Glivec, a drug used in the treatment of several cancers, is marketed by Novartis, one of the world's major pharmaceutical companies.
The pharmaceutical industry discovers, develops, produces, and markets drugs or pharmaceutical drugs for use as medications to be administered (or self-administered) to patients to cure them, vaccinate them, or alleviate a symptom. Pharmaceutical companies may deal in generic or brand medications and medical devices. They are subject to a variety of laws and regulations that govern the patenting, testing, safety, efficacy and marketing of drugs.
History
Mid-1800s – 1945: From botanicals to the first synthetic drugs
The
modern pharmaceutical industry traces its roots to two sources. The
first of these were local apothecaries that expanded from their
traditional role distributing botanical drugs such as morphine and quinine to wholesale manufacture in the mid 1800s. Rational drug discovery from plants started particularly with the isolation of morphine,
analgesic and sleep-inducing agent from opium, by the German apothecary
assistant Friedrich Sertürner who named the compound after the Greek
god of dreams, Morpheus. By the late 1880s, German dye manufacturers had perfected the purification of individual organic compounds from tar and other mineral sources and had also established rudimentary methods in organic chemical synthesis.
The development of synthetic chemical methods allowed scientists to
systematically vary the structure of chemical substances, and growth in
the emerging science of pharmacology expanded their ability to evaluate the biological effects of these structural changes.
Epinephrine, norepinephrine, and amphetamine
By the 1890s, the profound effect of adrenal
extracts on many different tissue types had been discovered, setting
off a search both for the mechanism of chemical signalling and efforts
to exploit these observations for the development of new drugs. The
blood pressure raising and vasoconstrictive effects of adrenal extracts
were of particular interest to surgeons as hemostatic
agents and as treatment for shock, and a number of companies developed
products based on adrenal extracts containing varying purities of the
active substance. In 1897, John Abel of Johns Hopkins University identified the active principle as epinephrine, which he isolated in an impure state as the sulfate salt. Industrial chemist Jokichi Takamine later developed a method for obtaining epinephrine in a pure state, and licensed the technology to Parke-Davis. Parke-Davis marketed epinephrine under the trade name Adrenalin. Injected epinephrine proved to be especially efficacious for the acute treatment of asthma attacks, and an inhaled version was sold in the United States until 2011 (Primatene Mist). By 1929 epinephrine had been formulated into an inhaler for use in the treatment of nasal congestion.
While highly effective, the requirement for injection limited the use of epinephrine and orally active derivatives were sought. A structurally similar compound, ephedrine, (actually more similar to norepinephrine,) was identified by Japanese chemists in the Ma Huang
plant and marketed by Eli Lilly as an oral treatment for asthma.
Following the work of Henry Dale and George Barger at
Burroughs-Wellcome, academic chemist Gordon Alles synthesized
amphetamine and tested it in asthma patients in 1929. The drug proved to
have only modest anti-asthma effects, but produced sensations of
exhilaration and palpitations. Amphetamine was developed by Smith, Kline and French as a nasal decongestant under the trade name Benzedrine Inhaler. Amphetamine was eventually developed for the treatment of narcolepsy, post-encephalitic parkinsonism,
and mood elevation in depression and other psychiatric indications. It
received approval as a New and Nonofficial Remedy from the American
Medical Association for these uses in 1937 and remained in common use
for depression until the development of tricyclic antidepressants in the 1960s.
Discovery and development of the barbiturates
Diethylbarbituric acid was the first marketed barbiturate. It was sold by Bayer under the trade name Veronal
In 1903, Hermann Emil Fischer and Joseph von Mering
disclosed their discovery that diethylbarbituric acid, formed from the
reaction of diethylmalonic acid, phosphorus oxychloride and urea,
induces sleep in dogs. The discovery was patented and licensed to Bayer pharmaceuticals, which marketed the compound under the trade name Veronal
as a sleep aid beginning in 1904. Systematic investigations of the
effect of structural changes on potency and duration of action led to
the discovery of phenobarbital
at Bayer in 1911 and the discovery of its potent anti-epileptic
activity in 1912. Phenobarbital was among the most widely used drugs
for the treatment of epilepsy through the 1970s, and as of 2014, remains on the World Health Organizations list of essential medications. The 1950s and 1960s saw increased awareness of the addictive properties
and abuse potential of barbiturates and amphetamines and led to
increasing restrictions on their use and growing government oversight of
prescribers. Today, amphetamine is largely restricted to use in the
treatment of attention deficit disorder and phenobarbital in the treatment of epilepsy.
Insulin
A series of experiments performed from the late 1800s to the early 1900s revealed that diabetes is caused by the absence of a substance normally produced by the pancreas. In 1869, Oskar Minkowski and Joseph von Mering found that diabetes could be induced in dogs by surgical removal of the pancreas. In 1921, Canadian professor Frederick Banting
and his student Charles Best repeated this study, and found that
injections of pancreatic extract reversed the symptoms produced by
pancreas removal. Soon, the extract was demonstrated to work in people,
but development of insulin therapy as a routine medical procedure was
delayed by difficulties in producing the material in sufficient quantity
and with reproducible purity. The researchers sought assistance from
industrial collaborators at Eli Lilly and Co. based on the company's
experience with large scale purification of biological materials.
