From Wikipedia, the free encyclopedia
Positron-emission tomography (
PET)
[1] is a
nuclear medicine functional imaging
technique that is used to observe metabolic processes in the body as an
aid to the diagnosis of disease. The system detects pairs of
gamma rays emitted indirectly by a
positron-emitting
radionuclide (
tracer),
which is introduced into the body on a biologically active molecule.
Three-dimensional images of tracer concentration within the body are
then constructed by computer analysis. In modern
PET-CT scanners, three-dimensional imaging is often accomplished with the aid of a
CT X-ray scan performed on the patient during the same session, in the same machine.
If the biologically active molecule chosen for PET is
fludeoxyglucose (FDG), an
analogue of
glucose,
the concentrations of tracer imaged will indicate tissue metabolic
activity as it corresponds to the regional glucose uptake. Use of this
tracer to explore the possibility of
cancer metastasis
(i.e., spreading to other sites) is the most common type of PET scan in
standard medical care (90% of current scans). Less often, other
radioactive tracers
are used to image the tissue concentration of other types of molecules
of interest. One of the disadvantages of PET scanners is their operating
cost.
[2]
Uses
PET/CT-System with 16-slice CT; the ceiling mounted device is an injection pump for CT contrast agent
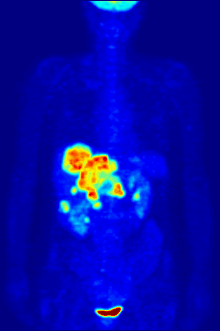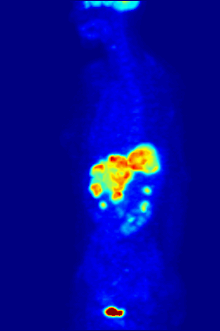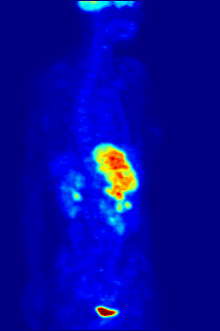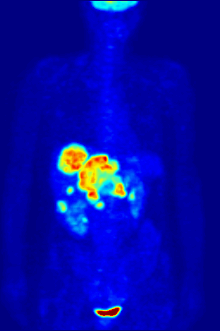
Whole-body PET scan using 18F-FDG
PET is both a medical and research tool. It is used heavily in clinical
oncology (
medical imaging of
tumours and the search for
metastases),
and for clinical diagnosis of certain diffuse brain diseases such as
those causing various types of dementias. PET is also an important
research tool to map normal human brain and heart function, and support
drug development.
PET is also used in pre-clinical studies using animals, where it
allows repeated investigations into the same subjects. This is
particularly valuable in cancer research, as it results in an increase
in the statistical quality of the data (subjects can act as their own
control) and substantially reduces the numbers of animals required for a
given study.
Alternative methods of scanning include
x-ray computed tomography (CT),
magnetic resonance imaging (MRI) and
functional magnetic resonance imaging (fMRI),
ultrasound and
single-photon emission computed tomography (SPECT).
While some imaging scans such as CT and MRI isolate organic anatomic
changes in the body, PET and SPECT are capable of detecting areas of
molecular biology
detail (even prior to anatomic change). PET scanning does this using
radiolabelled molecular probes that have different rates of uptake
depending on the type and function of tissue involved. Changing of
regional blood flow in various anatomic structures (as a measure of the
injected positron emitter) can be visualized and relatively quantified
with a PET scan.
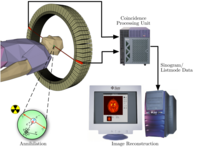
PET imaging is best performed using a dedicated PET scanner. It is
also possible to acquire PET images using a conventional dual-head
gamma camera
fitted with a coincidence detector. Although the quality of
gamma-camera PET is considerably lower and acquisition is slower, this
method allows institutions with low demand for PET to provide on-site
imaging, instead of referring patients to another centre or relying on a
visit by a mobile scanner.
