Eicosanoids are signaling molecules made by the enzymatic or non-enzymatic oxidation of arachidonic acid or other polyunsaturated fatty acids (PUFAs) that are, similar to arachidonic acid, 20 carbon units in length. Eicosanoids are a sub-category of oxylipins,
i.e. oxidized fatty acids of diverse carbon units in length, and are
distinguished from other oxylipins by their overwhelming importance as cell signaling molecules. Eicosanoids function in diverse physiological systems and pathological processes such as: mounting or inhibiting inflammation, allergy, fever and other immune responses; regulating the abortion of pregnancy and normal childbirth; contributing to the perception of pain; regulating cell growth; controlling blood pressure; and modulating the regional flow of blood to tissues. In performing these roles, eicosanoids most often act as autocrine signaling agents to impact their cells of origin or as paracrine signaling agents to impact cells in the proximity of their cells of origin. Eicosanoids may also act as endocrine agents to control the function of distant cells.
There are multiple subfamilies of eicosanoids, including most prominently the prostaglandins, thromboxanes, leukotrienes, lipoxins, resolvins, and eoxins.
For each subfamily, there is the potential to have at least 4 separate
series of metabolites, two series derived from ω-6 PUFAs (arachidonic
and dihomo-gamma-linolenic acids), one series derived from the ω-3 PUFA
(eicosapentaenoic acid), and one series derived from the ω-9 PUFA (mead
acid). This subfamily distinction is important. Mammals, including
humans, are unable to convert ω-6 into ω-3 PUFA. In consequence, tissue
levels of the ω-6 and ω-3 PUFAs and their corresponding eicosanoid
metabolites link directly to the amount of dietary ω-6 versus ω-3 PUFAs
consumed.
Since certain of the ω-6 and ω-3 PUFA series of metabolites have almost
diametrically opposing physiological and pathological activities, it
has often been suggested that the deleterious consequences associated
with the consumption of ω-6 PUFA-rich diets reflects excessive
production and activities of ω-6 PUFA-derived eicosanoids while the
beneficial effects associated with the consumption of ω-3 PUFA-rich
diets reflect the excessive production and activities of ω-3
PUFA-derived eicosanoids.
In this view, the opposing effects of ω-6 PUFA-derived and ω-3
PUFA-derived eicosanoids on key target cells underlie the detrimental
and beneficial effects of ω-6 and ω-3 PUFA-rich diets on inflammation and allergy reactions, atherosclerosis, hypertension, cancer growth, and a host of other processes.
Nomenclature
Fatty acid sources
"Eicosanoid" (eicosa-, Greek for "twenty"; see icosahedron) is the collective term[6] for straight-chain polyunsaturated fatty acids
(PUFAs) of 20 carbon units in length that have been metabolized or
otherwise converted to oxygen-containing products. The PUFA precursors
to the eicosanoids include:
- Arachidonic acid (AA), i.e. 5Z, 8Z,11Z,14Z-eicosatetraenoic acid is ω-6 fatty acid, with four double bonds in the cis configuration (see Cis–trans isomerism) each located between carbons 5-6, 8-9, 11-12, and 14-15.
- Adrenic acid (AdA), 7,10,13,16-docosatetraenoic acid, is an ω-6 fatty acid with four cis double bounds, each located between carbons 7-8, 10-11, 13-14, and 17-18.
- Eicosapentaenoic acid (EPA), i.e.i.e. 5Z, 8Z,11Z,14Z,17Z-eicosapentaenoic acid is an ω-3 fatty acid with five cis double bonds, each located between carbons 5-6, 8-9, 11-12, 14-15, and 17-18.
- Dihomo-gamma-linolenic acid (DGLA), 8Z, 11Z,14Z-eicosatrienoic acid is an ω-6 fatty acid with three cis double bonds, each located between carbons 8-9, 11-12, and 14-15.
- Mead acid, i.e. 5Z,8Z,11Z-eicosatrienoic acid, is an ω-9 fatty acid containing three cis double bonds, each located between carbons 5-6, 8-9, and 11-12.
Abbreviation
A particular eicosanoid is denoted by a four-character abbreviation, composed of:
- Its two-letter abbreviation (LT, EX or PG, as described above),
- One A-B-C sequence-letter,
- A subscript or plain script number following the designated eicosanoid's trivial name indicates the number of its double bonds. Examples are:
- The EPA-derived prostanoids have three double bonds (e.g. PGG3 or PGG3) while leukotrienes derived from EPA have five double bonds (e.g. LTB5 or LTB5).
- The AA-derived prostanoids have two double bonds (e.g. PGG2 or PGG2) while their AA-derived leukotrienes have four double bonds (e.g. LTB4 or LTB4).
