From Wikipedia, the free encyclopedia
The liquid drop model
The liquid drop model is one of the first models of
nuclear structure, proposed by
Carl Friedrich von Weizsäcker in 1935. It describes the nucleus as a
semiclassical fluid made up of
neutrons and
protons, with an internal repulsive
electrostatic force proportional to the number of protons. The
quantum mechanical nature of these particles appears via the
Pauli exclusion principle, which states that no two nucleons of the same kind can be at the same
state. Thus the fluid is actually what is known as a
Fermi liquid.
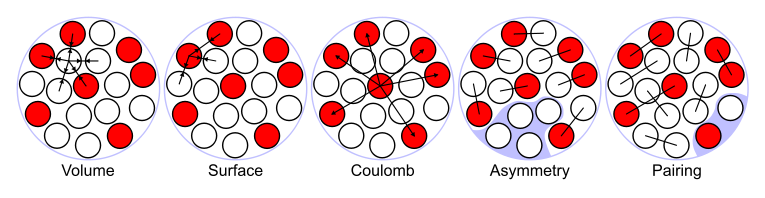
In this model, the binding energy of a nucleus with

protons and

neutrons is given by

where

is the total number of
nucleons (
Mass Number). The terms proportional to

and

represent the volume and surface energy of the liquid drop, the term proportional to

represents the electrostatic energy, the term proportional to

represents the Pauli exclusion principle and the last term

is the pairing term, which lowers the energy for even numbers of protons or neutrons.
The coefficients

