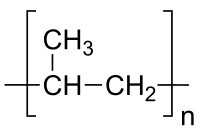A diamond cuboctahedron showing seven crystallographic planes, imaged with scanning electron microscopy
The interdisciplinary field of materials science, also commonly termed materials science and engineering is the design and discovery of new materials, particularly solids. The intellectual origins of materials science stem from the Enlightenment, when researchers began to use analytical thinking from chemistry, physics, and engineering to understand ancient, phenomenological observations in metallurgy and mineralogy. Materials science still incorporates elements of physics, chemistry, and engineering. As such, the field was long considered by academic institutions as a sub-field of these related fields. Beginning in the 1940s, materials science began to be more widely recognized as a specific and distinct field of science and engineering, and major technical universities around the world created dedicated schools of the study, within either the Science or Engineering schools, hence the naming.
- Materials science is a syncretic discipline hybridizing metallurgy, ceramics, solid-state physics, and chemistry. It is the first example of a new academic discipline emerging by fusion rather than fission.[3]
Materials scientists emphasize understanding how the history of a material (its processing) influences its structure, and thus the material's properties and performance. The understanding of processing-structure-properties relationships is called the § materials paradigm. This paradigm is used to advance understanding in a variety of research areas, including nanotechnology, biomaterials, and metallurgy. Materials science is also an important part of forensic engineering and failure analysis - investigating materials, products, structures or components which fail or do not function as intended, causing personal injury or damage to property. Such investigations are key to understanding, for example, the causes of various aviation accidents and incidents.
History
A late Bronze Age sword or dagger blade.
The material of choice of a given era is often a defining point. Phrases such as Stone Age, Bronze Age, Iron Age, and Steel Age are historic, if arbitrary examples. Originally deriving from the manufacture of ceramics and its putative derivative metallurgy, materials science is one of the oldest forms of engineering and applied science. Modern materials science evolved directly from metallurgy, which itself evolved from mining and (likely) ceramics and earlier from the use of fire. A major breakthrough in the understanding of materials occurred in the late 19th century, when the American scientist Josiah Willard Gibbs demonstrated that the thermodynamic properties related to atomic structure in various phases are related to the physical properties of a material. Important elements of modern materials science are a product of the space race: the understanding and engineering of the metallic alloys, and silica and carbon materials, used in building space vehicles enabling the exploration of space. Materials science has driven, and been driven by, the development of revolutionary technologies such as rubbers, plastics, semiconductors, and biomaterials.
Before the 1960s (and in some cases decades after), many materials science departments were named metallurgy departments, reflecting the 19th and early 20th century emphasis on metals. The growth of materials science in the United States was catalyzed in part by the Advanced Research Projects Agency, which funded a series of university-hosted laboratories in the early 1960s "to expand the national program of basic research and training in the materials sciences."[6] The field has since broadened to include every class of materials, including ceramics, polymers, semiconductors, magnetic materials, medical implant materials, biological materials, and nanomaterials, with modern materials classed within 3 distinct groups: Ceramic, Metal or Polymer. The prominent change in materials science during the last two decades is active usage of computer simulation methods to find new compounds, predict various properties, and as a result design new materials at a much greater rate than previous years.
Fundamentals
The materials paradigm represented in the form of a tetrahedron.
A material is defined as a substance (most often a solid, but other condensed phases can be included) that is intended to be used for certain applications.[7] There are a myriad of materials around us—they can be found in anything from buildings to spacecraft. Materials can generally be further divided into two classes: crystalline and non-crystalline. The traditional examples of materials are metals, semiconductors, ceramics and polymers.[8] New and advanced materials that are being developed include nanomaterials, biomaterials,[9] and energy materials to name a few.
The basis of materials science involves studying the structure of materials, and relating them to their properties. Once a materials scientist knows about this structure-property correlation, they can then go on to study the relative performance of a material in a given application. The major determinants of the structure of a material and thus of its properties are its constituent chemical elements and the way in which it has been processed into its final form. These characteristics, taken together and related through the laws of thermodynamics and kinetics, govern a material's microstructure, and thus its properties.
