produce free neutrons for use in
irradiation and in
neutron scattering experiments.
Description
Atomic nuclei are formed by a number of
protons, Z the
atomic number, and a number of neutrons, N the
neutron number, bound together by the
nuclear force. The atomic number defines the
chemical properties of the atom, and the neutron number determines the
isotope or
nuclide.
[8] The terms isotope and nuclide are often used
synonymously,
but they refer to chemical and nuclear properties, respectively.
Strictly speaking, isotopes are two or more nuclides with the same
number of protons; nuclides with the same number of neutrons are called
isotones. The
atomic mass number, symbol A, equals Z+N. Nuclides with the same atomic mass number are called
isobars. The nucleus of the most common
isotope of the
hydrogen atom (with the
chemical symbol 1H) is a lone proton. The nuclei of the heavy hydrogen isotopes
deuterium (D or
2H) and
tritium (T or
3H)
contain one proton bound to one and two neutrons, respectively. All
other types of atomic nuclei are composed of two or more protons and
various numbers of neutrons. The most common nuclide of the common
chemical element
lead,
208Pb, has 82 protons and 126 neutrons, for example. The
table of nuclides comprises all the known nuclides. Even though it is not a chemical element, the neutron is included in this table.
[10]
The free neutron has a mass of 939,565,413.3
eV/c
2, or
1.674927471×10−27 kg, or
1.00866491588 u.
[3] The neutron has a mean square
radius of about
0.8×10−15 m, or 0.8
fm,
[11] and it is a
spin-½ fermion.
[12]
The neutron has no measurable electric charge. With its positive electric charge, the proton is directly influenced by
electric fields, whereas the neutron is unaffected by electric fields. The neutron has a
magnetic moment, however, so the neutron is influenced by
magnetic fields. The neutron's magnetic moment has a negative value, because its orientation is opposite to the neutron's spin.
[13]
A free neutron is unstable,
decaying to a proton,
electron and
antineutrino with a
mean lifetime of just under 15 minutes (
881.5±1.5 s).
[14] This
radioactive decay, known as
beta decay,
is possible because the mass of the neutron is slightly greater than
the proton. The free proton is stable. Neutrons or protons bound in a
nucleus can be stable or unstable, however, depending on the
nuclide. Beta decay, in which neutrons decay to protons, or vice versa, is governed by the
weak force, and it requires the emission or absorption of electrons and neutrinos, or their antiparticles.
Nuclear
fission caused by absorption of a neutron by uranium-235. The heavy
nuclide fragments into lighter components and additional neutrons.
Protons and neutrons behave almost identically under the influence of the nuclear force within the nucleus. The concept of
isospin,
in which the proton and neutron are viewed as two quantum states of the
same particle, is used to model the interactions of nucleons by the
nuclear or weak forces. Because of the strength of the nuclear force at
short distances, the
binding energy of nucleons is more than seven orders of magnitude larger than the electromagnetic energy binding electrons in atoms.
Nuclear reactions (such as
nuclear fission) therefore have an
energy density that is more than ten million times that of
chemical reactions. Because of the
mass–energy equivalence,
nuclear binding energies add or subtract from the mass of nuclei.
Ultimately, the ability of the nuclear force to store energy arising
from the electromagnetic repulsion of nuclear components is the basis
for most of the energy that makes nuclear reactors or bombs possible. In
nuclear fission, the absorption of a neutron by a heavy nuclide (e.g.,
uranium-235)
causes the nuclide to become unstable and break into light nuclides and
additional neutrons. The positively charged light nuclides then repel,
releasing electromagnetic
potential energy.
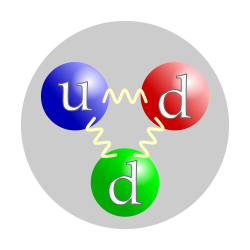
The neutron is classified as a
hadron, because it is a
composite particle made of
quarks. The neutron is also classified as a
baryon, because it is composed of three
valence quarks.
[15]
The finite size of the neutron and its magnetic moment indicates that
the neutron is a composite particle, as opposed to being an
elementary particle. A neutron contains two
down quarks with charge −
1⁄3 e and one
up quark with charge +
2⁄3 e.
Like protons, the quarks of the neutron are held together by the
strong force, mediated by
gluons.
[16] The nuclear force results from
secondary effects of the more fundamental strong force.
Discovery
The story of the discovery of the neutron and its properties is
central to the extraordinary developments in atomic physics that
occurred in the first half of the 20th century, leading ultimately to
the atomic bomb in 1945. In the 1911 Rutherford model, the atom
consisted of a small positively charged massive nucleus surrounded by a
much larger cloud of negatively charged electrons. In 1920, Rutherford
suggested that the nucleus consisted of positive protons and
neutrally-charged particles, suggested to be a proton and an electron
bound in some way.
[17] Electrons were assumed to reside within the nucleus because it was known that
beta radiation consisted of electrons emitted from the nucleus.
[17] Rutherford called these uncharged particles
neutrons, by the
Latin root for
neutralis (neuter) and the
Greek suffix
-on (a suffix used in the names of subatomic particles, i.e.
electron and
proton).
[18][19] References to the word
neutron in connection with the atom can be found in the literature as early as 1899, however.