- Hydroperoxy-, hydroxyl-, and oxo-eicosanoids possess a hydroperoxy (-OOH), hydroxy (-OH), or oxygen atom (=O) substituents link to a PUFA carbon by a single (-) or double (=) bond. Their trivial names indicate the substituent as: Hp or HP for a hydroperoxy residue (e.g. 5-hydroperooxy-eicosatraenoic acid or 5-HpETE or 5-HPETE); H for a hydroxy residue (e.g. 5-hydroxy-eicosatetraenoic acid or 5-HETE); and oxo- for an oxo residue (e.g. 5-oxo-eicosatetraenioic acid or 5-oxo-ETE or 5-oxoETE). The number of their double bounds is indicated by their full and trivial names: AA-derived hydroxy metabolites have four (i.e. 'tetra' or 'T') double bonds (e.g. 5-hydroxy-eicosatetraenoic acid or 5-HETE; EPA-derived hydroxy metabolites have five ('penta' or 'P') double bonds (e.g. 5-hydroxy-eicosapentaenoic acid or 5-HEPE); and DGLA-derived hydroxy metabolites have three ('tri' or 'Tr') double bonds (e.g. 5-hydroxy-eicosatrienoic acid or 5-HETrE).
The stereochemistry
of the eicosanoid products formed may differ among the pathways. For
prostaglandins, this is often indicated by Greek letters (e.g. PGF2α versus PGF2β). For hydroperoxy and hydroxy eicosanoids an S or R designates the chirality of their substituents (e.g. 5S-hydroxy-eicosateteraenoic acid [also termed 5(S)-, 5S-hydroxy-, and 5(S)-hydroxy-eicosatetraenoic acid] is given the trivial names of 5S-HETE, 5(S)-HETE, 5S-HETE, or 5(S)-HETE). Since eicosanoid-forming enzymes commonly make S isomer products either with marked preference or essentially exclusively, the use of S/R designations has often been dropped (e.g. 5S-HETE is 5-HETE). Nonetheless, certain eicosanoid-forming pathways do form R isomers and their S versus R isomeric products can exhibit dramatically different biological activities. Failing to specify S/R isomers can be misleading. Here, all hydroperoxy and hydroxy substituents have the S configuration unless noted otherwise.
Classic eicosanoids
Current usage limits the term eicosanoid to:
- ω-6 Series eicosanoids derived from arachidonic acid:
- Hydroxyeicosatetraenoic acids (HETE) include the following metabolites of arachidonic acid:
- 5-HETE, 12-HETE, 15-Hydroxyeicosatetraenoic acid (i.e. 15-HETE), 20-Hydroxyeicosatetraenoic acid (i.e. 20-HETE), and 19-HETE (see 20-Hydroxyeicosatetraenoic acid).
- Leukotrienes (LT) include the following metabolites of arachidonic acid:
- Eoxins (EX) include the following metabolites of arachidnoic acid:
- Prostanoids consisting of several different types:
- Prostaglandins (PG) include the following metabolites of arachidonic acid:
- PGG2, PGH2, PGE2, PGD2, PGF2alpha, PGA2, PGB2, (see Prostanoid and Specialized pro-resolving mediators#Prostaglandins and Isoprostanes).
- Prostacyclins include:
- PGI2.
- Thromboxanes (TX) include the following metabolites of aracidonic acid:
- Cyclopentenone prostaglandins include the following metabolites of arachidonic acid:
- PGA1, PGA2.
- Prostaglandins (PG) include the following metabolites of arachidonic acid:
- Hydroxyeicosatetraenoic acids (HETE) include the following metabolites of arachidonic acid:
- ω-6 Series eicosanoids derived from dihomo-gamma-linolenic acid.
These metabolites are analogs of arachidonic acid-derived eicosanoids
but lack a double bound between carbons 5 and 6 and therefore have 1
less double bound than their arachidonic acid-derived analogs. They the
following:
- PGA1, PGE1, and TXA1.
- ω-3 Series eicosanoids:
- Resolvins of the E series (RvE) (D series resolvins (RvD's are metabolites of the 22-carbon ω-6 fatty acid docosahexaenoic acid; see Specialized pro-resolving mediators#DHA-derived Resolvins). RvE's include the following metabolites of eicosapentaenoic acid:
- RvE1, 18S-RvE1, RvE2, and RvE3.
- Other ω-3 series eicosapentaenoic acid-derived eicosanoids are
analogs of ω-6 fatty acid-derived metabolites but contain a double bond
between carbon 17 and 18 and therefore have one more double bound than
their arachidonic acid-derived analogs. They include (HEPE is
hydroxy-eicsapentaenoic acid):
- 5-HEPE, 12-HEPE, 15-HEPE, and 20-HETE; LTA5, LTB5 (see Essential fatty acid interactions#counteractions), LTC5, LTD5, and LTE5 (see Arachidonate 5-lipoxygenase#eicosapentaenoic acid); PGE3, PGD3, PGF3α, and Δ(17)-6-keto PGF1α; PGI3; and TXA3 and TXB3.