Chemist George B. Walden
of Eli Lilly and Company found that careful adjustment of the pH of the
extract allowed a relatively pure grade of insulin to be produced.
Under pressure from Toronto University and a potential patent challenge
by academic scientists who had independently developed a similar
purification method, an agreement was reached for non-exclusive
production of insulin by multiple companies. Prior to the discovery and
widespread availability of insulin therapy the life expectancy of
diabetics was only a few months.
Early anti-infective research: Salvarsan, Prontosil, Penicillin and vaccines
The
development of drugs for the treatment of infectious diseases was a
major focus of early research and development efforts; in 1900
pneumonia, tuberculosis, and diarrhea were the three leading causes of
death in the United States and mortality in the first year of life
exceeded 10%.
In 1911 arsphenamine, the first synthetic anti-infective drug, was developed by Paul Ehrlich
and chemist Alfred Bertheim of the Institute of Experimental Therapy in
Berlin. The drug was given the commercial name Salvarsan. Ehrlich, noting both the general toxicity of arsenic
and the selective absorption of certain dyes by bacteria, hypothesized
that an arsenic-containing dye with similar selective absorption
properties could be used to treat bacterial infections. Arsphenamine
was prepared as part of a campaign to synthesize a series of such
compounds, and found to exhibit partially selective toxicity.
Arsphenamine proved to be the first effective treatment for syphilis, a disease which prior to that time was incurable and led inexorably to severe skin ulceration, neurological damage, and death.
Ehrlich's approach of systematically varying the chemical
structure of synthetic compounds and measuring the effects of these
changes on biological activity was pursued broadly by industrial
scientists, including Bayer scientists Josef Klarer, Fritz Mietzsch, and Gerhard Domagk. This work, also based in the testing of compounds available from the German dye industry, led to the development of Prontosil, the first representative of the sulfonamide class of antibiotics.
Compared to arsphenamine, the sulfonamides had a broader spectrum of
activity and were far less toxic, rendering them useful for infections
caused by pathogens such as streptococci. In 1939, Domagk received the Nobel Prize in Medicine for this discovery. Nonetheless, the dramatic decrease in deaths from infectious diseases that occurred prior to World War II
was primarily the result of improved public health measures such as
clean water and less crowded housing, and the impact of anti-infective
drugs and vaccines was significant mainly after World War II.
In 1928, Alexander Fleming discovered the antibacterial effects of penicillin,
but its exploitation for the treatment of human disease awaited the
development of methods for its large scale production and purification.
These were developed by a U.S. and British government-led consortium of
pharmaceutical companies during the Second World War.
Early progress toward the development of vaccines occurred
throughout this period, primarily in the form of academic and
government-funded basic research directed toward the identification of
the pathogens responsible for common communicable diseases. In 1885 Louis Pasteur and Pierre Paul Émile Roux created the first rabies vaccine. The first diphtheria vaccines were produced in 1914 from a mixture of diphtheria toxin and antitoxin
(produced from the serum of an inoculated animal), but the safety of
the inoculation was marginal and it was not widely used. The United
States recorded 206,000 cases of diphtheria in 1921 resulting in 15,520
deaths. In 1923 parallel efforts by Gaston Ramon at the Pasteur Institute and Alexander Glenny at the Wellcome Research Laboratories (later part of GlaxoSmithKline) led to the discovery that a safer vaccine could be produced by treating diphtheria toxin with formaldehyde. In 1944, Maurice Hilleman of Squibb Pharmaceuticals developed the first vaccine against Japanese encephelitis. Hilleman would later move to Merck where he would play a key role in the development of vaccines against measles, mumps, chickenpox, rubella, hepatitis A, hepatitis B, and meningitis.
Unsafe drugs and early industry regulation
In 1937 over 100 people died after ingesting a solution of the antibacterial sulfanilamide formulated in the toxic solvent diethylene glycol
Prior to the 20th century drugs were generally produced by small
scale manufacturers with little regulatory control over manufacturing or
claims of safety and efficacy. To the extent that such laws did exist,
enforcement was lax. In the United States, increased regulation of
vaccines and other biological drugs was spurred by tetanus outbreaks and
deaths caused by the distribution of contaminated smallpox vaccine and
diphtheria antitoxin.
The Biologics Control Act of 1902 required that federal government
grant premarket approval for every biological drug and for the process
and facility producing such drugs. This was followed in 1906 by the Pure Food and Drugs Act,
which forbade the interstate distribution of adulterated or misbranded
foods and drugs. A drug was considered misbranded if it contained
alcohol, morphine, opium, cocaine, or any of several other potentially
dangerous or addictive drugs, and if its label failed to indicate the
quantity or proportion of such drugs. The government's attempts to use
the law to prosecute manufacturers for making unsupported claims of
efficacy were undercut by a Supreme Court ruling restricting the federal
government's enforcement powers to cases of incorrect specification of
the drug's ingredients.
In 1937 over 100 people died after ingesting "Elixir Sulfanilamide" manufactured by S.E. Massengill Company of Tennessee. The product was formulated in diethylene glycol, a highly toxic solvent that is now widely used as antifreeze.
Under the laws extant at that time, prosecution of the manufacturer
was possible only under the technicality that the product had been
called an "elixir", which literally implied a solution in ethanol. In
response to this episode, the U.S. Congress passed the Federal Food, Drug, and Cosmetic Act of 1938,
which for the first time required pre-market demonstration of safety
before a drug could be sold, and explicitly prohibited false therapeutic
claims.