Structure
As mentioned above, structure is one of the most important components of the field of materials science. Materials science examines the structure of materials from the atomic scale, all the way up to the macro scale. Characterization is the way materials scientists examine the structure of a material. This involves methods such as diffraction with X-rays, electrons, or neutrons, and various forms of spectroscopy and chemical analysis such as Raman spectroscopy, energy-dispersive spectroscopy (EDS), chromatography, thermal analysis, electron microscope analysis, etc. Structure is studied at various levels, as detailed below.Atomic structure
This deals with the atoms of the materials, and how they are arranged to give molecules, crystals, etc. Much of the electrical, magnetic and chemical properties of materials arise from this level of structure. The length scales involved are in angstroms. The way in which the atoms and molecules are bonded and arranged is fundamental to studying the properties and behavior of any material.Nanostructure
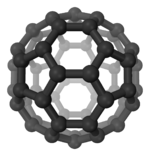
Buckminsterfullerene nanostructure.
Nanostructure deals with objects and structures that are in the 1—100 nm range.[10] In many materials, atoms or molecules agglomerate together to form objects at the nanoscale. This causes many interesting electrical, magnetic, optical, and mechanical properties.
In describing nanostructures it is necessary to differentiate between the number of dimensions on the nanoscale. Nanotextured surfaces have one dimension on the nanoscale, i.e., only the thickness of the surface of an object is between 0.1 and 100 nm. Nanotubes have two dimensions on the nanoscale, i.e., the diameter of the tube is between 0.1 and 100 nm; its length could be much greater. Finally, spherical nanoparticles have three dimensions on the nanoscale, i.e., the particle is between 0.1 and 100 nm in each spatial dimension. The terms nanoparticles and ultrafine particles (UFP) often are used synonymously although UFP can reach into the micrometre range. The term 'nanostructure' is often used when referring to magnetic technology. Nanoscale structure in biology is often called ultrastructure.
Materials which atoms and molecules form constituents in the nanoscale (i.e., they form nanostructure) are called nanomaterials. Nanomaterials are subject of intense research in the materials science community due to the unique properties that they exhibit.
Microstructure
Microstructure of pearlite.
Microstructure is defined as the structure of a prepared surface or thin foil of material as revealed by a microscope above 25× magnification. It deals with objects from 100 nm to a few cm. The microstructure of a material (which can be broadly classified into metallic, polymeric, ceramic and composite) can strongly influence physical properties such as strength, toughness, ductility, hardness, corrosion resistance, high/low temperature behavior, wear resistance, and so on. Most of the traditional materials (such as metals and ceramics) are microstructured.
The manufacture of a perfect crystal of a material is physically impossible. For example, any crystalline material will contain defects such as precipitates, grain boundaries (Hall–Petch relationship), vacancies, interstitial atoms or substitutional atoms. The microstructure of materials reveals these larger defects, so that they can be studied, with significant advances in simulation resulting in exponentially increasing understanding of how defects can be used to enhance material properties.
Macro structure
Macro structure is the appearance of a material in the scale millimeters to meters—it is the structure of the material as seen with the naked eye.Crystallography
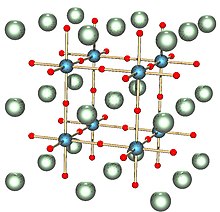
Crystal structure of a perovskite with a chemical formula ABX3.[11]
Crystallography is the science that examines the arrangement of atoms in crystalline solids. Crystallography is a useful tool for materials scientists. In single crystals, the effects of the crystalline arrangement of atoms is often easy to see macroscopically, because the natural shapes of crystals reflect the atomic structure. Further, physical properties are often controlled by crystalline defects. The understanding of crystal structures is an important prerequisite for understanding crystallographic defects. Mostly, materials do not occur as a single crystal, but in polycrystalline form, i.e., as an aggregate of small crystals with different orientations. Because of this, the powder diffraction method, which uses diffraction patterns of polycrystalline samples with a large number of crystals, plays an important role in structural determination. Most materials have a crystalline structure, but some important materials do not exhibit regular crystal structure. Polymers display varying degrees of crystallinity, and many are completely noncrystalline. Glass, some ceramics, and many natural materials are amorphous, not possessing any long-range order in their atomic arrangements. The study of polymers combines elements of chemical and statistical thermodynamics to give thermodynamic and mechanical, descriptions of physical properties.