and the strength of the pairing term may be estimated theoretically, or fit to data.
This simple model reproduces the main features of the
binding energy of nuclei.
The assumption of nucleus as a drop of
Fermi liquid
is still widely used in the form of Finite Range Droplet Model (FRDM),
due to the possible good reproduction of nuclear binding energy on the
whole chart, with the necessary accuracy for predictions of unknown
nuclei.
The shell model
The expression "shell model" is ambiguous in that it refers to two
different eras in the state of the art. It was previously used to
describe the existence of nucleon shells in the nucleus according to an
approach closer to what is now called
mean field theory.
Nowadays, it refers to a formalism analogous to the
configuration interaction formalism used in
quantum chemistry. We shall introduce the latter here.
Introduction to the shell concept
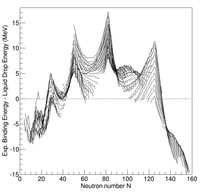
Difference between experimental binding energies and the liquid drop model prediction as a function of neutron number for Z>7
Systematic measurements of the
binding energy
of atomic nuclei show systematic deviations with respect to those
estimated from the liquid drop model. In particular, some nuclei having
certain values for the number of protons and/or neutrons are bound more
tightly together than predicted by the liquid drop model. These nuclei
are called singly/doubly
magic. This observation led scientists to assume the existence of a shell structure of
nucleons (protons and neutrons) within the nucleus, like that of
electrons within atoms.
Indeed, nucleons are
quantum objects.
Strictly speaking, one should not speak of energies of individual
nucleons, because they are all correlated with each other. However, as
an approximation one may envision an average nucleus, within which
nucleons propagate individually. Owing to their quantum character, they
may only occupy
discrete energy levels.
These levels are by no means uniformly distributed; some intervals of
energy are crowded, and some are empty, generating a gap in possible
energies. A shell is such a set of levels separated from the other ones
by a wide empty gap.
The energy levels are found by solving the
Schrödinger equation
for a single nucleon moving in the average potential generated by all
other nucleons. Each level may be occupied by a nucleon, or empty. Some
levels accommodate several different quantum states with the same
energy; they are said to be
degenerate. This occurs in particular if the average nucleus has some
symmetry.
The concept of shells allows one to understand why some nuclei
are bound more tightly than others. This is because two nucleons of the
same kind cannot be in the same state (
Pauli exclusion principle).
So the lowest-energy state of the nucleus is one where nucleons fill
all energy levels from the bottom up to some level. A nucleus with full
shells is exceptionally stable, as will be explained.
As with electrons in the
electron shell
model, protons in the outermost shell are relatively loosely bound to
the nucleus if there are only few protons in that shell, because they
are farthest from the center of the nucleus. Therefore, nuclei which
have a full outer proton shell will be more tightly bound and have a
higher binding energy than other nuclei with a similar total number of
protons. All this is also true for neutrons.
Furthermore, the energy needed to excite the nucleus (i.e. moving
a nucleon to a higher, previously unoccupied level) is exceptionally
high in such nuclei. Whenever this unoccupied level is the next after a
full shell, the only way to excite the nucleus is to raise one nucleon
across the gap,
thus spending a large amount of energy. Otherwise, if the highest
occupied energy level lies in a partly filled shell, much less energy is
required to raise a nucleon to a higher state in the same shell.
Some evolution of the shell structure observed in stable nuclei is expected away from the
valley of stability. For example, observations of unstable
isotopes have shown shifting and even a reordering of the single particle levels of which the shell structure is composed. This is sometimes observed as the creation of an
island of inversion or in the reduction of excitation energy gaps above the traditional magic numbers.
Basic hypotheses
Some basic hypotheses are made in order to give a precise conceptual framework to the shell model:
- The atomic nucleus is a quantum n-body system.
- The internal motion of nucleons within the nucleus is non-relativistic, and their behavior is governed by the Schrödinger equation.
- Nucleons are considered to be pointlike, without any internal structure.
Brief description of the formalism
The general process used in the shell model calculations is the following. First a
Hamiltonian
for the nucleus is defined. Usually, for computational practicality,
only one- and two-body terms are taken into account in this definition.
The interaction is an
effective theory: it contains free parameters which have to be fitted with experimental data.
The next step consists in defining a
basis of single-particle states, i.e. a set of
wavefunctions describing all possible nucleon states. Most of the time, this basis is obtained via a
Hartree–Fock computation. With this set of one-particle states,
Slater determinants are built, that is, wavefunctions for
Z proton variables or
N
neutron variables, which are antisymmetrized products of
single-particle wavefunctions (antisymmetrized meaning that under
exchange of variables for any pair of nucleons, the wavefunction only
changes sign).
In principle, the number of
quantum states available for a single nucleon at a finite energy is finite, say
n.
The number of nucleons in the nucleus must be smaller than the number
of available states, otherwise the nucleus cannot hold all of its
nucleons. There are thus several ways to choose
Z (or
N) states among the
n possible. In
combinatorial mathematics, the number of choices of
Z objects among
n is the
binomial coefficient C
Z
n. If
n is much larger than
Z (or
N), this increases roughly like
nZ. Practically, this number becomes so large that every computation is impossible for
A=
N+
Z larger than 8.
To obviate this difficulty, the space of possible single-particle states is divided into a core and a
valence shell, by analogy with
chemistry.
The core is a set of single-particles which are assumed to be inactive,
in the sense that they are the well bound lowest-energy states, and
that there is no need to reexamine their situation. They do not appear
in the Slater determinants, contrary to the states in the valence space,
which is the space of all single-particle states
not in the core, but possibly to be considered in the choice of the build of the (
Z-)
N-body wavefunction. The set of all possible Slater determinants in the valence space defines a
basis for
(
Z-)
N-body states.
The last step consists in computing the matrix of the Hamiltonian
within this basis, and to diagonalize it. In spite of the reduction of
the dimension of the basis owing to the fixation of the core, the