The post-war years, 1945–1970
Further advances in anti-infective research
The aftermath of World War II saw an explosion in the discovery of new classes of antibacterial drugs including the cephalosporins (developed by Eli Lilly based on the seminal work of Giuseppe Brotzu and Edward Abraham), streptomycin (discovered during a Merck-funded research program in Selman Waksman's laboratory), the tetracyclines (discovered at Lederle Laboratories, now a part of Pfizer), erythromycin (discovered at Eli Lilly and Co.)
and their extension to an increasingly wide range of bacterial
pathogens. Streptomycin, discovered during a Merck-funded research
program in Selman Waksman's laboratory at Rutgers in 1943, became the
first effective treatment for tuberculosis. At the time of its
discovery, sanitoriums for the isolation of tuberculosis-infected people
were an ubiquitous feature of cities in developed countries, with 50%
dying within 5 years of admission.
A Federal Trade Commission report issued in 1958 attempted to
quantify the effect of antibiotic development on American public health.
The report found that over the period 1946-1955, there was a 42% drop
in the incidence of diseases for which antibiotics were effective and
only a 20% drop in those for which antibiotics were not effective. The
report concluded that "it appears that the use of antibiotics, early
diagnosis, and other factors have limited the epidemic spread and thus
the number of these diseases which have occurred". The study further
examined mortality rates for eight common diseases for which antibiotics
offered effective therapy (syphilis, tuberculosis, dysentery, scarlet
fever, whooping cough, meningococcal infections, and pneumonia), and
found a 56% decline over the same period. Notable among these was a 75% decline in deaths due to tuberculosis.
Measles cases reported in the United States before and after introduction of the vaccine.
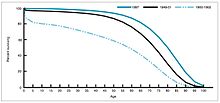
Percent surviving by age in 1900, 1950, and 1997.
During the years 1940-1955, the rate of decline in the U.S. death
rate accelerated from 2% per year to 8% per year, then returned to the
historical rate of 2% per year. The dramatic decline in the immediate
post-war years has been attributed to the rapid development of new
treatments and vaccines for infectious disease that occurred during
these years.
Vaccine development continued to accelerate, with the most notable achievement of the period being Jonas Salk's
1954 development of the polio vaccine under the funding of the
non-profit National Foundation for Infantile Paralysis. The vaccine
process was never patented, but was instead given to pharmaceutical
companies to manufacture as a low-cost generic. In 1960 Maurice Hilleman of Merck Sharp & Dohme identified the SV40
virus, which was later shown to cause tumors in many mammalian species.
It was later determined that SV40 was present as a contaminant in
polio vaccine lots that had been administered to 90% of the children in
the United States.
The contamination appears to have originated both in the original cell
stock and in monkey tissue used for production. In 2004 the United
States Cancer Institute announced that it had concluded that SV40 is not
associated with cancer in people.
Other notable new vaccines of the period include those for
measles (1962, John Franklin Enders of Children's Medical Center Boston,
later refined by Maurice Hilleman at Merck), Rubella (1969, Hilleman,
Merck) and mumps (1967, Hilleman, Merck)
The United States incidences of rubella, congenital rubella syndrome,
measles, and mumps all fell by more than 95% in the immediate aftermath of
widespread vaccination.
The first 20 years of licensed measles vaccination in the U.S.
prevented an estimated 52 million cases of the disease, 17,400 cases of mental retardation, and 5,200 deaths.
Development and marketing of antihypertensive drugs
Hypertension is a risk factor for atherosclerosis, heart failure, coronary artery disease, stroke, renal disease, and peripheral arterial disease, and is the most important risk factor for cardiovascular morbidity and mortality, in industrialized countries.
Prior to 1940 approximately 23% of all deaths among persons over age 50
were attributed to hypertension. Severe cases of hypertension were
treated by surgery.
Early developments in the field of treating hypertension included
quaternary ammonium ion sympathetic nervous system blocking agents, but
these compounds were never widely used due to their severe side
effects, because the long term health consequences of high blood
pressure had not yet been established, and because they had to be
administered by injection.
In 1952 researchers at Ciba discovered the first orally available vasodilator, hydralazine. A major shortcoming of hydralazine monotherapy was that it lost its effectiveness over time (tachyphylaxis). In the mid-1950s Karl H. Beyer, James M. Sprague, John E. Baer, and Frederick C. Novello of Merck and Co. discovered and developed chlorothiazide, which remains the most widely used antihypertensive drug today. This development was associated with a substantial decline in the mortality rate among people with hypertension. The inventors were recognized by a Public Health Lasker Award
in 1975 for "the saving of untold thousands of lives and the
alleviation of the suffering of millions of victims of hypertension".
A 2009 Cochrane review concluded that thiazide antihypertensive drugs reduce the risk of death (RR
0.89), stroke (RR 0.63), coronary heart disease (RR 0.84), and
cardiovascular events (RR 0.70) in people with high blood pressure.
In the ensuring years other classes of antihypertensive drug were
developed and found wide acceptance in combination therapy, including
loop diuretics (Lasix/furosemide, Hoechst Pharmaceuticals, 1963), beta blockers (ICI Pharmaceuticals, 1964) ACE inhibitors, and angiotensin receptor blockers.
