From Wikipedia, the free encyclopedia
Molecular modelling encompasses all methods, theoretical and computational, used to
model or mimic the behaviour of
molecules. The methods are used in the fields of
computational chemistry,
drug design,
computational biology and
materials science
to study molecular systems ranging from small chemical systems to large
biological molecules and material assemblies. The simplest calculations
can be performed by hand, but inevitably computers are required to
perform molecular modelling of any reasonably sized system. The common
feature of molecular modelling methods is the atomistic level
description of the molecular systems. This may include treating atoms as
the smallest individual unit (a
molecular mechanics approach), or explicitly modelling electrons of each atom (a
quantum chemistry approach).
Molecular mechanics
Molecular mechanics is one aspect of molecular modelling, as it involves the use of
classical mechanics (
Newtonian mechanics)
to describe the physical basis behind the models. Molecular models
typically describe atoms (nucleus and electrons collectively) as point
charges with an associated mass. The interactions between neighbouring
atoms are described by spring-like interactions (representing
chemical bonds) and
Van der Waals forces. The
Lennard-Jones potential is commonly used to describe the latter. The electrostatic interactions are computed based on
Coulomb's law. Atoms are assigned coordinates in Cartesian space or in
internal coordinates,
and can also be assigned velocities in dynamical simulations. The
atomic velocities are related to the temperature of the system, a
macroscopic quantity. The collective mathematical expression is termed a
potential function
and is related to the system internal energy (U), a thermodynamic
quantity equal to the sum of potential and kinetic energies. Methods
which minimize the potential energy are termed energy minimization
methods (e.g.,
steepest descent and
conjugate gradient), while methods that model the behaviour of the system with propagation of time are termed
molecular dynamics.


This function, referred to as a
potential function,
computes the molecular potential energy as a sum of energy terms that
describe the deviation of bond lengths, bond angles and torsion angles
away from equilibrium values, plus terms for non-bonded pairs of atoms
describing van der Waals and electrostatic interactions. The set of
parameters consisting of equilibrium bond lengths, bond angles, partial
charge values, force constants and van der Waals parameters are
collectively termed a
force field. Different implementations of molecular mechanics use different mathematical expressions and different parameters for the
potential function.
The common force fields in use today have been developed by using high
level quantum calculations and/or fitting to experimental data. The
method, termed energy minimization, is used to find positions of zero
gradient for all atoms, in other words, a local energy minimum. Lower
energy states are more stable and are commonly investigated because of
their role in chemical and biological processes. A
molecular dynamics
simulation, on the other hand, computes the behaviour of a system as a
function of time. It involves solving Newton's laws of motion,
principally the second law,

.
Integration of Newton's laws of motion, using different integration
algorithms, leads to atomic trajectories in space and time. The force on
an atom is defined as the negative gradient of the potential energy
function. The energy minimization method is useful to obtain a static
picture for comparing between states of similar systems, while molecular
dynamics provides information about the dynamic processes with the
intrinsic inclusion of temperature effects.
Variables
Molecules
can be modelled either in vacuum, or in the presence of a solvent such
as water. Simulations of systems in vacuum are referred to as
gas-phase simulations, while those that include the presence of solvent molecules are referred to as
explicit solvent
simulations. In another type of simulation, the effect of solvent is
estimated using an empirical mathematical expression; these are termed
implicit solvation simulations.
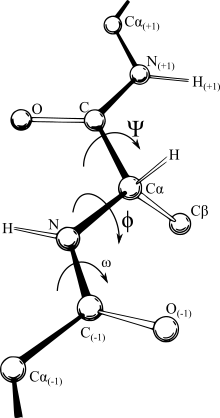
Coordinate representations
Most
force fields are distance-dependent, making the most convenient
expression for these Cartesian coordinates. Yet the comparatively rigid
nature of bonds which occur between specific atoms, and in essence,
defines what is meant by the designation
molecule, make an
internal coordinate system the most logical representation. In some
fields the IC representation (bond length, angle between bonds, and
twist angle of the bond as shown in the figure) is termed the
Z-matrix
or torsion angle representation. Unfortunately, continuous motions in
Cartesian space often require discontinuous angular branches in internal
coordinates, making it relatively hard to work with force fields in the
internal coordinate representation, and conversely a simple
displacement of an atom in Cartesian space may not be a straight line
trajectory due to the prohibitions of the interconnected bonds. Thus,
it is very common for computational optimizing programs to flip back and
forth between representations during their iterations. This can
dominate the calculation time of the potential itself and in long chain
molecules introduce cumulative numerical inaccuracy. While all
conversion algorithms produce mathematically identical results, they
differ in speed and numerical accuracy.
[1] Currently, the fastest and most accurate torsion to Cartesian
conversion is the Natural Extension Reference Frame (NERF) method.
[1]
Applications
Molecular
modelling methods are now used routinely to investigate the structure,
dynamics, surface properties, and thermodynamics of inorganic,
biological, and polymeric systems. The types of biological activity that
have been investigated using molecular modelling include
protein folding,
enzyme catalysis, protein stability, conformational changes associated with biomolecular function, and molecular recognition of proteins,
DNA, and membrane complexes.
 .
Integration of Newton's laws of motion, using different integration
algorithms, leads to atomic trajectories in space and time. The force on
an atom is defined as the negative gradient of the potential energy
function. The energy minimization method is useful to obtain a static
picture for comparing between states of similar systems, while molecular
dynamics provides information about the dynamic processes with the
intrinsic inclusion of temperature effects.
.
Integration of Newton's laws of motion, using different integration
algorithms, leads to atomic trajectories in space and time. The force on
an atom is defined as the negative gradient of the potential energy
function. The energy minimization method is useful to obtain a static
picture for comparing between states of similar systems, while molecular
dynamics provides information about the dynamic processes with the
intrinsic inclusion of temperature effects.