Oncology
PET scanning with the tracer
fluorine-18 (F-18)
fluorodeoxyglucose (FDG), called FDG-PET, is widely used in clinical
oncology. This tracer is a
glucose analog that is taken up by glucose-using cells and phosphorylated by
hexokinase (whose
mitochondrial form is greatly elevated in rapidly growing
malignant tumors). A typical dose of FDG used in an oncological scan has an effective radiation dose of 14
mSv.
[3] Because the
oxygen atom that is replaced by F-18 to generate FDG is required for the next step in
glucose metabolism
in all cells, no further reactions occur in FDG. Furthermore, most
tissues (with the notable exception of liver and kidneys) cannot remove
the
phosphate added by
hexokinase. This means that FDG is trapped in any cell that takes it up until it decays, since
phosphorylated
sugars, due to their ionic charge, cannot exit from the cell. This
results in intense radiolabeling of tissues with high glucose uptake,
such as the brain, the liver, and most cancers. As a result, FDG-PET can
be used for diagnosis, staging, and monitoring treatment of cancers,
particularly in
Hodgkin's lymphoma,
non-Hodgkin lymphoma, and
lung cancer.
[citation needed]
A few other isotopes and radiotracers are slowly being introduced into oncology for specific purposes. For example,
11C-labelled
metomidate (11C-metomidate), has been used to detect tumors of
adrenocortical origin.
[4][5] Also,
FDOPA PET-CT, in centers which offer it, has proven to be a more sensitive alternative to finding, and also localizing,
pheochromocytoma than the
MIBG scan.
[6][7][8]
Neuroimaging
-

PET scan of the human brain
-
Neurology: PET neuroimaging
is based on an assumption that areas of high radioactivity are
associated with brain activity. What is actually measured indirectly is
the flow of blood to different parts of the brain, which is, in general,
believed to be correlated, and has been measured using the tracer oxygen-15. Because of its 2-minute half-life, O-15 must be piped directly from a medical cyclotron
for such uses, which is difficult. In practice, since the brain is
normally a rapid user of glucose, and since brain pathologies such as Alzheimer's disease
greatly decrease brain metabolism of both glucose and oxygen in tandem,
standard FDG-PET of the brain, which measures regional glucose use, may
also be successfully used to differentiate Alzheimer's disease from
other dementing processes, and also to make early diagnoses of
Alzheimer's disease. The advantage of FDG-PET for these uses is its much
wider availability. PET imaging with FDG can also be used for
localization of seizure focus: A seizure focus will appear as
hypometabolic during an interictal scan. Several radiotracers (i.e. radioligands) have been developed for PET that are ligands for specific neuroreceptor subtypes such as [11C] raclopride, [18F] fallypride and [18F] desmethoxyfallypride for dopamine D2/D3 receptors, [11C] McN 5652 and [11C] DASB for serotonin transporters, [18F] Mefway for serotonin 5HT1A receptors, [18F] Nifene for nicotinic acetylcholine receptors or enzyme substrates (e.g. 6-FDOPA for the AADC enzyme).
These agents permit the visualization of neuroreceptor pools in the
context of a plurality of neuropsychiatric and neurologic illnesses.
- The development of a number of novel probes for noninvasive, in vivo
PET imaging of neuroaggregate in human brain has brought amyloid
imaging to the doorstep of clinical use. The earliest amyloid imaging
probes included 2-(1-{6-[(2-[18F]fluoroethyl)(methyl)amino]-2-naphthyl}ethylidene)malononitrile ([18F]FDDNP)[9] developed at the University of California, Los Angeles and N-methyl-[11C]2-(4'-methylaminophenyl)-6-hydroxybenzothiazole[10] (termed Pittsburgh compound B) developed at the University of Pittsburgh. These amyloid imaging probes permit the visualization of amyloid
plaques in the brains of Alzheimer's patients and could assist
clinicians in making a positive clinical diagnosis of AD pre-mortem and
aid in the development of novel anti-amyloid therapies. [11C]PMP (N-[11C]methylpiperidin-4-yl
propionate) is a novel radiopharmaceutical used in PET imaging to
determine the activity of the acetylcholinergic neurotransmitter system
by acting as a substrate for acetylcholinesterase. Post-mortem
examination of AD patients have shown decreased levels of
acetylcholinesterase. [11C]PMP is used to map the
acetylcholinesterase activity in the brain, which could allow for
pre-mortem diagnoses of AD and help to monitor AD treatments.[11] Avid Radiopharmaceuticals of Philadelphia has developed a compound called 18F-AV-45 that uses the longer-lasting radionuclide fluorine-18 to detect amyloid plaques using PET scans.[12]
Cardiology
Cardiology,
atherosclerosis and vascular disease study: In clinical
cardiology, FDG-PET can identify so-called "
hibernating myocardium", but its
cost-effectiveness in this role versus
SPECT is unclear. FDG-PET imaging of
atherosclerosis to detect patients at risk of
stroke is also feasible and can help test the efficacy of novel anti-atherosclerosis therapies.