ACE inhibitors reduce the risk of new onset kidney disease [RR 0.71]
and death [RR 0.84] in diabetic patients, irrespective of whether they
have hypertension.
Oral Contraceptives
Prior
to the second world war, birth control was prohibited in many
countries, and in the United States even the discussion of contraceptive
methods sometimes led to prosecution under Comstock laws. The history of the development of oral contraceptives is thus closely tied to the birth control movement and the efforts of activists Margaret Sanger, Mary Dennett, and Emma Goldman. Based on fundamental research performed by Gregory Pincus and synthetic methods for progesterone developed by Carl Djerassi at Syntex and by Frank Colton at G.D. Searle & Co., the first oral contraceptive, Enovid,
was developed by E.D. Searle and Co. and approved by the FDA in 1960.
The original formulation incorporated vastly excessive doses of
hormones, and caused severe side effects. Nonetheless, by 1962, 1.2
million American women were on the pill, and by 1965 the number had
increased to 6.5 million.
The availability of a convenient form of temporary contraceptive led to
dramatic changes in social mores including expanding the range of
lifestyle options available to women, reducing the reliance of women on
men for contraceptive practice, encouraging the delay of marriage, and
increasing pre-marital co-habitation.
Thalidomide and the Kefauver-Harris Amendments
Malformation of a baby born to a mother who had taken thalidomide while pregnant.
In the U.S., a push for revisions of the FD&C Act emerged from Congressional hearings led by Senator Estes Kefauver
of Tennessee in 1959. The hearings covered a wide range of policy
issues, including advertising abuses, questionable efficacy of drugs,
and the need for greater regulation of the industry. While momentum for
new legislation temporarily flagged under extended debate, a new tragedy
emerged that underscored the need for more comprehensive regulation and
provided the driving force for the passage of new laws.
On 12 September 1960, an American licensee, the William S.
Merrell Company of Cincinnati, submitted a new drug application for
Kevadon (thalidomide), a sedative that had been marketed in Europe since 1956. The FDA medical officer in charge of reviewing the compound, Frances Kelsey,
believed that the data supporting the safety of thalidomide was
incomplete. The firm continued to pressure Kelsey and the FDA to approve
the application until November 1961, when the drug was pulled off the
German market because of its association with grave congenital
abnormalities. Several thousand newborns in Europe and elsewhere
suffered the teratogenic
effects of thalidomide. Without approval from the FDA, the firm
distributed Kevadon to over 1,000 physicians there under the guise of
investigational use. Over 20,000 Americans received thalidomide in this
"study," including 624 pregnant patients, and about 17 known newborns
suffered the effects of the drug.
The thalidomide tragedy resurrected Kefauver's bill to enhance drug regulation that had stalled in Congress, and the Kefauver-Harris Amendment
became law on 10 October 1962. Manufacturers henceforth had to prove to
FDA that their drugs were effective as well as safe before they could
go on the US market. The FDA received authority to regulate advertising
of prescription drugs and to establish good manufacturing practices.
The law required that all drugs introduced between 1938 and 1962 had to
be effective. An FDA - National Academy of Sciences collaborative study
showed that nearly 40 percent of these products were not effective. A
similarly comprehensive study of over-the-counter products began ten
years later.
1970–1980s
Statins
In
1971, Akira Endo, a Japanese biochemist working for the pharmaceutical
company Sankyo, identified mevastatin (ML-236B), a molecule produced by
the fungus Penicillium citrinum, as an inhibitor of HMG-CoA reductase, a
critical enzyme used by the body to produce cholesterol. Animal trials showed very good inhibitory effect as in clinical trials,
however a long term study in dogs found toxic effects at higher doses
and as a result mevastatin was believed to be too toxic for human use.
Mevastatin was never marketed, because of its adverse effects of tumors,
muscle deterioration, and sometimes death in laboratory dogs.
P. Roy Vagelos, chief scientist and later CEO of Merck & Co, was interested, and made several trips to Japan starting in 1975. By 1978, Merck had isolated lovastatin (mevinolin, MK803) from the fungus Aspergillus terreus, first marketed in 1987 as Mevacor.
In April 1994, the results of a Merck-sponsored study, the Scandinavian Simvastatin Survival Study, were announced. Researchers tested simvastatin,
later sold by Merck as Zocor, on 4,444 patients with high cholesterol
and heart disease. After five years, the study concluded the patients
saw a 35% reduction in their cholesterol, and their chances of dying of a
heart attack were reduced by 42%. In 1995, Zocor and Mevacor both made Merck over US$1 billion. Endo was awarded the 2006 Japan Prize, and the Lasker-DeBakey Clinical Medical Research Award in 2008. For his "pioneering research into a new class of molecules" for "lowering cholesterol,"
Research and development
Drug discovery is the process by which potential drugs
are discovered or designed. In the past most drugs have been discovered
either by isolating the active ingredient from traditional remedies or
by serendipitous discovery. Modern biotechnology often focuses on understanding the metabolic pathways related to a disease state or pathogen, and manipulating these pathways using molecular biology or biochemistry. A great deal of early-stage drug discovery has traditionally been carried out by universities and research institutions.
Drug development refers to activities undertaken after a
compound is identified as a potential drug in order to establish its
suitability as a medication. Objectives of drug development are to
determine appropriate formulation and dosing, as well as to establish safety. Research in these areas generally includes a combination of in vitro studies, in vivo studies, and clinical trials. The cost of late stage development has meant it is usually done by the larger pharmaceutical companies.