matrices to be diagonalized reach easily dimensions of the order of 10
9, and demand specific diagonalization techniques.
The shell model calculations give in general an excellent fit
with experimental data. They depend however strongly on two main
factors:
- The way to divide the single-particle space into core and valence.
- The effective nucleon–nucleon interaction.
Mean field theories
The independent-particle model
The
interaction between nucleons, which is a consequence of
strong interactions
and binds the nucleons within the nucleus, exhibits the peculiar
behaviour of having a finite range: it vanishes when the distance
between two nucleons becomes too large; it is attractive at medium
range, and repulsive at very small range. This last property correlates
with the
Pauli exclusion principle according to which two
fermions (nucleons are fermions) cannot be in the same quantum state. This results in a very large
mean free path predicted for a nucleon within the nucleus.
The main idea of the Independent Particle approach is that a
nucleon moves inside a certain potential well (which keeps it bound to
the nucleus) independently from the other nucleons. This amounts to
replacing a
N-body problem (
N particles interacting) by
N
single-body problems. This essential simplification of the problem is
the cornerstone of mean field theories. These are also widely used in
atomic physics, where electrons move in a mean field due to the central nucleus and the electron cloud itself.
The independent particle model and mean field theories (we shall
see that there exist several variants) have a great success in
describing the properties of the nucleus starting from an effective
interaction or an effective potential, thus are a basic part of atomic
nucleus theory. One should also notice that they are modular enough, in
that it is quite easy to
extend the model to introduce effects such as nuclear pairing, or collective motions of the nucleon like
rotation, or
vibration,
adding the corresponding energy terms in the formalism. This implies
that in many representations, the mean field is only a starting point
for a more complete description which introduces correlations
reproducing properties like collective excitations and nucleon transfer.
Nuclear potential and effective interaction
A large part of the practical difficulties met in mean field theories is the definition (or calculation) of the
potential of the mean field itself. One can very roughly distinguish between two approaches:
- The phenomenological approach is a parameterization of the nuclear potential by an appropriate mathematical function. Historically, this procedure was applied with the greatest success by Sven Gösta Nilsson, who used as a potential a (deformed) harmonic oscillator
potential. The most recent parameterizations are based on more
realistic functions, which account more accurately for scattering
experiments, for example. In particular the form known as the Woods–Saxon potential can be mentioned.
- The self-consistent or Hartree–Fock
approach aims to deduce mathematically the nuclear potential from an
effective nucleon–nucleon interaction. This technique implies a
resolution of the Schrödinger equation
in an iterative fashion, starting from an ansatz wavefunction and
improving it variationally, since the potential depends there upon the
wavefunctions to be determined. The latter are written as Slater determinants.
In the case of the Hartree–Fock approaches, the trouble is not to
find the mathematical function which describes best the nuclear
potential, but that which describes best the nucleon–nucleon
interaction. Indeed, in contrast with
atomic physics where the interaction is known (it is the
Coulomb interaction), the nucleon–nucleon interaction within the nucleus is not known analytically.
There are two main reasons for this fact. First, the strong interaction acts essentially among the
quarks forming the nucleons. The
nucleon–nucleon interaction in vacuum is a mere
consequence of the quark–quark interaction. While the latter is well understood in the framework of the
Standard Model at high energies, it is much more complicated in low energies due to
color confinement and
asymptotic freedom.
Thus there is yet no fundamental theory allowing one to deduce the
nucleon–nucleon interaction from the quark–quark interaction.
Furthermore, even if this problem were solved, there would remain a
large difference between the ideal (and conceptually simpler) case of
two nucleons interacting in vacuum, and that of these nucleons
interacting in the nuclear matter. To go further, it was necessary to
invent the concept of
effective interaction.
The latter is basically a mathematical function with several arbitrary
parameters, which are adjusted to agree with experimental data.
Most modern interaction are zero-range so they act only when the two nucleons are in contact, as introduced by
Tony Skyrme.
The self-consistent approaches of the Hartree–Fock type
In the
Hartree–Fock approach of the
n-body problem, the starting point is a
Hamiltonian containing
n kinetic energy
terms, and potential terms. As mentioned before, one of the mean field
theory hypotheses is that only the two-body interaction is to be taken
into account. The potential term of the Hamiltonian represents all
possible two-body interactions in the set of
n fermions. It is the first hypothesis.
The second step consists in assuming that the
wavefunction of the system can be written as a
Slater determinant of one-particle
spin-orbitals. This statement is the mathematical translation of the independent-particle model. This is the second hypothesis.
There remains now to determine the components of this Slater determinant, that is, the individual
wavefunctions
of the nucleons. To this end, it is assumed that the total wavefunction
(the Slater determinant) is such that the energy is minimum. This is
the third hypothesis.
Technically, it means that one must compute the
mean value of the (known) two-body
Hamiltonian on the (unknown) Slater determinant, and impose that its mathematical
variation
vanishes. This leads to a set of equations where the unknowns are the
individual wavefunctions: the Hartree–Fock equations. Solving these
equations gives the wavefunctions and individual energy levels of
nucleons, and so the total energy of the nucleus and its wavefunction.
This short account of the
Hartree–Fock method explains why it is called also the
variational
approach. At the beginning of the calculation, the total energy is a
"function of the individual wavefunctions" (a so-called functional), and
everything is then made in order to optimize the choice of these
wavefunctions so that the functional has a minimum – hopefully absolute,
and not only local. To be more precise, there should be mentioned that
the energy is a functional of the
density, defined as the sum of the individual squared wavefunctions. Let us note also that the Hartree–Fock method is also used in
atomic physics and
condensed matter physics as Density Functional Theory, DFT.
The process of solving the Hartree–Fock equations can only be iterative, since these are in fact a