[20]
Throughout the 1920s, physicists assumed that the atomic nucleus was composed of protons and "nuclear electrons"
[21][22] but there were obvious problems. It was difficult to reconcile the proton–electron model for nuclei with the
Heisenberg uncertainty relation of quantum mechanics.
[23][24] The
Klein paradox,
[25] discovered by
Oskar Klein in 1928, presented further quantum mechanical objections to the notion of an electron confined within a nucleus.
[23]
Observed properties of atoms and molecules were inconsistent with the
nuclear spin expected from the proton–electron hypothesis. Since both
protons and electrons carry an intrinsic spin of ½
ħ, there is no way to arrange an odd number of spins ±½
ħ to give a spin integer multiple of
ħ. Nuclei with integer spin are common, e.g.,
14N.
In 1931,
Walther Bothe and
Herbert Becker found that if
alpha particle radiation from
polonium fell on
beryllium,
boron, or
lithium,
an unusually penetrating radiation was produced. The radiation was not
influenced by an electric field, so Bothe and Becker assumed it was
gamma radiation.
[26][27] The following year
Irène Joliot-Curie and
Frédéric Joliot-Curie in Paris showed that if this "gamma" radiation fell on
paraffin, or any other
hydrogen-containing compound, it ejected protons of very high energy.
[28] Neither Rutherford nor
James Chadwick at the
Cavendish Laboratory in
Cambridge were convinced by the gamma ray interpretation.
[29]
Chadwick quickly performed a series of experiments that showed that the
new radiation consisted of uncharged particles with about the same mass
as the
proton.
[6][30][31] These particles were neutrons. Chadwick won the
Nobel Prize in Physics for this discovery in 1935.
[2]
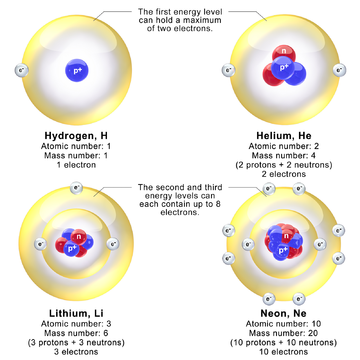
Models
depicting the nucleus and electron energy levels in hydrogen, helium,
lithium, and neon atoms. In reality, the diameter of the nucleus is
about 100,000 times smaller than the diameter of the atom.
Models for atomic nucleus consisting of protons and neutrons were quickly developed by
Werner Heisenberg[32][33][34] and others.
[35][36] The proton–neutron model explained the puzzle of nuclear spins. The origins of beta radiation were explained by
Enrico Fermi in 1934 by the
process of beta decay, in which the neutron decays to a proton by
creating an electron and a (as yet undiscovered)
neutrino.
[37] In 1935 Chadwick and his doctoral student
Maurice Goldhaber, reported the first accurate measurement of the mass of the neutron.
[38][39]
By 1934, Fermi had bombarded heavier elements with neutrons to
induce radioactivity in elements of high atomic number. In 1938, Fermi
received the Nobel Prize in Physics
"for his demonstrations of the
existence of new radioactive elements produced by neutron irradiation,
and for his related discovery of nuclear reactions brought about by slow neutrons".
[40] In 1938
Otto Hahn,
Lise Meitner, and
Fritz Strassmann discovered
nuclear fission, or the fractionation of uranium nuclei into light elements, induced by neutron bombardment.
[41][42][43] In 1945 Hahn received the 1944
Nobel Prize in Chemistry "for his discovery of the fission of heavy atomic nuclei."[44][45][46] The discovery of nuclear fission would lead to the development of nuclear power and the atomic bomb by the end of World War II.
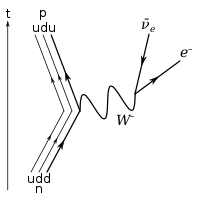
Beta decay and the stability of the nucleus
Under the
Standard Model of particle physics, the only possible decay mode for the neutron that
conserves baryon number is for one of the neutron's quarks to
change flavour via the
weak interaction. The decay of one of the neutron's down quarks into a lighter up quark can be achieved by the emission of a
W boson. By this process, the Standard Model description of
beta decay, the neutron decays into a
proton (which contains one down and two up quarks), an
electron, and an
electron antineutrino.
Since interacting protons have a mutual
electromagnetic repulsion that is stronger than their attractive
nuclear interaction, neutrons are a necessary constituent of any atomic nucleus that contains more than one proton (see
diproton and
neutron–proton ratio).
[47] Neutrons bind with protons and one another in the nucleus via the
nuclear force, effectively moderating the repulsive forces between the protons and stabilizing the nucleus.
Free neutron decay
Outside the nucleus, free neutrons are unstable and have a
mean lifetime of
881.5±1.5 s (about 14 minutes, 42 seconds); therefore the
half-life for this process (which differs from the mean lifetime by a factor of
ln(2) = 0.693) is
611.0±1.0 s (about 10 minutes, 11 seconds).
[14] Beta decay of the neutron, described above, can be denoted by the
radioactive decay:
[48]
n0 →
p+ +
e− +
ν
e
where
p+,
e−, and
ν
e denote the proton, electron and electron antineutrino, respectively.
For the free neutron the
decay energy
for this process (based on the masses of the neutron, proton, and
electron) is 0.782343 MeV. The maximal energy of the beta decay electron
(in the process wherein the neutrino receives a vanishingly small
amount of kinetic energy) has been measured at 0.782 ± 0.013 MeV.