[17]
Infectious diseases
Imaging infections with
molecular imaging
technologies can improve diagnosis and treatment follow-up. PET has
been widely used to image bacterial infections clinically by using
fluorodeoxyglucose (FDG) to identify the infection-associated inflammatory response.
Three different PET contrast agents have been developed to image bacterial infections in vivo: [
18F]
maltose,
[18] [
18F]maltohexaose and [
18F]2-fluorodeoxy
sorbitol (FDS).
[19] FDS has also the added benefit of being able to target only
Enterobacteriaceae.
Pharmacokinetics
Pharmacokinetics: In pre-clinical trials, it is possible to
radiolabel
a new drug and inject it into animals. Such scans are referred to as
biodistribution studies. The uptake of the drug, the tissues in which it
concentrates, and its eventual elimination, can be monitored far more
quickly and cost effectively than the older technique of killing and
dissecting the animals to discover the same information. Much more
commonly, drug occupancy at a purported site of action can be inferred
indirectly by competition studies between unlabeled drug and
radiolabeled compounds known apriori to bind with specificity to the
site. A single radioligand can be used this way to test many potential
drug candidates for the same target. A related technique involves
scanning with radioligands that compete with an endogenous (naturally
occurring) substance at a given receptor to demonstrate that a drug
causes the release of the natural substance.
[citation needed]
Small animal imaging
PET
technology for small animal imaging: A miniature PE tomograph has been
constructed that is small enough for a fully conscious and mobile rat to
wear on its head while walking around.
[20] This RatCAP (Rat Conscious Animal PET) allows animals to be scanned without the confounding effects of
anesthesia.
PET scanners designed specifically for imaging rodents, often referred
to as microPET, as well as scanners for small primates, are marketed for
academic and pharmaceutical research. The scanners are apparently based
on microminiature scintillators and amplified avalanche photodiodes
(APDs) through a new system recently invented uses single chip silicon
photomultipliers.
Musculo-skeletal imaging
Musculoskeletal
imaging: PET has been shown to be a feasible technique for studying
skeletal muscles during exercises like walking.
[21]
One of the main advantages of using PET is that it can also provide
muscle activation data about deeper lying muscles such as the
vastus intermedialis and the
gluteus minimus, as compared to other muscle studying techniques like
electromyography,
which can be used only on superficial muscles (i.e., directly under the
skin). A clear disadvantage is that PET provides no timing information
about muscle activation because it has to be measured after the exercise
is completed. This is due to the time it takes for FDG to accumulate in
the activated muscles.
Safety
PET scanning is non-invasive, but it does involve exposure to
ionizing radiation.
[2]
18F-FDG, which is now the standard radiotracer used for PET neuroimaging and cancer patient management,
[22] has an effective radiation dose of 14
mSv.
[3]
The amount of radiation in 18F-FDG is similar to the effective dose of spending one year in the American city of
Denver, Colorado (12.4
mSv/year).
[23]
For comparison, radiation dosage for other medical procedures range
from 0.02 mSv for a chest x-ray and 6.5–8 mSv for a CT scan of the
chest.
[24][25] Average civil aircrews are exposed to 3 mSv/year,
[26] and the whole body occupational dose limit for nuclear energy workers in the USA is 50mSv/year.
[27] For scale, see
Orders of magnitude (radiation).
For
PET-CT scanning, the radiation exposure may be substantial—around 23–26
mSv (for a 70 kg person—dose is likely to be higher for higher body weights).