Schrödinger equation in which the potential depends on the
density, that is, precisely on the
wavefunctions
to be determined. Practically, the algorithm is started with a set of
individual grossly reasonable wavefunctions (in general the
eigenfunctions of a
harmonic oscillator).
These allow to compute the density, and therefrom the Hartree–Fock
potential. Once this done, the Schrödinger equation is solved anew, and
so on. The calculation stops – convergence is reached – when the
difference among wavefunctions, or energy levels, for two successive
iterations is less than a fixed value. Then the mean field potential is
completely determined, and the Hartree–Fock equations become standard
Schrödinger equations. The corresponding Hamiltonian is then called the
Hartree–Fock Hamiltonian.
The relativistic mean field approaches
Born first in the 1970s with the works of
John Dirk Walecka on
quantum hadrodynamics, the
relativistic
models of the nucleus were sharpened up towards the end of the 1980s by
P. Ring and coworkers. The starting point of these approaches is the
relativistic
quantum field theory. In this context, the nucleon interactions occur via the exchange of
virtual particles called
mesons. The idea is, in a first step, to build a
Lagrangian containing these interaction terms. Second, by an application of the
least action principle, one gets a set of equations of motion. The real particles (here the nucleons) obey the
Dirac equation, whilst the virtual ones (here the mesons) obey the
Klein–Gordon equations.
In view of the non-
perturbative
nature of strong interaction, and also in view of the fact that the
exact potential form of this interaction between groups of nucleons is
relatively badly known, the use of such an approach in the case of
atomic nuclei requires drastic approximations. The main simplification
consists in replacing in the equations all field terms (which are
operators in the mathematical sense) by their
mean value (which are
functions). In this way, one gets a system of coupled
integro-differential equations, which can be solved numerically, if not analytically.
The interacting boson model
The
interacting boson model
(IBM) is a model in nuclear physics in which nucleons are represented
as pairs, each of them acting as a boson particle, with integral spin of
0, 2 or 4. This makes calculations feasible for larger nuclei.
There are several branches of this model - in one of them (IBM-1) one
can group all types of nucleons in pairs, in others (for instance -
IBM-2) one considers protons and neutrons in pairs separately.
Spontaneous breaking of symmetry in nuclear physics
One of the focal points of all physics is
symmetry. The nucleon–nucleon interaction and all
effective interactions used in practice have certain symmetries. They are invariant by
translation (changing the frame of reference so that directions are not altered), by
rotation (turning the frame of reference around some axis), or
parity
(changing the sense of axes) in the sense that the interaction does not
change under any of these operations. Nevertheless, in the Hartree–Fock
approach, solutions which are not invariant under such a symmetry can
appear. One speaks then of
spontaneous symmetry breaking.
Qualitatively, these spontaneous symmetry breakings can be
explained in the following way: in the mean field theory, the nucleus is
described as a set of independent particles. Most additional
correlations among nucleons which do not enter the mean field are
neglected. They can appear however by a breaking of the symmetry of the
mean field Hamiltonian, which is only approximate. If the density used
to start the iterations of the Hartree–Fock process breaks certain
symmetries, the final Hartree–Fock Hamiltonian may break these
symmetries, if it is advantageous to keep these broken from the point of
view of the total energy.
It may also converge towards a symmetric solution. In any case,
if the final solution breaks the symmetry, for example, the rotational
symmetry, so that the nucleus appears not to be spherical, but elliptic,
all configurations deduced from this deformed nucleus by a rotation are
just as good solutions for the Hartree–Fock problem. The ground state
of the nucleus is then
degenerate.
A similar phenomenon happens with the nuclear pairing, which violates the conservation of the number of baryons (see below).
Extensions of the mean field theories
Nuclear pairing phenomenon
The
most common extension to mean field theory is the nuclear pairing.
Nuclei with an even number of nucleons are systematically more bound
than those with an odd one. This implies that each nucleon binds with
another one to form a pair, consequently the system cannot be described
as independent particles subjected to a common mean field. When the
nucleus has an even number of protons and neutrons, each one of them
finds a partner. To excite such a system, one must at least use such an
energy as to break a pair. Conversely, in the case of odd number of
protons or neutrons, there exists an unpaired nucleon, which needs less
energy to be excited.
This phenomenon is closely analogous to that of Type 1
superconductivity in solid state physics. The first theoretical description of nuclear pairing was proposed at the end of the 1950s by
Aage Bohr,
Ben Mottelson, and
David Pines (which contributed to the reception of the Nobel Prize in Physics in 1975 by Bohr and Mottelson). It was close to the
BCS theory
of Bardeen, Cooper and Schrieffer, which accounts for metal
superconductivity. Theoretically, the pairing phenomenon as described by
the BCS theory combines with the mean field theory: nucleons are both
subject to the mean field potential and to the pairing interaction.
The
Hartree–Fock–Bogolyubov (HFB) method is a more sophisticated approach, enabling one to consider the pairing and mean field interactions
consistently on equal footing. HFB is now the de facto standard in the
mean field treatment of nuclear systems.
Symmetry restoration
Peculiarity of mean field methods is the calculation of nuclear property by explicit
symmetry breaking.
The calculation of the mean field with self-consistent methods (e.g.
Hartree-Fock), breaks rotational symmetry, and the calculation of
pairing property breaks particle-number.
Several techniques for symmetry restoration by projecting on good quantum numbers have been developed.
Particle vibration coupling
Mean
field methods (eventually considering symmetry restoration) are a good
approximation for the ground state of the system, even postulating a
system of independent particles. Higher-order corrections consider the
fact that the particles interact together by the means of correlation.
These correlations can be introduced taking into account the coupling of
independent particle degrees of freedom, low-energy collective
excitation of systems with even number of protons and neutrons.
In these way excited states can be reproduced by the means of
random phase approximation (RPA), and eventually consistently calculating also corrections to the ground state (e.g. by the means of
nuclear field theory).