- Resolvins of the E series (RvE) (D series resolvins (RvD's are metabolites of the 22-carbon ω-6 fatty acid docosahexaenoic acid; see Specialized pro-resolving mediators#DHA-derived Resolvins). RvE's include the following metabolites of eicosapentaenoic acid:
- ω-9 Series eicosanoids
- Hydroxy are derived form mead acid, is metabolized to the 3 double bond-containing analog of 5-HETE viz., 5-HETrE (see arachidonate 5-lipoxygenase#mead acid).
Hydroxyeicosatetraenoic acids, leukotrienes, eoxins and prostanoids are sometimes termed "classic eicosanoids"
Nonclassic eicosanoids
In
contrast to the classic eicosanoids, several other classes of PUFA
metabolites have been termed 'novel', 'eicosanoid-like' or 'nonclassic eicosanoids'. These included the following classes:
- Oxoeicosanoids (oxo-ETE) include the following metabolites:
- 5-oxo-eicosatetraenoic acid (5-oxo-ETE), 12-oxo-ETE, and 15-oxo-ETE, which are metabolites of arachidonic acid and 5-oxo-ETrE which is a metabolite of mead acid.
- Hepoxilins (Hx) include the following arachidonic acid metabolites:
- HxA3 and HxB3.
- Lipoxins (Lx) include the following metabolites of arachidonic acid:
- LxA4 and LxB4.
- Epi-lipoxins (epi-Lx) include the following metabolites of arachidonic acid:
- 15-epi-LxA4 (also termed AT-LxA4) and 15-epi-LxB4 (also termed AT-LxB4)(see Specialized pro-resolving mediators).
- Epoxyeicosatrienoic acids (EET) include the following metabolites of arachidonic acid:
- 5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET.
- Epoxyeicosatetraenoic acid (EEQ) include the following metabolites of eicosapentaenoic acid:
- 5,6-EEQ, 8,9-EEQ, 11,12-EEQ, 14,15-EEQ, and 15,16-EEQ.
- Isoprostanes (isoP) are non-enzymatically formed derivatives of polyunsaturated fatty acids studied as markers of oxidative stress; they include the following arachidonic acid-derived isoP's which are named based on their structural similarities to PGs:
- D2-isoPs, E2-isoPs, A2-isoPs, and J2-isoPs; and two epoxide-containing isoPs, 5,6-epoxyisoprostane E2 and 5,6-epoxyisoprostane A2. Some of these isoPs have been shown to possess anti-inflammatory activity.
- Isofurans are non-enzymatically formed dervatives of polyunsaturated fatty acids that possess a Furan ring structure; they are studied as markers of oxidative stress. There are 256 potentially different furan ring-containing isomers that can be derived from arachidonic acid.
- Endocannabinoids are certain glycerolipids or dopamine that are esterified to polyunsaturated fatty acids that activate cannabinoid receptors. They include the following arachidonic acid-esterified agents:
Metabolism of eicosapentaenoic acid to HEPEs, leukotrienes,
prostanoids, and epoxyeicosatetraenoic acids as well as the metabolism
of dihomo-gamma-linolenic acid to prostanoids and mead acid to
5(S)-hydroxy-6E,8Z,11Z-eicosatrienoic acid (5-HETrE),
5-oxo-6,8,11-eicosatrienoic acid (5-oxo-ETrE), LTA3, and LTC3 involve
the same enzymatic pathways that make their arachidonic acid-derived
analogs.
Biosynthesis
Eicosanoids typically are not stored within cells but rather synthesized as required. They derive from the fatty acids that make up the cell membrane and nuclear membrane.
These fatty acids must be released from their membrane sites and then
metabolized initially to products which most often are further
metabolized through various pathways to make the large array of products
we recognize as bioactive eicosanoids.
Fatty acid mobilization
Eicosanoid biosynthesis begins when a cell is activated by mechanical trauma, ischemia, other physical perturbations, attack by pathogens, or stimuli made by nearby cells, tissues, or pathogens such as chemotactic factors, cytokines, growth factors, and even certain eicosanoids. The activated cells then mobilize enzymes, termed phospholipase A2's (PLA2s), capable of releasing ω-6 and ω-3 fatty acids from membrane storage. These fatty acids are bound in ester linkage to the SN2 position of membrane phospholipids; PLA2s act as esterases to release the fatty acid. There are several classes of PLA2s with type IV cytosolic PLA2s (cPLA2s) appearing to be responsible for releasing the fatty acids under many conditions of cell activation. The cPLA2s act specifically on phospholipids that contain AA, EPA or GPLA at their SN2 position. cPLA2 may also release the lysophospholipid that becomes platelet-activating factor.
Peroxidation and reactive oxygen species
Next, the free fatty acid is oxygenated along any of several pathways; see the Pathways table. The eicosanoid pathways (via lipoxygenase or COX) add molecular oxygen (O2). Although the fatty acid is symmetric, the resulting eicosanoids are chiral; the oxidations proceed with high stereoselectivity (enzymatic oxidations are considered practically stereospecific).
