| Transcranial direct-current stimulation | |
|---|---|
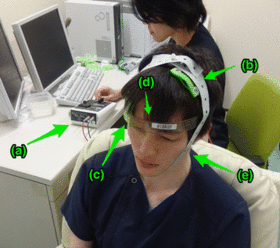
tDCS administration. Anodal (b) and cathodal (c) electrodes with 35-cm2
size are put on F3 and right supraorbital region, respectively. A head
strap is used (d) for convenience and reproducibility, and a rubber band
(e) for reducing resistance.
| |
| MeSH | D065908 |
Transcranial direct current stimulation (tDCS) is a form of neuromodulation that uses constant, low direct current delivered via electrodes on the head. It can be contrasted with cranial electrotherapy stimulation, which generally uses alternating current the same way.
It was originally developed to help patients with brain injuries or psychiatric conditions like major depressive disorder. There is increasing evidence for tDCS as a treatment for depression. However, there is mixed evidence about whether tDCS is useful for cognitive enhancement in healthy people. Several reviews have found evidence of small yet significant cognitive improvements. Other reviews found no evidence at all, although one of them has been criticized for overlooking within-subject effects and evidence from multiple-session tDCS trials. There is no good evidence that tDCS is useful for memory deficits in Parkinson's disease and Alzheimer's disease, schizophrenia, non-neuropathic pain, or improving upper limb function after stroke.
Medical use
In
2015, the British National Institute for Health and Care Excellence
(NICE) found tDCS to be safe and to appear effective for depression
treatment, although more and larger studies was needed at that point .
Since then, several studies and meta-analysis have been conducted that
add to the evidence of tDCS as a safe and effective treatment for
depression.
There is also evidence that tDCS is useful in treating neuropathic pain after spinal cord injury and improving activities of daily living assessment after stroke.
Adverse effects and contraindications
As of 2017, at stimulation up to 60 min and up to 4 mA over two weeks, adverse effects include skin irritation, a phosphene at the start of stimulation, nausea, headache, dizziness, and itching under the electrode. Adverse effects of long term treatment were not known as of 2017. Nausea most commonly occurs when the electrodes are placed above the mastoid for stimulation of the vestibular system. A phosphene is a brief flash of light that can occur if an electrode is placed near the eye.
Studies have been completed to determine the current density at which overt brain damage occurs in rats. It was found that in cathodal stimulation, a current density of 142.9 A/m2 delivering a charge density of 52400 C/m2 or higher caused a brain lesion in the rat. This is over two orders of magnitude higher than protocols that were in use as of 2009.
Mechanism of action
One
of the aspects of tDCS is its ability to achieve cortical changes even
after the stimulation is ended. The duration of this change depends on
the length of stimulation as well as the intensity of stimulation. The
effects of stimulation increase as the duration of stimulation increases
or the strength of the current increases. The way that the stimulation changes brain function is either by causing the neuron’s resting membrane potential to depolarize or hyperpolarize.
When positive stimulation (anodal tDCS) is delivered, the current
causes a depolarization of the resting membrane potential, which
increases neuronal excitability and allows for more spontaneous cell
firing. When negative stimulation (cathodal tDCS) is delivered, the
current causes a hyperpolarization of the resting membrane potential.
This decreases neuron excitability due to the decreased spontaneous cell
firing.
tDCS has been proposed to promote both long term potentiation and long term depression.
Operation
Transcranial direct current stimulation works by sending constant, low direct current through the electrodes.
When these electrodes are placed in the region of interest, the
current induces intracerebral current flow. This current flow then
either increases or decreases the neuronal excitability in the specific
area being stimulated based on which type of stimulation is being used.
This change of neuronal excitability leads to alteration of brain
function, which can be used in various therapies as well as to provide
more information about the functioning of the human brain.