Often, large multinational corporations exhibit vertical integration,
participating in a broad range of drug discovery and development,
manufacturing and quality control, marketing, sales, and distribution.
Smaller organizations, on the other hand, often focus on a specific
aspect such as discovering drug candidates or developing formulations.
Often, collaborative agreements between research organizations and large
pharmaceutical companies are formed to explore the potential of new
drug substances. More recently, multi-nationals are increasingly relying
on contract research organizations to manage drug development.
The cost of innovation
Drug
discovery and development is very expensive; of all compounds
investigated for use in humans only a small fraction are eventually approved in most nations by government appointed medical institutions or boards, who have to approve new drugs before they can be marketed in those countries. In 2010 18 NMEs (New Molecular Entities) were approved and three biologics
by the FDA, or 21 in total, which is down from 26 in 2009 and 24 in
2008. On the other hand, there were only 18 approvals in total in 2007
and 22 back in 2006. Since 2001, the Center for Drug Evaluation and
Research has averaged 22.9 approvals a year.
This approval comes only after heavy investment in pre-clinical development and clinical trials, as well as a commitment to ongoing safety monitoring.
Drugs which fail part-way through this process often incur large costs,
while generating no revenue in return. If the cost of these failed
drugs is taken into account, the cost of developing a successful new
drug (new chemical entity, or NCE), has been estimated at about US$1.3 billion (not including marketing expenses).
Professors Light and Lexchin reported in 2012, however, that the rate
of approval for new drugs has been a relatively stable average rate of
15 to 25 for decades.
Industry-wide research and investment reached a record $65.3 billion in 2009.
While the cost of research in the U.S. was about $34.2 billion between
1995 and 2010, revenues rose faster (revenues rose by $200.4 billion in
that time).
A study by the consulting firm Bain & Company
reported that the cost for discovering, developing and launching (which
factored in marketing and other business expenses) a new drug (along
with the prospective drugs that fail) rose over a five-year period to
nearly $1.7 billion in 2003. According to Forbes, by 2010 development costs were between $4 billion to $11 billion per drug.
Some of these estimates also take into account the opportunity cost of investing capital many years before revenues are realized.
Because of the very long time needed for discovery, development, and
approval of pharmaceuticals, these costs can accumulate to nearly half
the total expense. A direct consequence within the pharmaceutical
industry value chain is that major pharmaceutical multinationals tend to
increasingly outsource risks related to fundamental research, which
somewhat reshapes the industry ecosystem with biotechnology companies
playing an increasingly important role, and overall strategies being
redefined accordingly. Some approved drugs, such as those based on re-formulation of an existing active ingredient (also referred to as Line-extensions) are much less expensive to develop.
Controversies
Due
to repeated accusations and findings that some clinical trials
conducted or funded by pharmaceutical companies may report only positive
results for the preferred medication, the industry has been looked at
much more closely by independent groups and government agencies.
In response to specific cases in which unfavorable data from pharmaceutical company-sponsored research was not published, the Pharmaceutical Research and Manufacturers of America
have published new guidelines urging companies to report all findings
and limit the financial involvement in drug companies of researchers. US congress signed into law a bill which requires phase II and phase III clinical trials to be registered by the sponsor on the clinicaltrials.gov website run by the NIH.
Drug researchers not directly employed by pharmaceutical
companies often look to companies for grants, and companies often look
to researchers for studies that will make their products look favorable.
Sponsored researchers are rewarded by drug companies, for example with
support for their conference/symposium costs. Lecture scripts and even
journal articles presented by academic researchers may actually be
"ghost-written" by pharmaceutical companies.
An investigation by ProPublica
found that at least 21 doctors have been paid more than $500,000 for
speeches and consulting by drugs manufacturers since 2009, with half of
the top earners working in psychiatry, and about $2 billion in total paid to doctors for such services. AstraZeneca, Johnson & Johnson and Eli Lilly
have paid billions of dollars in federal settlements over allegations
that they paid doctors to promote drugs for unapproved uses. Some
prominent medical schools have since tightened rules on faculty
acceptance of such payments by drug companies.
In contrast to this viewpoint, an article and associated editorial in the New England Journal of Medicine
in May 2015 emphasized the importance of pharmaceutical
industry-physician interactions for the development of novel treatments,
and argued that moral outrage over industry malfeasance had
unjustifiably led many to overemphasize the problems created by
financial conflicts of interest. The article noted that major
healthcare organizations such as National Center for Advancing
Translational Sciences of the National Institutes of Health, the
President's Council of Advisors on Science and Technology, the World
Economic Forum, the Gates Foundation, the Wellcome Trust, and the Food
and Drug Administration had encouraged greater interactions between
physicians and industry in order to bring greater benefits to patients.
Product approval
In the United States, new pharmaceutical products must be approved by the Food and Drug Administration (FDA) as being both safe and effective. This process generally involves submission of an Investigational New Drug
filing with sufficient pre-clinical data to support proceeding with
human trials. Following IND approval, three phases of progressively
larger human clinical trials may be conducted. Phase I generally studies
toxicity using healthy volunteers. Phase II can include pharmacokinetics and dosing
in patients, and Phase III is a very large study of efficacy in the
intended patient population. Following the successful completion of
phase III testing, a New Drug Application
is submitted to the FDA. The FDA review the data and if the product is
seen as having a positive benefit-risk assessment, approval to market
the product in the US is granted.