[28]
Operation
Schematic view of a detector block and ring of a PET scanner
Radionuclides and radiotracers
Radionuclides used in PET scanning are typically
isotopes with short
half-lives [2] such as
carbon-11 (~20 min),
nitrogen-13 (~10 min),
oxygen-15 (~2 min),
fluorine-18 (~110 min),
gallium-68 (~67 min),
zirconium-89 (~78.41 hours), or
rubidium-82(~1.27 min). These radionuclides are incorporated either into compounds normally used by the body such as
glucose (or glucose analogues),
water, or
ammonia, or into molecules that bind to receptors or other sites of drug action. Such labelled compounds are known as
radiotracers.
PET technology can be used to trace the biologic pathway of any
compound in living humans (and many other species as well), provided it
can be radiolabeled with a PET isotope. Thus, the specific processes
that can be probed with PET are virtually limitless, and radiotracers
for new target molecules and processes are continuing to be synthesized;
as of this writing there are already dozens in clinical use and
hundreds applied in research. At present,
[when?] by far the most commonly used radiotracer in clinical PET scanning is
fluorodeoxyglucose
(also called FDG or fludeoxyglucose), an analogue of glucose that is
labeled with fluorine-18. This radiotracer is used in essentially all
scans for oncology and most scans in neurology, and thus makes up the
large majority of all of the radiotracer (> 95%) used in PET and
PET-CT scanning.
Due to the short half-lives of most positron-emitting radioisotopes, the radiotracers have traditionally been produced using a
cyclotron
in close proximity to the PET imaging facility. The half-life of
fluorine-18 is long enough that radiotracers labeled with fluorine-18
can be manufactured commercially at offsite locations and shipped to
imaging centers. Recently
rubidium-82 generators have become commercially available.
[29] These contain strontium-82, which decays by
electron capture to produce positron-emitting rubidium-82.
Emission
To conduct the scan, a short-lived radioactive tracer
isotope
is injected into the living subject (usually into blood circulation).
Each tracer atom has been chemically incorporated into a biologically
active molecule. There is a waiting period while the active molecule
becomes concentrated in tissues of interest; then the subject is placed
in the imaging scanner. The molecule most commonly used for this purpose
is F-18 labeled
fluorodeoxyglucose
(FDG), a sugar, for which the waiting period is typically an hour.
During the scan, a record of tissue concentration is made as the tracer
decays.
Schema of a PET acquisition process
As the radioisotope undergoes
positron emission decay (also known as positive
beta decay), it emits a positron, an antiparticle of the
electron
with opposite charge. The emitted positron travels in tissue for a
short distance (typically less than 1 mm, but dependent on the isotope
[30]), during which time it loses kinetic energy, until it decelerates to a point where it can interact with an electron.
[31] The encounter annihilates both electron and positron, producing a pair of
annihilation (
gamma)
photons moving in approximately opposite directions. These are detected when they reach a
scintillator in the scanning device, creating a burst of light which is detected by
photomultiplier tubes or silicon
avalanche photodiodes
(Si APD). The technique depends on simultaneous or coincident detection
of the pair of photons moving in approximately opposite directions
(they would be exactly opposite in their
center of mass frame,
but the scanner has no way to know this, and so has a built-in slight
direction-error tolerance). Photons that do not arrive in temporal
"pairs" (i.e. within a timing-window of a few nanoseconds) are ignored.
Localization of the positron annihilation event
The
most significant fraction of electron–positron annihilations results in
two 511 keV gamma photons being emitted at almost 180 degrees to each
other; hence, it is possible to localize their source along a straight
line of coincidence (also called the
line of response, or
LOR).
In practice, the LOR has a non-zero width as the emitted photons are
not exactly 180 degrees apart. If the resolving time of the detectors is
less than 500
picoseconds rather than about 10
nanoseconds, it is possible to localize the event to a segment of a
chord, whose length is determined by the detector timing resolution. As the timing resolution improves, the
signal-to-noise ratio
(SNR) of the image will improve, requiring fewer events to achieve the
same image quality. This technology is not yet common, but it is
available on some new systems.