[49] The latter number is not well-enough measured to determine the comparatively tiny rest mass of the
neutrino
(which must in theory be subtracted from the maximal electron kinetic
energy) as well as neutrino mass is constrained by many other methods.
A small fraction (about one in 1000) of free neutrons decay with
the same products, but add an extra particle in the form of an emitted
gamma ray:
n0 →
p+ +
e− +
ν
e +
γ
This gamma ray may be thought of as a sort of "internal
bremsstrahlung"
that arises as the emitted beta particle interacts with the charge of
the proton in an electromagnetic way. Internal bremsstrahlung gamma ray
production is also a minor feature of beta decays of bound neutrons (as
discussed below).
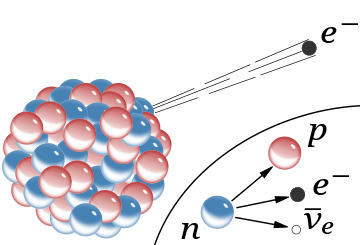
A
schematic of the
nucleus of an atom indicating
β−
radiation, the emission of a fast electron from the nucleus (the
accompanying antineutrino is omitted). In the Rutherford model for the
nucleus, red spheres were protons with positive charge and blue spheres
were protons tightly bound to an electron with no net charge.
The
inset shows beta decay of a free neutron as it is understood today; an electron and antineutrino are created in this process.
A very small minority of neutron decays (about four per million) are
so-called "two-body (neutron) decays", in which a proton, electron and
antineutrino are produced as usual, but the electron fails to gain the
13.6 eV necessary energy to escape the proton (the
ionization energy of
hydrogen), and therefore simply remains bound to it, as a neutral
hydrogen atom (one of the "two bodies"). In this type of free neutron decay, almost all of the neutron
decay energy
is carried off by the antineutrino (the other "body"). (The hydrogen
atom recoils with a speed of only about (decay energy)/(hydrogen rest
energy) times the speed of light, or 250 km/s.)
The transformation of a free proton to a neutron (plus a positron
and a neutrino) is energetically impossible, since a free neutron has a
greater mass than a free proton. But a high-energy collision of a
proton and an electron or neutrino can result in a neutron.
Bound neutron decay
While a free neutron has a half life of about 10.2 min, most neutrons within nuclei are stable. According to the
nuclear shell model, the protons and neutrons of a
nuclide are a
quantum mechanical system organized into discrete
energy levels with unique
quantum numbers.
For a neutron to decay, the resulting proton requires an available
state at lower energy than the initial neutron state. In stable nuclei
the possible lower energy states are all filled, meaning they are each
occupied by two protons with
spin up and spin down. The
Pauli exclusion principle
therefore disallows the decay of a neutron to a proton within stable
nuclei. The situation is similar to electrons of an atom, where
electrons have distinct
atomic orbitals and are prevented from decaying to lower energy states, with the emission of a
photon, by the exclusion principle.
Neutrons in unstable nuclei can decay by
beta decay
as described above. In this case, an energetically allowed quantum
state is available for the proton resulting from the decay. One example
of this decay is
carbon-14 (6 protons, 8 neutrons) that decays to
nitrogen-14 (7 protons, 7 neutrons) with a half-life of about 5,730 years.
Inside a nucleus, a proton can transform into a neutron via
inverse beta decay, if an energetically allowed quantum state is available for the neutron. This transformation occurs by emission of a
positron and an electron
neutrino:
p+ →
n0 +
e+ +
ν
e
The transformation of a proton to a neutron inside of a nucleus is also possible through
electron capture:
p+ +
e− →
n0 +
ν
e
Positron capture by neutrons in nuclei that contain an excess of
neutrons is also possible, but is hindered because positrons are
repelled by the positive nucleus, and quickly
annihilate when they encounter electrons.
Competition of beta decay types
Three types of beta decay in competition are illustrated by the single isotope
copper-64
(29 protons, 35 neutrons), which has a half-life of about 12.7 hours.
This isotope has one unpaired proton and one unpaired neutron, so either
the proton or the neutron can decay. This particular nuclide is almost
equally likely to undergo proton decay (by
positron emission, 18% or by
electron capture, 43%) or neutron decay (by electron emission, 39%).
Intrinsic properties
Mass
The mass of a neutron cannot be directly determined by
mass spectrometry due to lack of electric charge. However, since the masses of a proton and of a
deuteron
can be measured with a mass spectrometer, the mass of a neutron can be
deduced by subtracting proton mass from deuteron mass, with the
difference being the mass of the neutron plus the
binding energy of deuterium (expressed as a positive emitted energy). The latter can be directly measured by measuring the energy (

) of the single
0.7822 MeV
gamma photon emitted when neutrons are captured by protons (this is
exothermic and happens with zero-energy neutrons), plus the small recoil
kinetic energy (

) of the deuteron (about 0.06% of the total energy).

The energy of the gamma ray can be measured to high precision by
X-ray diffraction techniques, as was first done by Bell and Elliot in
1948. The best modern (1986) values for neutron mass by this technique
are provided by Greene, et al.
[50] These give a neutron mass of:
- mneutron= 1.008644904(14) u
The value for the neutron mass in MeV is less accurately known, due to less accuracy in the known conversion of
u to MeV:
[51]
- mneutron= 939.56563(28) MeV/c2.