Parts
Transcranial
direct current stimulation is a relatively simple technique requiring
only a few parts. These include two electrodes and a battery-powered
device that delivers constant current. Control software can also be
used in experiments that require multiple sessions with differing
stimulation types so that neither the person receiving the stimulation
nor the experimenter knows which type is being administered. Each
device has an anodal, positively charged electrode and a cathodal,
negative electrode. Current is "conventionally" described as flowing
from the positive anode, through the intervening conducting tissue, to
the cathode, creating a circuit.
Note that in traditional electric circuits constructed from metal
wires, current flow is created by the motion of negatively charged
electrons, which actually flow from cathode to anode. However, in
biological systems, such as the head, current is usually created by the
flow of ions,
which may be positively or negatively charged—positive ions will flow
towards the cathode; negative ions will flow toward the anode. The
device may control the current as well as the duration of stimulation.
Setup
To set up
the tDCS device, the electrodes and the skin need to be prepared. This
ensures a low resistance connection between the skin and the electrode.
The careful placement of the electrodes is crucial to successful tDCS
technique. The electrode pads come in various sizes with benefits to
each size. A smaller sized electrode achieves a more focused
stimulation of a site while a larger electrode ensures that the entirety
of the region of interest is being stimulated.
If the electrode is placed incorrectly, a different site or more sites
than intended may be stimulated resulting in faulty results.[19]
One of the electrodes is placed over the region of interest and the
other electrode, the reference electrode, is placed in another location
in order to complete the circuit.
This reference electrode is usually placed on the neck or shoulder of
the opposite side of the body than the region of interest. Since the
region of interest may be small, it is often useful to locate this
region before placing the electrode by using a brain imaging technique such as fMRI or PET.
Once the electrodes are placed correctly, the stimulation can be
started. Many devices have a built-in capability that allows the
current to be "ramped up" or increased gradually until the necessary
current is reached. This decreases the amount of stimulation effects
felt by the person receiving the tDCS.
After the stimulation has been started, the current will continue for
the amount of time set on the device and then will automatically be shut
off. Recently a new approach has been introduced where instead of using
two large pads, multiple (more than two) smaller sized gel electrodes
are used to target specific cortical structures. This new approach is
called High Definition tDCS (HD-tDCS). In a pilot study, HD-tDCS was found to have greater and longer lasting motor cortex excitability changes than sponge tDCS.
Types of stimulation
There are three different types of stimulation: anodal, cathodal,
and sham. The anodal stimulation is positive (V+) stimulation that
increases the neuronal excitability of the area being stimulated.
Cathodal (V-) stimulation decreases the neuronal excitability of the
area being stimulated. Cathodal stimulation can treat psychiatric
disorders that are caused by the hyper-activity of an area of the brain. Sham stimulation is used as a control
in experiments. Sham stimulation emits a brief current but then
remains off for the remainder of the stimulation time. With sham
stimulation, the person receiving the tDCS does not know that they are
not receiving prolonged stimulation. By comparing the results in
subjects exposed to sham stimulation with the results of subjects
exposed to anodal or cathodal stimulation, researchers can see how much
of an effect is caused by the current stimulation, rather than by the placebo effect.
History
The basic design of tDCS, using direct current
(DC) to stimulate the area of interest, has existed for over 100 years.
There were a number of rudimentary experiments completed before the
19th century using this technique that tested animal and human
electricity. Luigi Galvani and Alessandro Volta
were two such researchers that utilized the technology of tDCS in their
explorations of the source of animal cell electricity. It was due to
these initial studies that tDCS was first brought into the clinical
scene. In 1801, Giovanni Aldini
(Galvani's nephew) started a study in which he successfully used the
technique of direct current stimulation to improve the mood of
melancholy patients.
There was a brief rise of interest in transcranial direct current
stimulation in the 1960s when studies by researcher D. J. Albert proved
that the stimulation could affect brain function by changing the
cortical excitability. He also discovered that positive and negative stimulation had different effects on the cortical excitability. Research continued, further fueled by knowledge gained from other techniques like TMS and fMRI.
Comparison to other devices
Transcranial
electrical stimulation techniques. While tDCS uses constant current
intensity, tRNS and tACS use oscillating current. The vertical axis
represents the current intensity in milliamp (mA), while the horizontal
axis illustrates the time-course.
