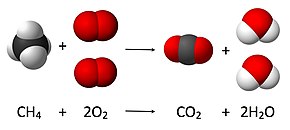
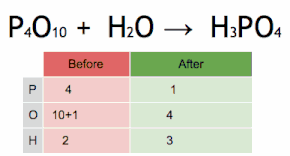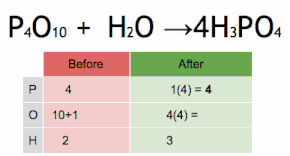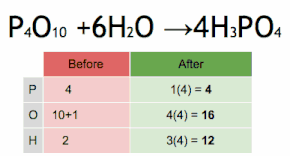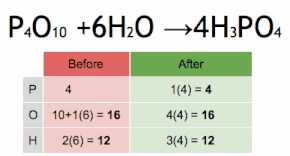
In mathematics and physics, vector is a term that refers informally to some quantities that cannot be expressed by a single number (a scalar), or to elements of some vector spaces.
Historically, vectors were introduced in geometry and physics (typically in mechanics) for quantities that have both a magnitude and a direction, such as displacements, forces and velocity. Such quantities are represented by geometric vectors in the same way as distances, masses and time are represented by real numbers.
The term vector is also used, in some contexts, for tuples, which are finite sequences (of numbers or other objects) of a fixed length.
Both geometric vectors and tuples can be added and scaled, and these vector operations led to the concept of a vector space, which is a set equipped with a vector addition and a scalar multiplication that satisfy some axioms generalizing the main properties of operations on the above sorts of vectors. A vector space formed by geometric vectors is called a Euclidean vector space, and a vector space formed by tuples is called a coordinate vector space.
Many vector spaces are considered in mathematics, such as extension fields, polynomial rings, algebras and function spaces. The term vector is generally not used for elements of these vector spaces, and is generally reserved for geometric vectors, tuples, and elements of unspecified vector spaces (for example, when discussing general properties of vector spaces).
Vectors in Euclidean geometry

In mathematics, physics, and engineering, a Euclidean vector or simply a vector (sometimes called a geometric vector or spatial vector) is a geometric object that has magnitude (or length) and direction. Euclidean vectors can be added and scaled to form a vector space. A Euclidean vector is frequently represented by a directed line segment, or graphically as an arrow connecting an initial point A with a terminal point B, and denoted by
A vector is what is needed to "carry" the point A to the point B; the Latin word vector means "carrier". It was first used by 18th century astronomers investigating planetary revolution around the Sun. The magnitude of the vector is the distance between the two points, and the direction refers to the direction of displacement from A to B. Many algebraic operations on real numbers such as addition, subtraction, multiplication, and negation have close analogues for vectors, operations which obey the familiar algebraic laws of commutativity, associativity, and distributivity. These operations and associated laws qualify Euclidean vectors as an example of the more generalized concept of vectors defined simply as elements of a vector space.
Vectors play an important role in physics: the velocity and acceleration of a moving object and the forces acting on it can all be described with vectors. Many other physical quantities can be usefully thought of as vectors. Although most of them do not represent distances (except, for example, position or displacement), their magnitude and direction can still be represented by the length and direction of an arrow. The mathematical representation of a physical vector depends on the coordinate system used to describe it. Other vector-like objects that describe physical quantities and transform in a similar way under changes of the coordinate system include pseudovectors and tensors.Vector spaces
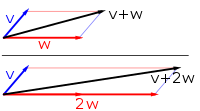
In mathematics and physics, a vector space (also called a linear space) is a set whose elements, often called vectors, may be added together and multiplied ("scaled") by numbers called scalars. Scalars are often real numbers, but can be complex numbers or, more generally, elements of any field. The operations of vector addition and scalar multiplication must satisfy certain requirements, called vector axioms. Real vector space and complex vector space are kinds of vector spaces based on different kinds of scalars: real coordinate space or complex coordinate space.
Vector spaces generalize Euclidean vectors, which allow modeling of physical quantities, such as forces and velocity, that have not only a magnitude, but also a direction. The concept of vector spaces is fundamental for linear algebra, together with the concept of matrices, which allows computing in vector spaces. This provides a concise and synthetic way for manipulating and studying systems of linear equations.
Vector spaces are characterized by their dimension, which, roughly speaking, specifies the number of independent directions in the space. This means that, for two vector spaces over a given field and with the same dimension, the properties that depend only on the vector-space structure are exactly the same (technically the vector spaces are isomorphic). A vector space is finite-dimensional if its dimension is a natural number. Otherwise, it is infinite-dimensional, and its dimension is an infinite cardinal. Finite-dimensional vector spaces occur naturally in geometry and related areas. Infinite-dimensional vector spaces occur in many areas of mathematics. For example, polynomial rings are countably infinite-dimensional vector spaces, and many function spaces have the cardinality of the continuum as a dimension.
Many vector spaces that are considered in mathematics are also endowed with other structures. This is the case of algebras, which include field extensions, polynomial rings, associative algebras and Lie algebras. This is also the case of topological vector spaces, which include function spaces, inner product spaces, normed spaces, Hilbert spaces and Banach spaces.Vectors in algebra
Every algebra over a field is a vector space, but elements of an algebra are generally not called vectors. However, in some cases, they are called vectors, mainly due to historical reasons.
- Vector quaternion, a quaternion with a zero real part
- Multivector or p-vector, an element of the exterior algebra of a vector space.
- Spinors, also called spin vectors, have been introduced for extending the notion of rotation vector. In fact, rotation vectors represent well rotations locally, but not globally, because a closed loop in the space of rotation vectors may induce a curve in the space of rotations that is not a loop. Also, the manifold of rotation vectors is orientable, while the manifold of rotations is not. Spinors are elements of a vector subspace of some Clifford algebra.
- Witt vector, an infinite sequence of elements of a commutative ring, which belongs to an algebra over this ring, and has been introduced for handling carry propagation in the operations on p-adic numbers.
Data represented by vectors
The set of tuples of n real numbers has a natural structure of vector space defined by component-wise addition and scalar multiplication. It is common to call these tuples vectors, even in contexts where vector-space operations do not apply. More generally, when some data can be represented naturally by vectors, they are often called vectors even when addition and scalar multiplication of vectors are not valid operations on these data. Here are some examples.
- Rotation vector, a Euclidean vector whose direction is that of the axis of a rotation and magnitude is the angle of the rotation.
- Burgers vector, a vector that represents the magnitude and direction of the lattice distortion of dislocation in a crystal lattice
- Interval vector, in musical set theory, an array that expresses the intervallic content of a pitch-class set
- Probability vector, in statistics, a vector with non-negative entries that sum to one.
- Random vector or multivariate random variable, in statistics, a set of real-valued random variables that may be correlated. However, a random vector may also refer to a random variable that takes its values in a vector space.
- Logical vector, a vector of 0s and 1s (Booleans).


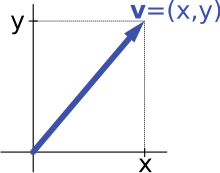








![{\displaystyle {\ce {2CH3OH->[{\overset {}{\ce {-H2O}}}]CH3OCH3}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a9322a7fd3bb1fb28dab61d3c7fa287ab389d3c7)