Another method to determine the mass of a neutron starts from the
beta decay of the neutron, when the momenta of the resulting proton and
electron are measured.
Electric charge
The total electric charge of the neutron is
0 e. This zero value has been tested experimentally, and the present experimental limit for the charge of the neutron is
−2(8)×10−22 e,
[4] or
−3(13)×10−41 C. This value is consistent with zero, given the experimental
uncertainties (indicated in parentheses). By comparison, the charge of the proton is
+1 e.
Magnetic moment
Even though the neutron is a neutral particle, the magnetic moment of
a neutron is not zero. The neutron is not affected by electric fields,
but it is affected by magnetic fields. The magnetic moment of the
neutron is an indication of its quark substructure and internal charge
distribution.
[52]
The value for the neutron's magnetic moment was first directly measured by
Luis Alvarez and
Felix Bloch at
Berkeley, California, in 1940,
[53]
using an extension of the magnetic resonance methods developed by Rabi.
Alvarez and Bloch determined the magnetic moment of the neutron to be
μn= −1.93(2) μN, where
μN is the
nuclear magneton.
In the
quark model for
hadrons, the neutron is composed of one up quark (charge +2/3
e) and two down quarks (charge −1/3
e).
[52] The magnetic moment of the neutron can be modeled as a sum of the magnetic moments of the constituent quarks.
[54]
The calculation assumes that the quarks behave like pointlike Dirac
particles, each having their own magnetic moment. Simplistically, the
magnetic moment of the neutron can be viewed as resulting from the
vector sum of the three quark magnetic moments, plus the orbital
magnetic moments caused by the movement of the three charged quarks
within the neutron.
In one of the early successes of the Standard Model (
SU(6) theory, now understood in terms of quark behavior), in 1964 Mirza A. B. Beg,
Benjamin W. Lee, and
Abraham Pais
theoretically calculated the ratio of proton to neutron magnetic
moments to be −3/2, which agrees with the experimental value to within
3%.
[55][56][57] The measured value for this ratio is
−1.45989805(34).
[3] A contradiction of the
quantum mechanical basis of this calculation with the
Pauli exclusion principle, led to the discovery of the
color charge for quarks by
Oscar W. Greenberg in 1964.
[55]
The above treatment compares neutrons with protons, allowing the
complex behavior of quarks to be subtracted out between models, and
merely exploring what the effects would be of differing quark charges
(or quark type). Such calculations are enough to show that the interior
of neutrons is very much like that of protons, save for the difference
in quark composition with a down quark in the neutron replacing an up
quark in the proton.
Attempts have been made to quantitatively recover the neutron magnetic moment from first principles. From the
nonrelativistic, quantum mechanical
wavefunction for
baryons
composed of three quarks, a straightforward calculation gives fairly
accurate estimates for the magnetic moments of neutrons, protons, and
other baryons.
[54] For a neutron, the end result of this calculation is that the magnetic moment of the neutron is given by
μn= 4/3 μd − 1/3 μu, where
μd and
μu
are the magnetic moments for the down and up quarks, respectively. This
result combines the intrinsic magnetic moments of the quarks with their
orbital magnetic moments, and assumes the three quarks are in a
particular, dominant quantum state.
| Baryon
|
Magnetic moment
of quark model
|
Computed
( ) )
|
Observed
( ) )
|
| p
|
4/3 μu − 1/3 μd
|
2.79
|
2.793
|
| n
|
4/3 μd − 1/3 μu
|
−1.86
|
−1.913
|
The results of this calculation are encouraging, but the masses of
the up or down quarks were assumed to be 1/3 the mass of a nucleon.
[54] The masses of the quarks are actually only about 1% that of a nucleon.
[58] The discrepancy stems from the complexity of the Standard Model for nucleons, where most of their mass originates in the
gluon fields, virtual particles, and their associated energy that are essential aspects of the
strong force.
[58][59] Furthermore, the complex system of quarks and gluons that constitute a neutron requires a relativistic treatment.
[60] The nucleon magnetic moment has been successfully computed numerically from
first principles,
however, including all the effects mentioned and using more realistic
values for the quark masses. The calculation gave results that were in
fair agreement with measurement, but it required significant computing
resources.
[61][62]
Spin
The neutron is a spin 1/2 particle, that is, it is a
fermion with intrinsic angular momentum equal to 1/2
ħ, where
ħ is the
reduced Planck constant. For many years after the discovery of the neutron, its exact spin was ambiguous. Although it was assumed to be a spin 1/2
Dirac particle,
the possibility that the neutron was a spin 3/2 particle lingered. The
interactions of the neutron's magnetic moment with an external magnetic
field were exploited to finally determine the spin of the neutron.
[63]
In 1949, Hughes and Burgy measured neutrons reflected from a
ferromagnetic mirror and found that the angular distribution of the
reflections was consistent with spin 1/2.
[64] In 1954, Sherwood, Stephenson, and Bernstein employed neutrons in a
Stern–Gerlach experiment
that used a magnetic field to separate the neutron spin states. They
recorded two such spin states, consistent with a spin 1/2 particle.
[63][65]
As a fermion, the neutron is subject to the
Pauli exclusion principle; two neutrons cannot have the same quantum numbers. This is the source of the
degeneracy pressure which makes
neutron stars possible.