[32]
Image reconstruction
The
raw data collected by a PET scanner are a list of 'coincidence events'
representing near-simultaneous detection (typically, within a window of 6
to 12 nanoseconds of each other) of annihilation photons by a pair of
detectors. Each coincidence event represents a line in space connecting
the two detectors along which the positron emission occurred (i.e., the
line of response (LOR)).
Analytical techniques, much like the reconstruction of
computed tomography (CT) and
single-photon emission computed tomography (SPECT) data, are commonly used, although the
data set
collected in PET is much poorer than CT, so reconstruction techniques
are more difficult. Coincidence events can be grouped into projection
images, called
sinograms.
The sinograms are sorted by the angle of each view and tilt (for 3D
images). The sinogram images are analogous to the projections captured
by
computed tomography
(CT) scanners, and can be reconstructed in a similar way. The
statistics of data thereby obtained are much worse than those obtained
through transmission tomography. A normal PET data set has millions of
counts for the whole acquisition, while the CT can reach a few billion
counts. This contributes to PET images appearing "noisier" than CT. Two
major sources of noise in PET are scatter (a detected pair of photons,
at least one of which was deflected from its original path by
interaction with matter in the field of view, leading to the pair being
assigned to an incorrect LOR) and random events (photons originating
from two different annihilation events but incorrectly recorded as a
coincidence pair because their arrival at their respective detectors
occurred within a coincidence timing window).
In practice, considerable pre-processing of the data is
required—correction for random coincidences, estimation and subtraction
of
scattered photons, detector
dead-time
correction (after the detection of a photon, the detector must "cool
down" again) and detector-sensitivity correction (for both inherent
detector sensitivity and changes in sensitivity due to angle of
incidence).
Filtered back projection
(FBP) has been frequently used to reconstruct images from the
projections. This algorithm has the advantage of being simple while
having a low requirement for computing resources. Disadvantages are that
shot noise
in the raw data is prominent in the reconstructed images, and areas of
high tracer uptake tend to form streaks across the image. Also, FBP
treats the data deterministically—it does not account for the inherent
randomness associated with PET data, thus requiring all the
pre-reconstruction corrections described above.
Statistical, likelihood-based approaches: Statistical, likelihood-based
[33] [34] iterative
expectation-maximization algorithms such as the Shepp-Vardi algorithm
[35]
are now the preferred method of reconstruction. These algorithms
compute an estimate of the likely distribution of annihilation events
that led to the measured data, based on statistical principles. The
advantage is a better noise profile and resistance to the streak
artifacts common with FBP, but the disadvantage is higher computer
resource requirements. A further advantage of statistical image
reconstruction techniques is that the physical effects that would need
to be pre-corrected for when using an analytical reconstruction
algorithm, such as scattered photons, random coincidences, attenuation
and detector dead-time, can be incorporated into the likelihood model
being used in the reconstruction, allowing for additional noise
reduction. Iterative reconstruction has also been shown to result in
improvements in the resolution of the reconstructed images, since more
sophisticated models of the scanner Physics can be incorporated into the
likelihood model than those used by analytical reconstruction methods,
allowing for improved quantification of the radioactivity distribution.
[36]
Research has shown that
Bayesian methods that involve a Poisson likelihood function and an appropriate
prior probability (e.g., a smoothing prior leading to
total variation regularization or a
Laplacian distribution leading to

-based regularization in a
wavelet or other domain), such as via
Ulf Grenander's
Sieve estimator[37] [38] or via Bayes penalty methods
[39] [40] or via
I.J. Good's roughness method
[41] ,
[42]
may yield superior performance to expectation-maximization-based
methods which involve a Poisson likelihood function but do not involve
such a prior.
[43][44][45]
Attenuation correction: Quantitative PET Imaging requires attenuation correction
[46]. In these systems attenuation correction is based on a transmission scan using
68Ge rotating rod source
[47].
transmission scans directly measure attenuation values at 511keV
[48]. Attenuation occurs when
photons
emitted by the radiotracer inside the body are absorbed by intervening
tissue between the detector and the emission of the photon. As different
LORs must traverse different thicknesses of tissue, the photons are
attenuated differentially. The result is that structures deep in the
body are reconstructed as having falsely low tracer uptake. Contemporary
scanners can estimate attenuation using integrated
x-ray CT equipment, in place of earlier equipment that offered a crude form of CT using a
gamma ray (
positron emitting) source and the PET detectors.