A fourth phase of post-approval surveillance is also often
required due to the fact that even the largest clinical trials cannot
effectively predict the prevalence of rare side-effects. Postmarketing surveillance
ensures that after marketing the safety of a drug is monitored closely.
In certain instances, its indication may need to be limited to
particular patient groups, and in others the substance is withdrawn from
the market completely.
The FDA provides information about approved drugs at the Orange Book site.
In the UK, the Medicines and Healthcare Products Regulatory Agency approves drugs for use, though the evaluation is done by the European Medicines Agency, an agency of the European Union based in London. Normally an approval in the UK and other European countries comes later than one in the USA. Then it is the National Institute for Health and Care Excellence (NICE), for England and Wales, who decides if and how the National Health Service (NHS) will allow (in the sense of paying for) their use. The British National Formulary is the core guide for pharmacists and clinicians.
In many non-US western countries a 'fourth hurdle' of cost effectiveness analysis has developed before new technologies can be provided. This focuses on the efficiency (in terms of the cost per QALY)
of the technologies in question rather than their efficacy. In England
and Wales NICE decides whether and in what circumstances drugs and
technologies will be made available by the NHS, whilst similar
arrangements exist with the Scottish Medicines Consortium in Scotland, and the Pharmaceutical Benefits Advisory Committee
in Australia. A product must pass the threshold for cost-effectiveness
if it is to be approved. Treatments must represent 'value for money' and
a net benefit to society.
Orphan drugs
There are special rules
for certain rare diseases ("orphan diseases") in several major drug
regulatory territories. For example, diseases involving fewer than
200,000 patients in the United States, or larger populations in certain
circumstances are subject to the Orphan Drug Act. Because medical research and development of drugs to treat such
diseases is financially disadvantageous, companies that do so are
rewarded with tax reductions, fee waivers, and market exclusivity on
that drug for a limited time (seven years), regardless of whether the
drug is protected by patents.
Global sales
| Company | Pharma Sales ($ billion) |
|---|---|
| Pfizer |
53,370 |
| GlaxoSmithKline |
38,30 |
| Sanofi-Aventis |
40,14 |
| Roche |
27,290 |
| AstraZeneca |
21,45 |
| Johnson & Johnson |
81,38 |
| Novartis |
52,67 |
| Merck & Co |
41,37 |
| Wyeth |
1,68 |
| Lilly |
24,28 |
| Bristol-Myers Squibb |
22,04 |
| Boehringer Ingelheim |
13,860 |
| Amgen |
23,32 |
| Abbott Laboratories |
30,40 |
| Bayer |
10,162 |
| Takeda |
8,716 |
| Schering-Plough |
8,561 |
| Teva |
19,75 |
| Genentech |
7,640 |
| Astellas |
7,390 |
| Novo Nordisk |
7,087 |
| Daiichi Sankyo |
6,790 |
| Baxter International |
6,461 |
| Merck KGaA |
5,643 |
| Eisai |
4,703 |
In 2011, global spending on prescription drugs topped $954 billion,
even as growth slowed somewhat in Europe and North America. The United
States accounts for more than a third of the global pharmaceutical
market, with $340 billion in annual sales followed by the EU and Japan. Emerging markets such as China, Russia, South Korea and Mexico outpaced that market, growing a huge 81 percent.
The top ten best-selling drugs of 2013 totaled $75.6 billion in sales, with the anti-inflammatory drug Humira
being the best-selling drug worldwide at $10.7 billion in sales. The
second and third best selling were Enbrel and Remicade, respectively.
The top three best-selling drugs in the United States in 2013 were
Abilify ($6.3 billion,) Nexium ($6 billion) and Humira ($5.4 billion). The best-selling drug ever, Lipitor, averaged $13 billion annually and netted $141 billion total over its lifetime before Pfizer's patent expired in November 2011.
IMS Health
publishes an analysis of trends expected in the pharmaceutical industry
in 2007, including increasing profits in most sectors despite loss of
some patents, and new 'blockbuster' drugs on the horizon.
Patents and generics
Depending on a number of considerations, a company may apply for and be granted a patent for the drug, or the process of producing the drug, granting exclusivity rights typically for about 20 years.
However, only after rigorous study and testing, which takes 10 to 15
years on average, will governmental authorities grant permission for the
company to market and sell the drug.
Patent protection enables the owner of the patent to recover the costs
of research and development through high profit margins for the branded drug. When the patent protection for the drug expires, a generic drug
is usually developed and sold by a competing company. The development
and approval of generics is less expensive, allowing them to be sold at a
lower price. Often the owner of the branded drug will introduce a
generic version before the patent expires in order to get a head start
in the generic market.
Restructuring has therefore become routine, driven by the patent
expiration of products launched during the industry's "golden era" in
the 1990s and companies' failure to develop sufficient new blockbuster
products to replace lost revenues.
Prescriptions
In
the U.S., the value of prescriptions increased over the period of 1995
to 2005 by 3.4 billion annually, a 61 percent increase. Retail sales of prescription drugs
jumped 250 percent from $72 billion to $250 billion, while the average
price of prescriptions more than doubled from $30 to $68.