Bonding
To obtain a full understanding of the material structure and how it relates to its properties, the materials scientist must study how the different atoms, ions and molecules are arranged and bonded to each other. This involves the study and use of quantum chemistry or quantum physics. Solid-state physics, solid-state chemistry and physical chemistry are also involved in the study of bonding and structure.Synthesis and processing
Synthesis and processing involves the creation of a material with the desired micro-nanostructure. From an engineering standpoint, a material cannot be used in industry if no economical production method for it has been developed. Thus, the processing of materials is vital to the field of materials science.Different materials require different processing or synthesis methods. For example, the processing of metals has historically been very important and is studied under the branch of materials science named physical metallurgy. Also, chemical and physical methods are also used to synthesize other materials such as polymers, ceramics, thin films, etc. As of the early 21st century, new methods are being developed to synthesize nanomaterials such as graphene.
Thermodynamics
A phase diagram for a binary system displaying a eutectic point.
Thermodynamics is concerned with heat and temperature and their relation to energy and work. It defines macroscopic variables, such as internal energy, entropy, and pressure, that partly describe a body of matter or radiation. It states that the behavior of those variables is subject to general constraints, that are common to all materials, not the peculiar properties of particular materials. These general constraints are expressed in the four laws of thermodynamics. Thermodynamics describes the bulk behavior of the body, not the microscopic behaviors of the very large numbers of its microscopic constituents, such as molecules. The behavior of these microscopic particles is described by, and the laws of thermodynamics are derived from, statistical mechanics.
The study of thermodynamics is fundamental to materials science. It forms the foundation to treat general phenomena in materials science and engineering, including chemical reactions, magnetism, polarizability, and elasticity. It also helps in the understanding of phase diagrams and phase equilibrium.
Kinetics
Chemical kinetics is the study of the rates at which systems that are out of equilibrium change under the influence of various forces. When applied to materials science, it deals with how a material changes with time (moves from non-equilibrium to equilibrium state) due to application of a certain field. It details the rate of various processes evolving in materials including shape, size, composition and structure. Diffusion is important in the study of kinetics as this is the most common mechanism by which materials undergo change.Kinetics is essential in processing of materials because, among other things, it details how the microstructure changes with application of heat.
In research
Materials science has received much attention from researchers. In most universities, many departments ranging from physics to chemistry to chemical engineering, along with materials science departments, are involved in materials research. Research in materials science is vibrant and consists of many avenues. The following list is in no way exhaustive. It serves only to highlight certain important research areas.Nanomaterials
A scanning electron microscopy image of carbon nanotubes bundles
Nanomaterials describe, in principle, materials of which a single unit is sized (in at least one dimension) between 1 and 1000 nanometers (10−9 meter) but is usually 1—100 nm.
Nanomaterials research takes a materials science-based approach to nanotechnology, leveraging advances in materials metrology and synthesis which have been developed in support of microfabrication research. Materials with structure at the nanoscale often have unique optical, electronic, or mechanical properties.
The field of nanomaterials is loosely organized, like the traditional field of chemistry, into organic (carbon-based) nanomaterials such as fullerenes, and inorganic nanomaterials based on other elements, such as silicon. Examples of nanomaterials include fullerenes, carbon nanotubes, nanocrystals, etc.
Biomaterials
A biomaterial is any matter, surface, or construct that interacts with biological systems. The study of biomaterials is called bio materials science. It has experienced steady and strong growth over its history, with many companies investing large amounts of money into developing new products. Biomaterials science encompasses elements of medicine, biology, chemistry, tissue engineering, and materials science.
Biomaterials can be derived either from nature or synthesized in a laboratory using a variety of chemical approaches using metallic components, polymers, bioceramics, or composite materials. They are often used and/or adapted for a medical application, and thus comprises whole or part of a living structure or biomedical device which performs, augments, or replaces a natural function. Such functions may be benign, like being used for a heart valve, or may be bioactive with a more interactive functionality such as hydroxylapatite coated hip implants. Biomaterials are also used every day in dental applications, surgery, and drug delivery. For example, a construct with impregnated pharmaceutical products can be placed into the body, which permits the prolonged release of a drug over an extended period of time. A biomaterial may also be an autograft, allograft or xenograft used as an organ transplant material.
Electronic, optical, and magnetic
Semiconductors, metals, and ceramics are used today to form highly complex systems, such as integrated electronic circuits, optoelectronic devices, and magnetic and optical mass storage media. These materials form the basis of our modern computing world, and hence research into these materials is of vital importance.
Semiconductors are a traditional example of these types of materials. They are materials that have properties that are intermediate between conductors and insulators. Their electrical conductivities are very sensitive to impurity concentrations, and this allows for the use of doping to achieve desirable electronic properties. Hence, semiconductors form the basis of the traditional computer.