While attenuation-corrected images are generally more faithful
representations, the correction process is itself susceptible to
significant artifacts. As a result, both corrected and uncorrected
images are always reconstructed and read together.
2D/3D reconstruction: Early PET scanners had only a single
ring of detectors, hence the acquisition of data and subsequent
reconstruction was restricted to a single transverse plane. More modern
scanners now include multiple rings, essentially forming a cylinder of
detectors.
There are two approaches to reconstructing data from such a scanner:
1) treat each ring as a separate entity, so that only coincidences
within a ring are detected, the image from each ring can then be
reconstructed individually (2D reconstruction), or 2) allow coincidences
to be detected between rings as well as within rings, then reconstruct
the entire volume together (3D).
3D techniques have better sensitivity (because more coincidences are
detected and used) and therefore less noise, but are more sensitive to
the effects of scatter and random coincidences, as well as requiring
correspondingly greater computer resources. The advent of sub-nanosecond
timing resolution detectors affords better random coincidence
rejection, thus favoring 3D image reconstruction.
Time-of-flight (TOF) PET: For modern systems with a higher
time resolution (roughly 3 nanoseconds) a technique called
"Time-of-flight" is used to improve the overall performance.
Time-of-flight PET makes use of very fast gamma-ray detectors and data
processing system which can more precisely decide the difference in time
between the detection of the two photons. Although it is technically
impossible to localize the point of origin of the annihilation event
exactly (currently within 10 cm) thus image reconstruction is still
needed, TOF technique gives a remarkable improvement in image quality,
especially signal-to-noise ratio.
Complete body PET-CT fusion image
Brain PET-MRI fusion image
Combination of PET with CT or MRI
PET scans are increasingly read alongside CT or
magnetic resonance imaging (MRI) scans, with the combination (called
"co-registration")
giving both anatomic and metabolic information (i.e., what the
structure is, and what it is doing biochemically). Because PET imaging
is most useful in combination with anatomical imaging, such as CT,
modern PET scanners are now available with integrated high-end
multi-detector-row CT scanners (so-called "PET-CT"). Because the two
scans can be performed in immediate sequence during the same session,
with the patient not changing position between the two types of scans,
the two sets of images are more precisely
registered,
so that areas of abnormality on the PET imaging can be more perfectly
correlated with anatomy on the CT images. This is very useful in showing
detailed views of moving organs or structures with higher anatomical
variation, which is more common outside the brain.
At the
Jülich Institute of Neurosciences and Biophysics, the world's largest PET-MRI device began operation in April 2009: a 9.4-
tesla
magnetic resonance tomograph (MRT) combined with a positron emission
tomograph (PET). Presently, only the head and brain can be imaged at
these high magnetic field strengths.
[49]
For brain imaging, registration of CT, MRI and PET scans may be
accomplished without the need for an integrated PET-CT or PET-MRI
scanner by using a device known as the
N-localizer.
[16][50][51][52]
Limitations
The
minimization of radiation dose to the subject is an attractive feature
of the use of short-lived radionuclides. Besides its established role as
a diagnostic technique, PET has an expanding role as a method to assess
the response to therapy, in particular, cancer therapy,
[53]
where the risk to the patient from lack of knowledge about disease
progress is much greater than the risk from the test radiation.
Limitations to the widespread use of PET arise from the high costs of
cyclotrons needed to produce the short-lived
radionuclides
for PET scanning and the need for specially adapted on-site chemical
synthesis apparatus to produce the radiopharmaceuticals after
radioisotope preparation. Organic radiotracer molecules that will
contain a positron-emitting radioisotope cannot be synthesized first and
then the radioisotope prepared within them, because bombardment with a
cyclotron to prepare the radioisotope destroys any organic carrier for
it. Instead, the isotope must be prepared first, then afterward, the
chemistry to prepare any organic radiotracer (such as
FDG)
accomplished very quickly, in the short time before the isotope decays.