Marketing
Advertising
is common in healthcare journals as well as through more mainstream
media routes. In some countries, notably the US, they are allowed to
advertise directly to the general public. Pharmaceutical companies
generally employ sales people (often called 'drug reps' or, an older
term, 'detail men') to market directly and personally to physicians and
other healthcare providers. In some countries, notably the US, pharmaceutical companies also employ lobbyists to influence politicians. Marketing of prescription drugs in the US is regulated by the federal Prescription Drug Marketing Act of 1987.
To healthcare professionals
The book Bad Pharma
also discusses the influence of drug representatives, how ghostwriters
are employed by the drug companies to write papers for academics to
publish, how independent the academic journals really are, how the drug
companies finance doctors' continuing education, and how patients'
groups are often funded by industry.
Direct to consumer advertising
Since the 1980s new methods of marketing for prescription drugs to
consumers have become important. Direct-to-consumer media advertising
was legalised in the FDA Guidance for Industry on Consumer-Directed
Broadcast Advertisements.
Controversy about drug marketing and lobbying
There
has been increasing controversy surrounding pharmaceutical marketing
and influence. There have been accusations and findings of influence on
doctors and other health professionals through drug reps including the
constant provision of marketing 'gifts' and biased information to health
professionals;
highly prevalent advertising in journals and conferences; funding
independent healthcare organizations and health promotion campaigns;
lobbying physicians and politicians (more than any other industry in the
US); sponsorship of medical schools or nurse training; sponsorship of continuing educational events, with influence on the curriculum; and hiring physicians as paid consultants on medical advisory boards.
Some advocacy groups, such as No Free Lunch and AllTrials,
have criticized the effect of drug marketing to physicians because they
say it biases physicians to prescribe the marketed drugs even when
others might be cheaper or better for the patient.
There have been related accusations of disease mongering (over-medicalising)
to expand the market for medications. An inaugural conference on that
subject took place in Australia in 2006. In 2009, the Government-funded National Prescribing Service launched the "Finding Evidence – Recognizing Hype" program, aimed at educating GPs on methods for independent drug analysis.
A 2005 review by a special committee of the UK government came to all the above conclusions in a European Union context whilst also highlighting the contributions and needs of the industry.
Meta-analyses have shown that psychiatric studies sponsored by
pharmaceutical companies are several times more likely to report
positive results, and if a drug company employee is involved the effect
is even larger. Influence has also extended to the training of doctors and nurses in medical schools, which is being fought.
It has been argued that the design of the Diagnostic and Statistical Manual of Mental Disorders and the expansion of the criteria represents an increasing medicalization of human nature, or "disease mongering", driven by drug company influence on psychiatry. The potential for direct conflict of interest
has been raised, partly because roughly half the authors who selected
and defined the DSM-IV psychiatric disorders had or previously had
financial relationships with the pharmaceutical industry.
In the US, starting in 2013, under the Physician Financial
Transparency Reports (part of the Sunshine Act), the Centers for
Medicare & Medicaid Services has to collect information from
applicable manufacturers and group purchasing organizations in order to
report information about their financial relationships with physicians
and hospitals. Data are made public in the Centers for Medicare &
Medicaid Services website. The expectation is that relationship between
doctors and Pharmaceutical industry will become fully transparent.
In a report conducted by the Center for Responsive Politics,
there were more than 1,100 lobbyists working in some capacity for the
pharmaceutical business in 2017. In the first quarter of 2017, the
health products and pharmaceutical industry spent $78 million on
lobbying member of the United States Congress.
Regulatory issues
Ben Goldacre has argued that regulators – such as the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK, or the Food and Drug Administration
(FDA) in the United States – advance the interests of the drug
companies rather than the interests of the public due to revolving door
exchange of employees between the regulator and the companies and
friendships develop between regulator and company employees.
He argues that regulators do not require that new drugs offer an
improvement over what is already available, or even that they be
particularly effective.
Others have argued that excessive regulation suppresses
therapeutic innovation, and that the current cost of regulator-required
clinical trials prevents the full exploitation of new genetic and
biological knowledge for the treatment of human disease. A 2012 report
by the President's Council of Advisors on Science and Technology made
several key recommendations to reduce regulatory burdens to new drug
development, including 1) expanding the FDA's use of accelerated
approval processes, 2) creating an expedited approval pathway for drugs
intended for use in narrowly defined populations, and 3) undertaking
pilot projects designed to evaluate the feasibility of a new, adaptive
drug approval process.
Pharmaceutical fraud
Pharmaceutical fraud involves deceptions which bring financial gain to a pharmaceutical company. It affects individuals and public and private insurers. There are several different schemes used to defraud the health care system
which are particular to the pharmaceutical industry. These include:
Good Manufacturing Practice (GMP) Violations, Off Label Marketing, Best
Price Fraud, CME Fraud, Medicaid Price Reporting, and Manufactured
Compound Drugs. Of this amount $2.5 billion was recovered through False Claims Act cases in FY 2010. Examples of fraud cases include the GlaxoSmithKline $3 billion settlement, Pfizer $2.3 billion settlement and Merck & Co. $650 million settlement. Damages from fraud can be recovered by use of the False Claims Act, most commonly under the qui tam provisions which rewards an individual for being a "whistleblower", or relator (law).