Four families of enzymes initiate or contribute to the initiation of the catalysis of fatty acids to eicosanoids:
- Cyclooxygenases (COXs): COX-1 and COX-2 initiate the metabolism of arachidonic acid to prostanoids that contain two double bonds, i.e. the prostaglandins (e.g. PGE2), prostacyclin (i.e. PGI2), and thromboxanes (e.g. TXA2). The two COX enzymes likewise initiate the metabolism of: a) eicosapentaenoic acid, which has 5 double bonds compared to the 4 double bonds of arachidonic acid, to prostanoid, prostacyclin, and thromboxane products that have three double bonds, e.g. PGE3, PGI3, and TXA3 and b) Dihomo-γ-linolenic acid, which has three double bonds, to prostanoid, prostacyclin, and thromboxane products that have only one double bond, e.g. PGE1, PGI1, and TXA1.
- Lipoxygenases (LOXs): 5-Lipoxygenase (5-LOX or ALOX5) initiates the metabolism of arachidonic acid to 5-hydroperoxyeicosatetraenoic acid (5-HpETE) which then may be rapidly reduced to 5-hydroxyeicosatetraenoic acid (5-HETE) or further metabolized to the leukotrienes (e.g. LTB4 and LTC4); 5-HETE may be oxidized to 5-oxo-eicosatetraenoic acid (5-oxo-ETE). In similar fashions, 15-lipoxygenase (15-lipoxygenase 1, 15-LOX, 15-LOX1, or ALOX15) initiates the metabolism of arachidonic acid to 15-HpETE, 15-HETE, eoxins, 8,15-dihydroxyeicosapentaenoic acid (i.e. 8,15-DiHETE), and 15-oxo-ETE and 12-lipoxygenase (12-LOX or ALOX12) initiates the metabolism of arachidonic acid to 12-HpETE, 12-HETE, hepoxilins, and 12-oxo-ETE. These enzymes also initiate the metabolism of; a) eicosatetraenoic acid to analogs of the arachidonic acid metabolites that contain 5 rather than four double bonds, e.g. 5-hydroxy-eicosapentaenoic acid (5-HEPE), LTB5, LTC5, 5-oxo-EPE, 15-HEPE, and 12-HEPE; b) the three double bond-containing dihomo-γ-linolenic acid to products that contain 3 double bonds, e.g. 8-hydroxy-eicosatrienoic acid (8-HETrE), 12-HETrE, and 15-HETrE (this fatty acid cannot be converted to leukotrienes); and the three double bond-containing mead acid (by ALOX5) to 5-hydroperoxy-eicosatrienoic acid (5-HpETrE), 5-HETrE, and 5-oxo-HETrE. In the most studied of these pathways, ALOX5 metabolizes eicosapentaenoic acid to 5-hydroperoxyeicosapentaenoic acid (5-HpEPE), 5-HEPE, and LTB5, and 5-oxo-EPE, all of which are less active than there arachidonic acid analogs. Since eicosapentaenoic acid competes with arachidonic acid for ALOX5, production of the eicosapentaenoate metabolites leads to a reduction in the eicosatetraenoate metabolites and therefore reduction in the latter metabolites' signaling. The initial mono-hydroperoxy and mono-hydroxy products made by the aforementioned lipoxygenases have their hydroperosy and hydroxyl residues positioned in the S chiral configuration and are more properly termed 5S-HpETE, 5S-HETE, 12S-HpETE, 12S-HETE, 15S-HpETE and, 15S-HETE. ALOX12B (i.e. arachidonate 12-lipoxygenase, 12R type) forms R chirality products, i.e. 12R-HpETE and 12R-HETE. Similarly, ALOXE3 (i.e. epidermis-type lipoxygenase 3 or eLOX3) metabolizes arachidonic acid to 12R-HpETE and 12R-HETE; however these are minor products that this enzyme forms only under a limited set of conditions. ALOXE3 preferentially metabolizes arachidonic acid to hepoxilins.