Few hospitals and universities are capable of maintaining such systems,
and most clinical PET is supported by third-party suppliers of
radiotracers that can supply many sites simultaneously. This limitation
restricts clinical PET primarily to the use of tracers labelled with
fluorine-18, which has a half-life of 110 minutes and can be transported
a reasonable distance before use, or to rubidium-82 (used as
rubidium-82 chloride) with a half-life of 1.27 minutes, which is created in a portable generator and is used for
myocardial perfusion
studies. Nevertheless, in recent years a few on-site cyclotrons with
integrated shielding and "hot labs" (automated chemistry labs that are
able to work with radioisotopes) have begun to accompany PET units to
remote hospitals. The presence of the small on-site cyclotron promises
to expand in the future as the cyclotrons shrink in response to the high
cost of isotope transportation to remote PET machines.
[54]
In recent years the shortage of PET scans has been alleviated in the
US, as rollout of radiopharmacies to supply radioisotopes has grown
30%/year.
[55]
Because the half-life of fluorine-18 is about two hours, the prepared
dose of a radiopharmaceutical bearing this radionuclide will undergo
multiple half-lives of decay during the working day. This necessitates
frequent recalibration of the remaining dose (determination of activity
per unit volume) and careful planning with respect to patient
scheduling.
History
The concept of emission and transmission
tomography was introduced by
David E. Kuhl,
Luke Chapman and Roy Edwards in the late 1950s. Their work later led to
the design and construction of several tomographic instruments at the
University of Pennsylvania. In 1975 tomographic imaging techniques were further developed by
Michel Ter-Pogossian,
Michael E. Phelps,
Edward J. Hoffman and others at
Washington University School of Medicine.
[56][57]
Work by Gordon Brownell, Charles Burnham and their associates at the
Massachusetts General Hospital
beginning in the 1950s contributed significantly to the development of
PET technology and included the first demonstration of annihilation
radiation for medical imaging.
[58]
Their innovations, including the use of light pipes and volumetric
analysis, have been important in the deployment of PET imaging. In 1961,
James Robertson and his associates at Brookhaven National Laboratory
built the first single-plane PET scan, nicknamed the "head-shrinker."
[59]
One of the factors most responsible for the acceptance of positron
imaging was the development of radiopharmaceuticals. In particular, the
development of labeled 2-fluorodeoxy-D-glucose (2FDG) by the Brookhaven
group under the direction of Al Wolf and Joanna Fowler was a major
factor in expanding the scope of PET imaging.
[60] The compound was first administered to two normal human volunteers by
Abass Alavi
in August 1976 at the University of Pennsylvania. Brain images obtained
with an ordinary (non-PET) nuclear scanner demonstrated the
concentration of FDG in that organ. Later, the substance was used in
dedicated positron tomographic scanners, to yield the modern procedure.
The logical extension of positron instrumentation was a design using
two 2-dimensional arrays. PC-I was the first instrument using this
concept and was designed in 1968, completed in 1969 and reported in
1972. The first applications of PC-I in tomographic mode as
distinguished from the computed tomographic mode were reported in 1970.
[61]
It soon became clear to many of those involved in PET development that a
circular or cylindrical array of detectors was the logical next step in
PET instrumentation. Although many investigators took this approach,
James Robertson
[62] and
Zang-Hee Cho[63] were the first to propose a ring system that has become the prototype of the current shape of PET.
The PET-CT scanner, attributed to Dr. David Townsend and Dr. Ronald
Nutt, was named by TIME Magazine as the medical invention of the year in
2000.
[64]
Cost
As of August 2008,
Cancer Care Ontario
reports that the current average incremental cost to perform a PET scan
in the province is Can$1,000–1,200 per scan. This includes the cost of
the radiopharmaceutical and a stipend for the physician reading the
scan.
[65]
In
England, the
NHS reference cost (2015-2016) for an adult outpatient PET scan is £798, and £242 for direct access services.
[66]
Quality Control
The overall performance of PET systems can be evaluated by quality control tools such as the
Jaszczak phantom.
[67]