Every major company selling the antipsychotics — Bristol-Myers Squibb, Eli Lilly, Pfizer, AstraZeneca and Johnson & Johnson
— has either settled recent government cases, under the False Claims
Act, for hundreds of millions of dollars or is currently under
investigation for possible health care fraud. Following charges of
illegal marketing, two of the settlements set records last year for the
largest criminal fines ever imposed on corporations. One involved Eli
Lilly's antipsychotic Zyprexa, and the other involved Bextra. In the Bextra case, the government also charged Pfizer with illegally marketing another antipsychotic, Geodon; Pfizer settled that part of the claim for $301 million, without admitting any wrongdoing.
On 2 July 2012, GlaxoSmithKline
pleaded guilty to criminal charges and agreed to a $3 billion
settlement of the largest health-care fraud case in the U.S. and the
largest payment by a drug company. The settlement is related to the company's illegal promotion of prescription drugs, its failure to report safety data, bribing doctors, and promoting medicines for uses for which they were not licensed. The drugs involved were Paxil, Wellbutrin, Advair, Lamictal, and Zofran for off-label, non-covered uses. Those and the drugs Imitrex, Lotronex, Flovent, and Valtrex were involved in the kickback scheme.
The following is a list of the four largest settlements reached
with pharmaceutical companies from 1991 to 2012, rank ordered by the
size of the total settlement. Legal claims against the pharmaceutical
industry have varied widely over the past two decades, including Medicare and Medicaid fraud, off-label promotion, and inadequate manufacturing practices.
| Company | Settlement | Violation(s) | Year | Product(s) | Laws allegedly violated (if applicable) |
|---|---|---|---|---|---|
| GlaxoSmithKline | $3 billion | Off-label promotion/ failure to disclose safety data |
2012 | Avandia/Wellbutrin/Paxil | False Claims Act/FDCA |
| Pfizer | $2.3 billion | Off-label promotion/kickbacks | 2009 | Bextra/Geodon/ Zyvox/Lyrica |
False Claims Act/FDCA |
| Abbott Laboratories | $1.5 billion | Off-label promotion | 2012 | Depakote | False Claims Act/FDCA |
| Eli Lilly | $1.4 billion | Off-label promotion | 2009 | Zyprexa | False Claims Act/FDCA |
Developing world
Patents
Patents have been criticized in the developing world, as they are thought to reduce access to existing medicines. Reconciling patents and universal access to medicine would require an efficient international policy of price discrimination. Moreover, under the TRIPS agreement of the World Trade Organization, countries must allow pharmaceutical products to be patented. In 2001, the WTO adopted the Doha Declaration,
which indicates that the TRIPS agreement should be read with the goals
of public health in mind, and allows some methods for circumventing
pharmaceutical monopolies: via compulsory licensing or parallel imports, even before patent expiration.
In March 2001, 40 multi-national pharmaceutical companies brought litigation against South Africa for its Medicines Act,
which allowed the generic production of antiretroviral drugs (ARVs) for
treating HIV, despite the fact that these drugs were on-patent. HIV was and is an epidemic
in South Africa, and ARVs at the time cost between 10,000 and US$15,000
per patient per year. This was unaffordable for most South African
citizens, and so the South African government committed to providing
ARVs at prices closer to what people could afford. To do so, they would
need to ignore the patents on drugs and produce generics within the
country (using a compulsory license), or import them from abroad. After
international protest in favour of public health rights (including the
collection of 250,000 signatures by MSF),
the governments of several developed countries (including The
Netherlands, Germany, France, and later the US) backed the South African
government, and the case was dropped in April of that year.
In 2016, GlaxoSmithKline (the worlds 6th largest Pharmaceutical)
announced that it would be dropping its patents in poor countries so as
to allow independent companies to make and sell versions of its drugs in
those areas, thereby widening the public access to them. GlaxoSmithKline published a list of 50 countries they would no longer hold patents in, affecting 1 billion people worldwide.
Charitable programs
In
2011 four of the top 20 corporate charitable donations and eight of the
top 30 corporate charitable donations came from pharmaceutical
manufacturers. The bulk of corporate charitable donations (69% as of
2012) comes by way of non-cash charitable donations, the majority of
which again were donations contributed by pharmaceutical companies.
Some of those large pharmaceutical companies are “patient assistance”
foundations, providing financial support to individuals in purchasing
prescription medicines, but pharmaceutical companies are also huge
givers of in-kind products, i.e. presumably their own drugs. Non-cash
donations of product can be "profit maximizing…as part of an inventory
control issue when they have excess inventories” for some corporations,
says Patrick Rooney of Giving USA in an interview with Nonprofit
Quarterly.
Charitable programs and drug discovery & development efforts by pharmaceutical companies include:
- "Merck's Gift", wherein billions of river blindness drugs were donated in Africa
- Pfizer's gift of free/discounted fluconazole and other drugs for AIDS in South Africa
- GSK's commitment to give free albendazole tablets to the WHO for, and until, the elimination of lymphatic filariasis worldwide.
- In 2006, Novartis committed US$755 million in corporate citizenship initiatives around the world, particularly focusing on improving access to medicines in the developing world through its Access to Medicine projects, including donations of medicines to patients affected by leprosy, tuberculosis, and malaria; Glivec patient assistance programs; and relief to support major humanitarian organisations with emergency medical needs.