- Epoxygenases: these are cytochrome P450 enzymes which generate nonclassic eicosanoid epoxides derived from: a) arachidonic acid viz., 5,6-epoxy-eicsattrienoic acid (5,6-EET), 8,9-EET, 11,12-EET, and 14,15-EET; b) eicosapentaenoic acid viz., 5,6,-epoxy-eicosatetraenoic acid (5,6-EEQ), 8,9-EEQ, 11,12-EEQ, 14,15-EEQ, and 17,18-EEQ; c) di-homo-γ-linolenic acid viz., 8,9-epoxy-eicosadienoic acid (8,9-EpEDE), 11,12-EpEDE, and 14,15-EpEDE; and d) adrenic acid viz., 7,8-epox-eicosatrienoic acid (7,8-EpETrR), 10,11-EpTrE, 13,14-EpTrE, and 16,17-EpETrE. All of these epoxides are converted, sometimes rapidly, to their dihydroxy metabolites, by various cells and tissues. For example, 5,6-EET is converted to 5,6-dihydroxy-eicosatrienoic acid (5,6-DiHETrE), 8,9-EEQ to 8,9-dihydroxy-eicosatetraenoic acid (8,9-DiHETE, 11,12-EpEDE to 11,12-dihydroxy-eicosadienoic acid (11,12DiHEDE), and 16,17-EpETrE to 16,17-dihydroxy-eicosatrienoic acid (16,17-DiETrE
- Cytochrome P450 microsome ω-hydroxylases: CYP4A11, CYP4A22, CYP4F2, and CYP4F3 metabolize arachidonic acid primarily to 20-Hydroxyeicosatetraenoic acid (20-HETE) but also to 16-HETE, 17-HETE, 18-HETE, and 19-HETE; they also metabolize eicosapentaenoic acid primarily to 20-hydroxy-eicosapentaenoic acid (20-HEPE) but also to 19-HEPE.
Two different enzymes may act in series on a PUFA to form more
complex metabolites. For example, ALOX5 acts with ALOX12 or
aspirin-treated COX-2 to metabolize arachidonic acid to lipoxins and with cytochrome P450 monooxygenase(s),
bacterial cytochrome P450 (in infected tissues), or aspirin-treated
COX2 to metabolize eicosapentaenoic acid to the E series resolvins (RvEs) (see Specialized pro-resolving mediators).
When this occurs with enzymes located in different cell types and
involves the transfer of one enzyme's product to a cell which uses the
second enzyme to make the final product it is referred to as
transcellular metabolism or transcellular biosynthesis.
The oxidation of lipids is hazardous to cells, particularly when
close to the nucleus.
There are elaborate mechanisms to prevent unwanted oxidation. COX, the
lipoxygenases, and the phospholipases are tightly controlled—there are
at least eight proteins activated to coordinate generation of
leukotrienes. Several of these exist in multiple isoforms.
Oxidation by either COX or lipoxygenase releases reactive oxygen species (ROS) and the initial products in eicosanoid generation are themselves highly reactive peroxides. LTA4 can form adducts with tissue DNA. Other reactions of lipoxygenases generate cellular damage; murine models implicate 15-lipoxygenase in the pathogenesis of atherosclerosis.
The oxidation in eicosanoid generation is compartmentalized; this limits the peroxides' damage.
The enzymes that are biosynthetic for eicosanoids (e.g., glutathione-S-transferases, epoxide hydrolases, and carrier proteins) belong to families whose functions are involved largely with cellular detoxification.
This suggests that eicosanoid signaling might have evolved from the detoxification of ROS.
The cell must realize some benefit from generating lipid hydroperoxides close-by its nucleus.
PGs and LTs may signal or regulate DNA-transcription there;
LTB4 is ligand for PPARα.
Prostanoid pathways
Both COX1 and COX2 (also termed prostaglandin-endoperoxide synthase-1 (PTGS1) and PTGS2, respectively) metabolize arachidonic acid by adding molecular O2 between carbons 9 and 11 to form an endoperoxide bridge between these two carbons, adding molecular O2 to carbon 15 to yield a 15-hydroperoxy product, creating a carbon-carbon bond between carbons 8 and 12 to create a cyclopentane
ring in the middle of the fatty acid, and in the process making PGG2, a
product that has two fewer double bonds than arachidonic acid. The
15-hydroperoxy residue of PGG2 is then reduced to a 15-hydroxyl residue thereby forming PGH2. PGH2 is the parent prostanoid to all other prostanoids. It is metabolized by (see diagram in Prostanoids: a) the Prostaglandin E synthase pathway in which any one of three isozymes, PTGES, PTGES2, or PTGES3, convert PGH2 to PGE2 (subsequent products of this pathway include PGA2 and PGB2; b) PGF synthase which converts PGH2 to PGF2α; c) Prostaglandin D2 synthase which converts PGH2 to PGD2 (subsequent products in this pathway include 15-dPGJ2; d) thromboxane synthase which converts PGH2 to TXA2 (subsequent products in this pathway include TXB2); and e) Prostacyclin synthase which converts PGH2 to PGI2 (subsequent products in this pathway include 6-keto-PGFα.
These pathways have been shown or in some cases presumed to metabolize
eicosapentaenoic acid to eicosanoid analogs of the sited products that
have three rather than two double bonds and therefore contain the number
3 in place of 2 attached to their names (e.g. PGE3 instead of PGE2).
The PGE2, PGE1, and PGD2 products formed in the pathways just cited can undergo a spontaneous dehydration reaction
to form PGA2, PGA1, and PGJ2, respectively; PGJ2 may then undergo a
spontaneous isomerization followed by a dehydration reaction to form in
series Δ12-PGJ2 and 15-deoxy-Δ12,14-PGJ2.