Structure and geometry of charge distribution
An
article published in 2007 featuring a model-independent analysis
concluded that the neutron has a negatively charged exterior, a
positively charged middle, and a negative core.
[66]
In a simplified classical view, the negative "skin" of the neutron
assists it to be attracted to the protons with which it interacts in the
nucleus. (However, the main attraction between neutrons and protons is
via the
nuclear force, which does not involve charge.)
The simplified classical view of the neutron's charge
distribution also "explains" the fact that the neutron magnetic dipole
points in the opposite direction from its spin angular momentum vector
(as compared to the proton). This gives the neutron, in effect, a
magnetic moment which resembles a negatively charged particle. This can
be reconciled classically with a neutral neutron composed of a charge
distribution in which the negative sub-parts of the neutron have a
larger average radius of distribution, and therefore contribute more to
the particle's magnetic dipole moment, than do the positive parts that
are, on average, nearer the core.
Electric dipole moment
The
Standard Model of particle physics predicts a tiny separation of positive and negative charge within the neutron leading to a permanent
electric dipole moment.
[67] The predicted value is, however, well below the current sensitivity of experiments. From several
unsolved puzzles in particle physics,
it is clear that the Standard Model is not the final and full
description of all particles and their interactions. New theories going
beyond the Standard Model
generally lead to much larger predictions for the electric dipole
moment of the neutron. Currently, there are at least four experiments
trying to measure for the first time a finite neutron electric dipole
moment, including:
Anti-neutron
The antineutron is the
antiparticle of the neutron. It was discovered by
Bruce Cork in the year 1956, a year after the
antiproton was discovered.
CPT-symmetry
puts strong constraints on the relative properties of particles and
antiparticles, so studying antineutrons yields provide stringent tests
on CPT-symmetry. The fractional difference in the masses of the neutron
and antineutron is
(9±6)×10−5. Since the difference is only about two
standard deviations away from zero, this does not give any convincing evidence of CPT-violation.
[14]
Neutron compounds
Dineutrons and tetraneutrons
The existence of stable clusters of 4 neutrons, or
tetraneutrons,
has been hypothesised by a team led by Francisco-Miguel Marqués at the
CNRS Laboratory for Nuclear Physics based on observations of the
disintegration of
beryllium-14 nuclei. This is particularly interesting because current theory suggests that these clusters should not be stable.
In February 2016, Japanese physicist Susumu Shimoura of
the University of Tokyo and co-workers reported they had observed the purported tetraneutrons for the first time experimentally.
[73]
Nuclear physicists around the world say this discovery, if confirmed,
would be a milestone in the field of nuclear physics and certainly would
deepen our understanding of the nuclear forces.
[74][75]
The
dineutron
is another hypothetical particle. In 2012, Artemis Spyrou from Michigan
State University and coworkers reported that they observed, for the
first time, the dineutron emission in the decay of
16Be. The
dineutron character is evidenced by a small emission angle between the
two neutrons. The authors measured the two-neutron separation energy to
be 1.35(10) MeV, in good agreement with shell model calculations, using
standard interactions for this mass region.
[76]
Neutronium and neutron stars
At extremely high pressures and temperatures, nucleons and electrons
are believed to collapse into bulk neutronic matter, called
neutronium. This is presumed to happen in
neutron stars.
The extreme pressure inside a neutron star may deform the neutrons into a cubic symmetry, allowing tighter packing of neutrons.
[77]
Detection
The common means of detecting a
charged particle by looking for a track of ionization (such as in a
cloud chamber)
does not work for neutrons directly. Neutrons that elastically scatter
off atoms can create an ionization track that is detectable, but the
experiments are not as simple to carry out; other means for detecting
neutrons, consisting of allowing them to interact with atomic nuclei,
are more commonly used. The commonly used methods to detect neutrons can
therefore be categorized according to the nuclear processes relied
upon, mainly
neutron capture or
elastic scattering.
[78]
Neutron detection by neutron capture
A common method for detecting neutrons involves converting the energy released from
neutron capture reactions into electrical signals. Certain nuclides have a high neutron capture
cross section,
which is the probability of absorbing a neutron. Upon neutron capture,
the compound nucleus emits more easily detectable radiation, for example
an alpha particle, which is then detected. The nuclides
3He
,
6Li
,
10B
,
233U
,
235U
,
237Np
, and
239Pu
are useful for this purpose.
Neutron detection by elastic scattering
Neutrons
can elastically scatter off nuclei, causing the struck nucleus to
recoil. Kinematically, a neutron can transfer more energy to a light
nucleus such as hydrogen or helium than to a heavier nucleus. Detectors
relying on elastic scattering are called fast neutron detectors.
Recoiling nuclei can ionize and excite further atoms through collisions.
Charge and/or scintillation light produced in this way can be collected
to produce a detected signal. A major challenge in fast neutron
detection is discerning such signals from erroneous signals produced by
gamma radiation in the same detector.
Fast neutron detectors have the advantage of not requiring a
moderator, and are therefore capable of measuring the neutron's energy,
time of arrival, and in certain cases direction of incidence.
Sources and production
Free neutrons are unstable, although they have the longest half-life
of any unstable subatomic particle by several orders of magnitude. Their
half-life is still only about 10 minutes, however, so they can be
obtained only from sources that produce them continuously.