In transcranial magnetic stimulation
(TMS), an electric coil is held above the region of interest on the
scalp that uses rapidly changing magnetic fields to induce small electrical currents
in the brain. There are two types of TMS: repetitive TMS and single
pulse TMS. Both are used in research therapy but effects lasting longer
than the stimulation period are only observed in repetitive TMS.
Similar to tDCS, an increase or decrease in neuronal activity can be
achieved using this technique, but the method of how this is induced is
very different. Transcranial direct current stimulation has the two
different directions of current that cause the different effects.
Increased neuronal activity is induced in repetitive TMS by using a
higher frequency and decreased neuronal activity is induced by using a
lower frequency.
Variants related to tDCS include tACS, tPCS and transcranial random noise stimulation (tRNS), a group of technologies commonly referred to as transcranial electrical stimulation, or TES.
Research
In 2016, European meta analysis has found level B evidence (probable efficacy) for fibromyalgia, depression and craving.
A 2015 review of results from hundreds of tDCS experiments found
that there was no statistically conclusive evidence to support any net
cognitive effect, positive or negative, of single session tDCS in
healthy populations - there is no evidence that tDCS is useful for cognitive enhancement.
A second study by the same authors found there was little-to-no
statistically reliable impact of tDCS on any neurophysiologic outcome.
A few clinical trials have been conducted on the use of tDCS to ameliorate memory deficits in Parkinson's disease and Alzheimer's disease and healthy subjects, with mixed results.
A 2016 Cochrane review found evidence that tDSC can improve activities
of daily living in Parkinson’s disease but the evidence was very low to
moderate quality.
As of 2014, there have been several small randomized clinical trials (RCT) in major depressive disorder
(MDD); most found alleviation of depressive symptoms. There have been
only two RCTs in treatment-resistant MDD; both were small, and one found
an effect and the other did not.
One meta-analysis of the data focused on reduction in symptoms and
found an effect compared to sham treatment, but another that was focused
on relapse found no effect compared to sham.
Research conducted as of 2013 in schizophrenia,
has found that while large effect sizes were initially found for
symptom improvement, later and larger studies have found smaller effect
sizes (see also section on use of tDCS in psychiatric disorders below).
Studies have mostly concentrated on positive symptoms like auditory
hallucinations; research on negative symptoms is lacking.
Research conducted as of 2012 on the use of tDCS to treat pain,
found that the research has been of low quality and cannot be used as a
basis to recommend use of tDCS to treat pain. In chronic pain following spinal cord injury, research is of high quality and has found tDCS to be ineffective.
In stroke, research conducted as of 2014, has found that tDCS is not effective for improving upper limb function after stroke. While some reviews have suggested an effect of tDCS for improving post-stroke aphasia, a 2015 Cochrane review could find no improvement from combining tDCS with conventional treatment. Research conducted as of 2013 suggests that tDCS may be effective for improve vision deficits following stroke.
tDCS has also been studied in various psychiatric disorders such as depression, and to reverse cognitive deficits in schizophrenia. Some researchers are investigating potential applications such as the improvement of focus and concentration. tDCS has also been studied in addiction.
tDCS has also been used in neuroscience research, particularly to try to link specific brain regions to specific cognitive tasks or psychological phenomena.
The cerebellum has been a focus of research, due to its high
concentration of neurons, its location immediately below the skull, and
its multiple reciprocal anatomical connections to motor and associative
parts of the brain.
Most such studies focus on the impact of cerebellar tDCS on motor,
cognitive, and affective functions in healthy and patient populations,
but some also employ tDCS over the cerebellum to study the functional
connectivity of the cerebellum to other areas of the brain.
Regulatory approvals
As of 2015, tDCS has not been approved for any use by the US FDA. An FDA briefing document prepared in 2012 stated that "there is no regulation for therapeutic tDCS". tDCS is a CE approved treatment for Major Depressive Disorder (MDD) in the EU, Australia, and Mexico.