PGH2 has a 5-carbon ring bridged by molecular oxygen. Its derived
PGS have lost this oxygen bridge and contain a single, unsaturated
5-carbon ring with the exception of thromboxane A2 which possesses a
6-member ring consisting of one oxygen and 5 carbon atoms. The 5-carbon
ring of prostacyclin is conjoined to a second ring consisting of 4
carbon and one oxygen atom. And, the 5 member ring of the cyclopentenone
prostaglandins possesses an unsaturated bond in a conjugated system with a carbonyl group that causes these PGs to form bonds with a diverse range of bioactive proteins.
Hydroxyeicosatetraenoate (HETE) and leukotriene (LT) pathways
The enzyme 5-lipoxygenase (5-LO or ALOX5) converts arachidonic acid into 5-hydroperoxyeicosatetraenoic acid (5-HPETE), which may be released and rapidly reduced to 5-hydroxyeicosatetraenoic acid (5-HETE) by ubiquitous cellular glutathione-dependent peroxidases. Alternately, ALOX5 uses its LTA synthase activity to act convert 5-HPETE to leukotriene A4 (LTA4). LTA4 is then metabolized either to LTB4 by Leukotriene A4 hydrolase or Leukotriene C4 (LTC4) by either LTC4 synthase or microsomal glutathione S-transferase 2 (MGST2). Either of the latter two enzymes act to attach the sulfur of cysteine's thio- (i.e. SH) group in the tripeptide glutamate-cysteine-glycine
to carbon 6 of LTA4 thereby forming LTC4. After release from its parent
cell, the glutamate and glycine residues of LTC4 are removed step-wise
by gamma-glutamyltransferase and a dipeptidase to form sequentially LTD4 and LTE4.
The decision to form LTB4 versus LTC4 depends on the relative content
of LTA4 hydrolase versus LTC4 synthase (or glutathione S=transferase in
cells; Eosinophils, mast cells, and alveolar macrophages
possess relatively high levels of LTC4 synthase and accordingly form
LTC4 rather than or to a far greater extent than LTB4. 5-LOX may also
work in series with cytochrome P450 oxygenases or aspirin-treated COX2
to form Resolvins RvE1, RvE2, and 18S-RvE1.
The enzyme arachidonate 12-lipoxygenase (12-LO or ALOX12) metabolizes arachidonic acid to the S stereoisomer of 12-hydroperoxyeicosatetraenoic acid (5-HPETE) which is rapidly reduced by cellular peroxidases to the S stereoisomer of 12-hydroxyeicosatetraenoic acid (12-HETE) or further metabolized to hepoxilins (Hx) such as HxA3 and HxB.
The enzymes 15-lipoxygenase-1 (15-LO-1 or ALOX15) and 15-lipoxygenase-2 (15-LO-2, ALOX15B) metabolize arachidonic acid to the S stereoisomer of 15-Hydroperoxyeicosatetraenoic acid (15(S)-HPETE) which is rapidly reduced by cellular peroxidases to the S stereoisomer of 15-Hydroxyicosatetraenoic acid (15(S)-HETE).
The 15-lipoxygenases (particularly ALOX15) may also act in series with
5-lipoxygenase, 12-lipoxygenase, or aspirin-treated COX2 to form the
lipoxins and epi-lipoxins or with P450 oxygenases or aspirin-treated
COX2
to form Resolvin E3 (see Specialized pro-resolving mediators#EPA-derived resolvins.
A subset of cytochrome P450 (CYP450) microsome-bound ω-hydroxylases (see 20-Hydroxyeicosatetraenoic acid) metabolize arachidonic acid to 20-Hydroxyeicosatetraenoic acid (20-HETE) and 19-hydroxyeicosatetraenoic acid by an omega oxidation reaction.
Epoxyeicosanoid pathway
The
human cytochrome P450 (CYP) epoxygenases, CYP1A1, CYP1A2, CYP2C8,
CYP2C9, CYP2C18, CYP2C19, CYP2E1, CYP2J2, and CYP2S1 metabolize
arachidonic acid to the non-classic Epoxyeicosatrienoic acids (EETs) by converting one of the fatty acid's double bonds to its epoxide to form one or more of the following EETs, 14,15-ETE, 11,12-EET, 8,9-ETE, and 4,5-ETE. 14,15-EET and 11,12-EET are the major EETs produced by mammalian, including human, tissues. The same CYPs but also CYP4A1, CYP4F8, and CYP4F12 metabolize eicosapentaenoic acid to five epoxide epoxyeicosatetraenoic acids (EEQs) viz., 17,18-EEQ, 14,15-EEQ, 11,12-EEQ. 8,9-EEQ, and 5,6-EEQ.