This field also includes new areas of research such as superconducting materials, spintronics, metamaterials, etc. The study of these materials involves knowledge of materials science and solid-state physics or condensed matter physics.
Computational science and theory
With the increase in computing power, simulating the behavior of materials has become possible. This enables materials scientists to discover properties of materials formerly unknown, as well as to design new materials. Up until now, new materials were found by time-consuming trial and error processes. But, now it is hoped that computational methods could drastically reduce that time, and allow tailoring materials properties. This involves simulating materials at all length scales, using methods such as density functional theory, molecular dynamics, etc.In industry
Radical materials advances can drive the creation of new products or even new industries, but stable industries also employ materials scientists to make incremental improvements and troubleshoot issues with currently used materials. Industrial applications of materials science include materials design, cost-benefit tradeoffs in industrial production of materials, processing methods (casting, rolling, welding, ion implantation, crystal growth, thin-film deposition, sintering, glassblowing, etc.), and analytic methods (characterization methods such as electron microscopy, X-ray diffraction, calorimetry, nuclear microscopy (HEFIB), Rutherford backscattering, neutron diffraction, small-angle X-ray scattering (SAXS), etc.).Besides material characterization, the material scientist or engineer also deals with extracting materials and converting them into useful forms. Thus ingot casting, foundry methods, blast furnace extraction, and electrolytic extraction are all part of the required knowledge of a materials engineer. Often the presence, absence, or variation of minute quantities of secondary elements and compounds in a bulk material will greatly affect the final properties of the materials produced. For example, steels are classified based on 1/10 and 1/100 weight percentages of the carbon and other alloying elements they contain. Thus, the extracting and purifying methods used to extract iron in a blast furnace can affect the quality of steel that is produced.
Ceramics and glasses
Si3N4 ceramic bearing parts
Another application of material science is the structures of ceramics and glass typically associated with the most brittle materials. Bonding in ceramics and glasses uses covalent and ionic-covalent types with SiO2 (silica or sand) as a fundamental building block. Ceramics are as soft as clay or as hard as stone and concrete. Usually, they are crystalline in form. Most glasses contain a metal oxide fused with silica. At high temperatures used to prepare glass, the material is a viscous liquid. The structure of glass forms into an amorphous state upon cooling. Windowpanes and eyeglasses are important examples. Fibers of glass are also available. Scratch resistant Corning Gorilla Glass is a well-known example of the application of materials science to drastically improve the properties of common components. Diamond and carbon in its graphite form are considered to be ceramics.
Engineering ceramics are known for their stiffness and stability under high temperatures, compression and electrical stress. Alumina, silicon carbide, and tungsten carbide are made from a fine powder of their constituents in a process of sintering with a binder. Hot pressing provides higher density material. Chemical vapor deposition can place a film of a ceramic on another material. Cermets are ceramic particles containing some metals. The wear resistance of tools is derived from cemented carbides with the metal phase of cobalt and nickel typically added to modify properties.
Composites
A 6 μm diameter carbon filament (running from bottom left to top right) siting atop the much larger human hair.
Filaments are commonly used for reinforcement in composite materials.
Another application of materials science in industry is making composite materials. These are structured materials composed of two or more macroscopic phases. Applications range from structural elements such as steel-reinforced concrete, to the thermal insulating tiles which play a key and integral role in NASA's Space Shuttle thermal protection system which is used to protect the surface of the shuttle from the heat of re-entry into the Earth's atmosphere. One example is reinforced Carbon-Carbon (RCC), the light gray material which withstands re-entry temperatures up to 1,510 °C (2,750 °F) and protects the Space Shuttle's wing leading edges and nose cap. RCC is a laminated composite material made from graphite rayon cloth and impregnated with a phenolic resin. After curing at high temperature in an autoclave, the laminate is pyrolized to convert the resin to carbon, impregnated with furfural alcohol in a vacuum chamber, and cured-pyrolized to convert the furfural alcohol to carbon. To provide oxidation resistance for reuse ability, the outer layers of the RCC are converted to silicon carbide.
Other examples can be seen in the "plastic" casings of television sets, cell-phones and so on. These plastic casings are usually a composite material made up of a thermoplastic matrix such as acrylonitrile butadiene styrene (ABS) in which calcium carbonate chalk, talc, glass fibers or carbon fibers have been added for added strength, bulk, or electrostatic dispersion. These additions may be termed reinforcing fibers, or dispersants, depending on their purpose.