Natural neutron background. A small natural background
flux of free neutrons exists everywhere on Earth. In the atmosphere and
deep into the ocean, the "neutron background" is caused by
muons produced by
cosmic ray
interaction with the atmosphere. These high-energy muons are capable of
penetration to considerable depths in water and soil. There, in
striking atomic nuclei, among other reactions they induce spallation
reactions in which a neutron is liberated from the nucleus. Within the
Earth's crust a second source is neutrons produced primarily by
spontaneous fission of uranium and thorium present in crustal minerals.
The neutron background is not strong enough to be a biological hazard,
but it is of importance to very high resolution particle detectors that
are looking for very rare events, such as (hypothesized) interactions
that might be caused by particles of
dark matter.
[9] Recent research has shown that even thunderstorms can produce neutrons with energies of up to several tens of MeV.
[79] Recent research has shown that the fluence of these neutrons lies between 10
−9 and 10
−13 per ms and per m
2
depending on the detection altitude. The energy of most of these
neutrons, even with initial energies of 20 MeV, decreases down to the
keV range within 1 ms.
[80]
Even stronger neutron background radiation is produced at the
surface of Mars, where the atmosphere is thick enough to generate
neutrons from cosmic ray muon production and neutron-spallation, but not
thick enough to provide significant protection from the neutrons
produced. These neutrons not only produce a Martian surface neutron
radiation hazard from direct downward-going neutron radiation but may
also produce a significant hazard from reflection of neutrons from the
Martian surface, which will produce reflected neutron radiation
penetrating upward into a Martian craft or habitat from the floor.
[81]
Sources of neutrons for research. These include certain types of
radioactive decay (
spontaneous fission and
neutron emission), and from certain
nuclear reactions.
Convenient nuclear reactions include tabletop reactions such as natural
alpha and gamma bombardment of certain nuclides, often beryllium or
deuterium, and induced
nuclear fission,
such as occurs in nuclear reactors. In addition, high-energy nuclear
reactions (such as occur in cosmic radiation showers or accelerator
collisions) also produce neutrons from disintegration of target nuclei.
Small (tabletop)
particle accelerators optimized to produce free neutrons in this way, are called
neutron generators.
In practice, the most commonly used small laboratory sources of
neutrons use radioactive decay to power neutron production. One noted
neutron-producing
radioisotope,
californium-252 decays (half-life 2.65 years) by
spontaneous fission 3% of the time with production of 3.7 neutrons per fission, and is used alone as a neutron source from this process.
Nuclear reaction sources (that involve two materials) powered by radioisotopes use an
alpha decay source plus a beryllium target, or else a source of high-energy gamma radiation from a source that undergoes
beta decay followed by
gamma decay, which produces
photoneutrons on interaction of the high-energy
gamma ray with ordinary stable beryllium, or else with the
deuterium in
heavy water. A popular
source of the latter type is radioactive
antimony-124
plus beryllium, a system with a half-life of 60.9 days, which can be
constructed from natural antimony (which is 42.8% stable antimony-123)
by activating it with neutrons in a nuclear reactor, then transported to
where the neutron source is needed.
[82]
Nuclear fission reactors naturally produce free neutrons; their role is to sustain the energy-producing
chain reaction. The intense
neutron radiation can also be used to produce various radioisotopes through the process of
neutron activation, which is a type of
neutron capture.
Experimental
nuclear fusion reactors
produce free neutrons as a waste product. However, it is these neutrons
that possess most of the energy, and converting that energy to a useful
form has proved a difficult engineering challenge. Fusion reactors that
generate neutrons are likely to create radioactive waste, but the waste
is composed of neutron-activated lighter isotopes, which have
relatively short (50–100 years) decay periods as compared to typical
half-lives of 10,000 years
[citation needed] for fission waste, which is long due primarily to the long half-life of alpha-emitting transuranic actinides.
[83]
Neutron beams and modification of beams after production
Free neutron beams are obtained from
neutron sources by
neutron transport. For access to intense neutron sources, researchers must go to a specialized
neutron facility that operates a
research reactor or a
spallation source.
The neutron's lack of total electric charge makes it difficult to
steer or accelerate them. Charged particles can be accelerated,
decelerated, or deflected by
electric or
magnetic fields.
These methods have little effect on neutrons. However, some effects may
be attained by use of inhomogeneous magnetic fields because of the
neutron's magnetic moment. Neutrons can be controlled by methods that include
moderation,
reflection, and
velocity selection.
Thermal neutrons can be polarized by transmission through
magnetic materials in a method analogous to the
Faraday effect for
photons. Cold neutrons of wavelengths of 6–7 angstroms can be produced in beams of a high degree of polarization, by use of
magnetic mirrors and magnetized interference filters.
[84]
Applications
The neutron plays an important role in many nuclear reactions. For example, neutron capture often results in
neutron activation, inducing
radioactivity. In particular, knowledge of neutrons and their behavior has been important in the development of
nuclear reactors and
nuclear weapons. The
fissioning of elements like
uranium-235 and
plutonium-239 is caused by their absorption of neutrons.
Cold, thermal, and hot neutron radiation is commonly employed in
neutron scattering facilities, where the radiation is used in a similar way one uses
X-rays for the analysis of
condensed matter. Neutrons are complementary to the latter in terms of atomic contrasts by different scattering
cross sections; sensitivity to magnetism; energy range for inelastic neutron spectroscopy; and deep penetration into matter.