Function, pharmacology, and clinical significance
The
following table lists a sampling of the major eicosanoids that possess
clinically relevant biological activity, the cellular receptors
that they stimulate or, where noted, antagonize to attain this
activity, some of the major functions which they regulate (either
promote or inhibit) in humans and mouse models, and some of their
relevancies to human diseases.
| Eicosanoid | Targeted receptors | Functions regulated | Clinical relevancy |
|---|---|---|---|
| PGE2 | PTGER1, PTGER2, PTGER3, PTGER4 | inflammation; fever; pain perception; allodynia; parturition | NSAIDs inhibit its production to reduce inflammation, fever, and pain; used to promote labor in childbirth; an Abortifacient |
| PGD2 | Prostaglandin DP1 receptor 1, Prostaglandin DP2 receptor | allergy reactions; allodynia; hair growth | NSAIDs may target it to inhibit allodynia and male-pattern hair loss |
| TXA2 | Thromboxane receptor α and β | blood platelet aggregation; blood clotting; allergic reactions | NSAIDs inhibit its production to reduce incidence of strokes and heart attacks |
| PGI2 | Prostacyclin receptor | platelet aggregation, vascular smooth muscle contraction | PGI2 analogs used to treat vascular disorders like pulmonary hypertension, Raynaud's syndrome, and Buerger's disease |
| 15-d-Δ12,14-PGJ2 | PPARγ, Prostaglandin DP2 receptor | inhibits inflammation and cell growth | Inhibits diverse inflammatory responses in animal models; structural model for developing anti-inflammatory agents |
| 20-HETE | ? | vasoconstriction, inhibits platelets | inactivating mutations in the 20-HETE-forming enzyme, CYP2U1, associated with Hereditary spastic paraplegia |
| 5-Oxo-ETE | OXER1 | chemotactic factor for and activator of eosinophils | studies needed to determine if inhibiting its production or action inhibits allergic reactons |
| LTB4 | LTB4R, LTB4R2 | chemotactic factor for and activator of leukocytes; inflammation | studies to date shown no clear benefits of LTB4 receptor antagonists for human inflammatory diseases |
| LTC4 | CYSLTR1, CYSLTR2, GPR17 | vascular permeability; vascular smooth muscle contraction; allergy | antagonists of CYSLTR1 used in asthma as well as other allergic and allergic-like reactions |
| LTD4 | CYSLTR1, CYSLTR2, GPR17 | vascular permeability; vascular smooth muscle contraction; allergy | antagonists of CYSLTR1 used in asthma as well as other allergic and allergic-like reactions |
| LTE4 | GPR99 | increases vascular permeability and airway mucin secretion | thought to contribute to asthma as well as other allergic and allergic-like reactions |
| LxA4 | FPR2 | inhibits functions of pro-inflammatory cells | Specialized pro-resolving mediators class of inflammatory reaction suppressors |
| LxB4 | FPR2, GPR32, AHR | inhibits functions of pro-inflammatory cells | Specialized pro-resolving mediators class of inflammatory reaction suppressors |
| RvE1 | CMKLR1, inhibits BLT, TRPV1, TRPV3, NMDAR, TNFR | inhibits functions of pro-inflammatory cells | Specialized pro-resolving mediators class of inflammatory reaction suppressors; also suppresses pain perception |
| RvE2 | CMKLR1, receptor antagonist of BLT | inhibits functions of pro-inflammatory cells | Specialized pro-resolving mediators class of inflammatory reaction suppressors |
| 14,15-EET | ? | vasodilation, inhibits platelets and pro-inflammatory cells | role(s) in human disease not yet proven |
Prostanoids
Many of the prostanoids are known to mediate local symptoms of inflammation: vasoconstriction or vasodilation, coagulation, pain, and fever. Inhibition of COX-1 and/or the inducible COX-2 isoforms, is the hallmark of NSAIDs (non-steroidal anti-inflammatory drugs), such as aspirin. Prostanoids also activate the PPARγ members of the steroid/thyroid family of nuclear hormone receptors, and directly influence gene transcription.
Prostanoids have numerous other relevancies to clinical medicine as
evidence by their use, the use of their more stable pharmacological
analogs, of the use of their receptor antagonists as indicated in the
following chart.
| Medicine | Type | Medical condition or use | Medicine | Type | Medical condition or use | |
|---|---|---|---|---|---|---|
| Alprostadil | PGE1 | Erectile dysfunction, maintaining a patent ductus arteriosus in the fetus | Beraprost | PGI1 analog | Pulmonary hypertension, avoiding reperfusion injury | |
| Bimatoprost | PGF2α analog | Glaucoma, ocular hypertension | Carboprost | PGF2α analog | Labor induction, abortifacient in early pregnancy | |
| Dinoprostone | PGE2 | labor induction | Iloprost | PGI2 analog | pulmonary artery hypertension | |
| Latanoprost | PGF2α analog | Glaucoma, ocular hypertension | Misoprostol | PGE1 analog | stomach ulcers labor induction, abortifacient | |
| Travoprost | PGF2α analog | Glaucoma, ocular hypertension | U46619 | Longer lived TX analog Longer lived TX analog | Research only |
Cyclopentenone prostaglandins
PGA1, PGA2, PGJ2, Δ12-PGJ2, and 15-deox-Δ12,14-PGJ2 exhibit a wide
range of anti-inflammatory and inflammation-resolving actions in diverse
animal models. They therefore appear to function in a manner similar to Specialized pro-resolving mediators
although one of their mechanisms of action, forming covalent bonds with
key signaling proteins, differs from those of the specialized
pro-resolving mediators.