Polymers
The repeating unit of the polymer polypropylene
Expanded polystyrene polymer packaging.
Polymers are chemical compounds made up of a large number of identical components linked together like chains. They are an important part of materials science. Polymers are the raw materials (the resins) used to make what are commonly called plastics and rubber. Plastics and rubber are really the final product, created after one or more polymers or additives have been added to a resin during processing, which is then shaped into a final form. Plastics which have been around, and which are in current widespread use, include polyethylene, polypropylene, polyvinyl chloride (PVC), polystyrene, nylons, polyesters, acrylics, polyurethanes, and polycarbonates and also rubbers which have been around are natural rubber, styrene-butadiene rubber, chloroprene, and butadiene rubber. Plastics are generally classified as commodity, specialty and engineering plastics.
Polyvinyl chloride (PVC) is widely used, inexpensive, and annual production quantities are large. It lends itself to a vast array of applications, from artificial leather to electrical insulation and cabling, packaging, and containers. Its fabrication and processing are simple and well-established. The versatility of PVC is due to the wide range of plasticisers and other additives that it accepts. The term "additives" in polymer science refers to the chemicals and compounds added to the polymer base to modify its material properties.
Polycarbonate would be normally considered an engineering plastic (other examples include PEEK, ABS). Such plastics are valued for their superior strengths and other special material properties. They are usually not used for disposable applications, unlike commodity plastics.
Specialty plastics are materials with unique characteristics, such as ultra-high strength, electrical conductivity, electro-fluorescence, high thermal stability, etc.
The dividing lines between the various types of plastics is not based on material but rather on their properties and applications. For example, polyethylene (PE) is a cheap, low friction polymer commonly used to make disposable bags for shopping and trash, and is considered a commodity plastic, whereas medium-density polyethylene (MDPE) is used for underground gas and water pipes, and another variety called ultra-high-molecular-weight polyethylene (UHMWPE) is an engineering plastic which is used extensively as the glide rails for industrial equipment and the low-friction socket in implanted hip joints.
Metal alloys
Wire rope made from steel alloy.
The study of metal alloys is a significant part of materials science. Of all the metallic alloys in use today, the alloys of iron (steel, stainless steel, cast iron, tool steel, alloy steels) make up the largest proportion both by quantity and commercial value. Iron alloyed with various proportions of carbon gives low, mid and high carbon steels. An iron-carbon alloy is only considered steel if the carbon level is between 0.01% and 2.00%. For the steels, the hardness and tensile strength of the steel is related to the amount of carbon present, with increasing carbon levels also leading to lower ductility and toughness. Heat treatment processes such as quenching and tempering can significantly change these properties, however. Cast Iron is defined as an iron–carbon alloy with more than 2.00% but less than 6.67% carbon. Stainless steel is defined as a regular steel alloy with greater than 10% by weight alloying content of Chromium. Nickel and Molybdenum are typically also found in stainless steels.
Other significant metallic alloys are those of aluminium, titanium, copper and magnesium. Copper alloys have been known for a long time (since the Bronze Age), while the alloys of the other three metals have been relatively recently developed. Due to the chemical reactivity of these metals, the electrolytic extraction processes required were only developed relatively recently. The alloys of aluminium, titanium and magnesium are also known and valued for their high strength-to-weight ratios and, in the case of magnesium, their ability to provide electromagnetic shielding. These materials are ideal for situations where high strength-to-weight ratios are more important than bulk cost, such as in the aerospace industry and certain automotive engineering applications.
Semiconductors
The study of semiconductors is a significant part of materials science. A semiconductor is a material that has a resistivity between a metal and insulator. Its electronic properties can be greatly altered through intentionally introducing impurities or doping. From these semiconductor materials, things such as diodes, transistors, light-emitting diodes (LEDs), and analog and digital electric circuits can be built, making them materials of interest in industry. Semiconductor devices have replaced thermionic devices (vacuum tubes) in most applications. Semiconductor devices are manufactured both as single discrete devices and as integrated circuits (ICs), which consist of a number—from a few to millions—of devices manufactured and interconnected on a single semiconductor substrate.[14]Of all the semiconductors in use today, silicon makes up the largest portion both by quantity and commercial value. Monocrystalline silicon is used to produce wafers used in the semiconductor and electronics industry. Second to silicon, gallium arsenide (GaAs) is the second most popular semiconductor used. Due to its higher electron mobility and saturation velocity compared to silicon, it is a material of choice for high-speed electronics applications. These superior properties are compelling reasons to use GaAs circuitry in mobile phones, satellite communications, microwave point-to-point links and higher frequency radar systems. Other semiconductor materials include germanium, silicon carbide, and gallium nitride and have various applications.