The development of "neutron lenses" based on total internal
reflection within hollow glass capillary tubes or by reflection from
dimpled aluminum plates has driven ongoing research into neutron
microscopy and neutron/gamma ray tomography.
[85][86][87]
A major use of neutrons is to excite delayed and prompt
gamma rays from elements in materials. This forms the basis of
neutron activation analysis (NAA) and
prompt gamma neutron activation analysis (PGNAA). NAA is most often used to analyze small samples of materials in a
nuclear reactor whilst PGNAA is most often used to analyze subterranean rocks around
bore holes and industrial bulk materials on conveyor belts.
Another use of neutron emitters is the detection of light nuclei,
in particular the hydrogen found in water molecules. When a fast
neutron collides with a light nucleus, it loses a large fraction of its
energy. By measuring the rate at which slow neutrons return to the probe
after reflecting off of hydrogen nuclei, a
neutron probe may determine the water content in soil.
Medical therapies
Because neutron radiation is both penetrating and ionizing, it can be
exploited for medical treatments. Neutron radiation can have the
unfortunate side-effect of leaving the affected area radioactive,
however.
Neutron tomography is therefore not a viable medical application.
Fast neutron therapy utilizes high-energy neutrons typically greater than 20 MeV to treat cancer.
Radiation therapy
of cancers is based upon the biological response of cells to ionizing
radiation. If radiation is delivered in small sessions to damage
cancerous areas, normal tissue will have time to repair itself, while
tumor cells often cannot.
[88] Neutron radiation can deliver energy to a cancerous region at a rate an order of magnitude larger than
gamma radiation[89]
Beams of low-energy neutrons are used in
boron capture therapy
to treat cancer. In boron capture therapy, the patient is given a drug
that contains boron and that preferentially accumulates in the tumor to
be targeted. The tumor is then bombarded with very low-energy neutrons
(although often higher than thermal energy) which are captured by the
boron-10 isotope in the boron, which produces an excited state of boron-11 that then decays to produce
lithium-7 and an
alpha particle
that have sufficient energy to kill the malignant cell, but
insufficient range to damage nearby cells. For such a therapy to be
applied to the treatment of cancer, a neutron source having an intensity
of the order of a thousand million (10
9) neutrons per second per cm
2 is preferred. Such fluxes require a research nuclear reactor.
Protection
Exposure
to free neutrons can be hazardous, since the interaction of neutrons
with molecules in the body can cause disruption to
molecules and
atoms, and can also cause reactions that give rise to other forms of
radiation
(such as protons). The normal precautions of radiation protection
apply: Avoid exposure, stay as far from the source as possible, and keep
exposure time to a minimum. Some particular thought must be given to
how to protect from neutron exposure, however. For other types of
radiation, e.g.,
alpha particles,
beta particles, or
gamma rays, material of a high atomic number and with high density make for good shielding; frequently,
lead
is used. However, this approach will not work with neutrons, since the
absorption of neutrons does not increase straightforwardly with atomic
number, as it does with alpha, beta, and gamma radiation. Instead one
needs to look at the particular interactions neutrons have with matter
(see the section on detection above). For example,
hydrogen-rich
materials are often used to shield against neutrons, since ordinary
hydrogen both scatters and slows neutrons. This often means that simple
concrete blocks or even paraffin-loaded plastic blocks afford better
protection from neutrons than do far more dense materials. After
slowing, neutrons may then be absorbed with an isotope that has high
affinity for slow neutrons without causing secondary capture radiation,
such as lithium-6.
Hydrogen-rich
ordinary water affects neutron absorption in
nuclear fission reactors: Usually, neutrons are so strongly absorbed by normal water that fuel enrichment with fissionable isotope is required. The
deuterium in
heavy water
has a very much lower absorption affinity for neutrons than does
protium (normal light hydrogen). Deuterium is, therefore, used in
CANDU-type reactors, in order to slow (
moderate) neutron velocity, to increase the probability of
nuclear fission compared to
neutron capture.
Neutron temperature
Thermal neutrons
A
thermal neutron is a
free neutron that is
Boltzmann distributed with kT=
0.0253 eV (
4.0×10−21 J)
at room temperature. This gives characteristic (not average, or median)
speed of 2.2 km/s. The name 'thermal' comes from their energy being
that of the room temperature gas or material they are permeating. (see
kinetic theory
for energies and speeds of molecules). After a number of collisions
(often in the range of 10–20) with nuclei, neutrons arrive at this
energy level, provided that they are not absorbed.
In many substances, thermal neutron reactions show a much larger
effective cross-section than reactions involving faster neutrons, and
thermal neutrons can therefore be absorbed more readily (i.e., with
higher probability) by any
atomic nuclei that they collide with, creating a heavier — and often
unstable —
isotope of the
chemical element as a result.
Most
fission reactors use a
neutron moderator to slow down, or
thermalize the neutrons that are emitted by
nuclear fission so that they are more easily captured, causing further fission. Others, called
fast breeder reactors, use fission energy neutrons directly.
Cold neutrons
Cold neutrons are thermal neutrons that have been equilibrated in a very cold substance such as liquid
deuterium. Such a
cold source is placed in the moderator of a research reactor or spallation source. Cold neutrons are particularly valuable for
neutron scattering experiments.