Relation to other fields
Materials science evolved—starting from the 1960s—because it was recognized that to create, discover and design new materials, one had to approach it in a unified manner. Thus, materials science and engineering emerged at the intersection of various fields such as metallurgy, solid state physics, chemistry, chemical engineering, mechanical engineering and electrical engineering.The field is inherently interdisciplinary, and the materials scientists/engineers must be aware and make use of the methods of the physicist, chemist and engineer. The field thus maintains close relationships with these fields. Also, many physicists, chemists and engineers also find themselves working in materials science.
The overlap between physics and materials science has led to the offshoot field of materials physics, which is concerned with the physical properties of materials. The approach is generally more macroscopic and applied than in condensed matter physics. See important publications in materials physics for more details on this field of study.
The field of materials science and engineering is important both from a scientific perspective, as well as from an engineering one. When discovering new materials, one encounters new phenomena that may not have been observed before. Hence, there is a lot of science to be discovered when working with materials. Materials science also provides a test for theories in condensed matter physics.
Materials are of the utmost importance for engineers, as the usage of the appropriate materials is crucial when designing systems. As a result, materials science is an increasingly important part of an engineer's education.
Emerging technologies in materials science
| Emerging technology | Status | Potentially marginalized technologies | Potential applications | Related articles |
|---|---|---|---|---|
| Aerogel | Hypothetical, experiments, diffusion, early uses[15] | Traditional insulation, glass | Improved insulation, insulative glass if it can be made clear, sleeves for oil pipelines, aerospace, high-heat & extreme cold applications | |
| Amorphous metal | Experiments | Kevlar | Armor | |
| Conductive polymers | Research, experiments, prototypes | Conductors | Lighter and cheaper wires, antistatic materials, organic solar cells | |
| Femtotechnology, picotechnology | Hypothetical | Present nuclear | New materials; nuclear weapons, power | |
| Fullerene | Experiments, diffusion | Synthetic diamond and carbon nanotubes (e.g., Buckypaper) | Programmable matter | |
| Graphene | Hypothetical, experiments, diffusion, early uses[16][17] | Silicon-based integrated circuit | Components with higher strength to weight ratios, transistors that operate at higher frequency, lower cost of display screens in mobile devices, storing hydrogen for fuel cell powered cars, filtration systems, longer-lasting and faster-charging batteries, sensors to diagnose diseases[18] | Potential applications of graphene |
| High-temperature superconductivity | Cryogenic receiver front-end (CRFE) RF and microwave filter systems for mobile phone base stations; prototypes in dry ice; Hypothetical and experiments for higher temperatures[19] | Copper wire, semiconductor integral circuits | No loss conductors, frictionless bearings, magnetic levitation, lossless high-capacity accumulators, electric cars, heat-free integral circuits and processors | |
| LiTraCon | Experiments, already used to make Europe Gate | Glass | Building skyscrapers, towers, and sculptures like Europe Gate | |
| Metamaterials | Hypothetical, experiments, diffusion[20] | Classical optics | Microscopes, cameras, metamaterial cloaking, cloaking devices | |
| Metal foam | Research, commercialization | Hulls | Space colonies, floating cities | |
| Multi-function structures[21] | Hypothetical, experiments, some prototypes, few commercial | Composite materials mostly | Wide range, e.g., self health monitoring, self healing material, morphing, ... | |
| Nanomaterials: carbon nanotubes | Hypothetical, experiments, diffusion, early uses[22][23] | Structural steel and aluminium | Stronger, lighter materials, space elevator | Potential applications of carbon nanotubes, carbon fiber |
| Programmable matter | Hypothetical, experiments[24][25] | Coatings, catalysts | Wide range, e.g., claytronics, synthetic biology | |
| Quantum dots | Research, experiments, prototypes[26] | LCD, LED | Quantum dot laser, future use as programmable matter in display technologies (TV, projection), optical data communications (high-speed data transmission), medicine (laser scalpel) | |
| Silicene | Hypothetical, research | Field-effect transistors | ||
| Superalloy | Research, diffusion | Aluminum, titanium, composite materials | Aircraft jet engines | |
| Synthetic diamond | early uses (drill bits, jewelry) | Silicon transistors | Electronics |