[citation needed]
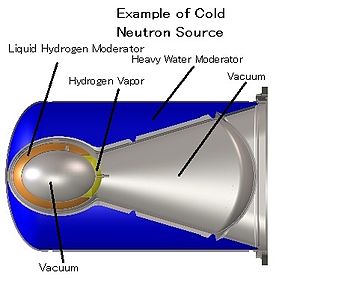
Cold neutron source providing neutrons at about the temperature of liquid hydrogen
Ultracold neutrons
Ultracold neutrons are produced by elastically scattering cold neutrons in substances with a temperature of a few kelvins, such as solid
deuterium or superfluid
helium. An alternative production method is the mechanical deceleration of cold neutrons.
Fission energy neutrons
A
fast neutron is a free neutron with a kinetic energy level close to
1 MeV (
1.6×10−13 J), hence a speed of ~
14000 km/s (~ 5% of the speed of light). They are named
fission energy or
fast
neutrons to distinguish them from lower-energy thermal neutrons, and
high-energy neutrons produced in cosmic showers or accelerators. Fast
neutrons are produced by nuclear processes such as
nuclear fission. Neutrons produced in fission, as noted above, have a
Maxwell–Boltzmann distribution of kinetic energies from 0 to ~14 MeV, a mean energy of 2 MeV (for U-235 fission neutrons), and a
mode
of only 0.75 MeV, which means that more than half of them do not
qualify as fast (and thus have almost no chance of initiating fission in
fertile materials, such as U-238 and Th-232).
Fast neutrons can be made into thermal neutrons via a process called moderation. This is done with a
neutron moderator. In reactors, typically
heavy water,
light water, or
graphite are used to moderate neutrons.
Fusion neutrons
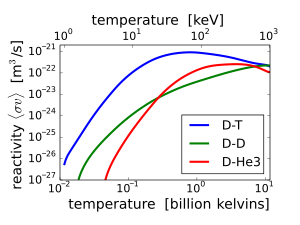
The
fusion reaction rate increases rapidly with temperature until it
maximizes and then gradually drops off. The DT rate peaks at a lower
temperature (about 70 keV, or 800 million kelvins) and at a higher value
than other reactions commonly considered for fusion energy.
D–T (
deuterium–
tritium) fusion is the
fusion reaction that produces the most energetic neutrons, with 14.1
MeV of
kinetic energy and traveling at 17% of the
speed of light.
D–T fusion is also the easiest fusion reaction to ignite, reaching
near-peak rates even when the deuterium and tritium nuclei have only a
thousandth as much kinetic energy as the 14.1 MeV that will be produced.
14.1 MeV neutrons have about 10 times as much energy as fission neutrons, and are very effective at fissioning even non-
fissile heavy nuclei,
and these high-energy fissions produce more neutrons on average than
fissions by lower-energy neutrons. This makes D–T fusion neutron sources
such as proposed
tokamak power reactors useful for
transmutation of transuranic waste. 14.1 MeV neutrons can also produce neutrons by
knocking them loose from nuclei.
On the other hand, these very high-energy neutrons are less likely to simply
be captured without causing fission or spallation. For these reasons,
nuclear weapon design extensively utilizes D–T fusion 14.1 MeV neutrons to
cause more fission. Fusion neutrons are able to cause fission in ordinarily non-fissile materials, such as
depleted uranium (uranium-238), and these materials have been used in the jackets of
thermonuclear weapons.
Fusion neutrons also can cause fission in substances that are
unsuitable or difficult to make into primary fission bombs, such as
reactor grade plutonium. This physical fact thus causes ordinary non-weapons grade materials to become of concern in certain
nuclear proliferation discussions and treaties.
Other fusion reactions produce much less energetic neutrons. D–D fusion produces a 2.45 MeV neutron and
helium-3 half of the time, and produces
tritium and a proton but no neutron the rest of the time. D–
3He fusion produces no neutron.
Intermediate-energy neutrons
A fission energy neutron that has slowed down but not yet reached thermal energies is called an epithermal neutron.
Cross sections for both
capture and
fission reactions often have multiple
resonance peaks at specific energies in the epithermal energy range.
These are of less significance in a
fast neutron reactor, where most neutrons are absorbed before slowing down to this range, or in a well-
moderated thermal reactor, where epithermal neutrons interact mostly with moderator nuclei, not with either
fissile or
fertile actinide
nuclides.
However, in a partially moderated reactor with more interactions of
epithermal neutrons with heavy metal nuclei, there are greater
possibilities for
transient changes in
reactivity that might make reactor control more difficult.
Ratios of capture reactions to fission reactions are also worse (more captures without fission) in most
nuclear fuels such as
plutonium-239,
making epithermal-spectrum reactors using these fuels less desirable,
as captures not only waste the one neutron captured but also usually
result in a
nuclide that is not
fissile with thermal or epithermal neutrons, though still
fissionable with fast neutrons. The exception is
uranium-233 of the
thorium cycle, which has good capture-fission ratios at all neutron energies.
High-energy neutrons
High-energy neutrons have much more energy than fission energy neutrons and are generated as secondary particles by
particle accelerators or in the atmosphere from
cosmic rays. These high-energy neutrons are extremely efficient at
ionization and far more likely to cause
cell death than
X-rays or protons.