HETEs and oxo-ETEs
As indicated in their individual Wikipedia pages, 5-hydroxyeicosatetraenoic acid (which, like 5-oxo-eicosatetraenoic acid, acts through the OXER1 receptor), 5-oxo-eicosatetraenoic acid, 12-Hydroxyeicosatetraenoic acid, 15-Hydroxyeicosatetraenoic acid, and 20-Hydroxyeicosatetraenoic acid
show numerous activities in animal and human cells as well as in animal
models that are related to, for example, inflammation, allergic
reactions, cancer cell growth, blood flow to tissues, and/or blood
pressure. However, their function and relevancy to human physiology and
pathology have not as yet been shown.
Leukotrienes
The three cysteinyl leukotrienes, LTC4, LTD4, and LTE4, are potent
bronchoconstrictors, increasers of vascular permeability in
postcapillary venules, and stimulators of mucus
secretion that are released from the lung tissue of asthmatic subjects
exposed to specific allergens. They play a pathophysiological role in
diverse types of immediate hypersensitivity reactions. Drugs that block their activation of the CYSLTR1 receptor viz., montelukast, zafirlukast, and pranlukast, are used clinically as maintenance treatment for allergen-induced asthma and rhinitis; nonsteroidal anti-inflammatory drug-induced asthma and rhinitis (see Aspirin-induced asthma); exercise- and cold-air induced asthma (see Exercise-induced bronchoconstriction); and childhood sleep apnea due to adenotonsillar hypertrophy. When combined with antihistamine drug therapy, they also appear useful for treating urticarial diseases such as hives.
Lipoxins and epi-lipoxins
LxA4, LxB4, 15-epi-LxA4, and 15-epi-LXB4, like other members of the specialized pro-resolving mediators) class of eicosanoids, possess anti-inflammatory and inflammation resolving activity. In a randomized controlled trial, AT-LXA4 and a comparatively stable analog of LXB4, 15R/S-methyl-LXB4, reduced the severity of eczema in a study of 60 infants and, in another study, inhaled LXA4 decreased LTC4-initiated bronchoprovocation in patients with asthma.
Eoxins
The eoxins (EXC4, EXD4, EXE5) are newly described. They stimulate
vascular permeability in an ex vivo human vascular endothelial model
system,
and in a small study of 32 volunteers EXC4 production by eosinophils
isolated from severe and aspirin-intolerant asthmatics was greater than
that from healthy volunteers and mild asthmatic patients; these findings
have been suggested to indicate that the eoxins have pro-inflammatory
actions and therefore potentially involved in various allergic
reactions. Production of eoxins by Reed-Sternburg cells has also led to suggestion that they are involve in Hodgkins disease. However, the clinical significance of eoxins has not yet been demonstrated.
Resolvin metabolites of eicosapentaenoic acid
RvE1, 18S-RvE1, RvE2, and RvE3, like other members of the specialized
pro-resolving mediators) class of eicosanoids, possess
anti-inflammatory and inflammation resolving activity. A synthetic
analog of RvE1 is in clinical phase III testing for the treatment of the inflammation-based dry eyesyndrome;
along with this study, other clinical trials (NCT01639846, NCT01675570,
NCT00799552 and NCT02329743) using an RvE1 analogue to treat various
ocular conditions are underway. RvE1 is also in clinical development studies for the treatment of neurodegenerative diseases and hearing loss.
Other metabolites of eicosapentaenoic acid
The
metabolites of eicosapentaenoic acid that are analogs of their
arachidonic acid-derived prostanoid, HETE, and LT counterparts include:
the 3-series prostanoids (e.g. PGE3, PGD3, PGF3α, PGI3, and TXA3), the
hydroxyeicosapentaenoic acids (e.g. 5-HEPE, 12-HEPE, 15-HEPE, and
20-HEPE), and the 5-series LTs (e.g. LTB5, LTC5, LTD5, and LTE5). Many
of the 3-series prostanoids, the hydroxyeicosapentaenoic acids, and the
5-series LT have been shown or thought to be weaker stimulators of their
target cells and tissues than their arachidonic acid-derived analogs.
They are proposed to reduce the actions of their aracidonate-derived
analogs by replacing their production with weaker analogs. Eicosapentaenoic acid-derived counterparts of the Eoxins have not been described.
Epoxyeicosanoids
The epoxy eicostrienoic acids (or EETs)—and, presumably, the epoxy
eicosatetraenoic acids—have vasodilating actions on heart, kidney, and
other blood vessels as well as on the kidney's reabsorption of sodium
and water, and act to reduce blood pressure and ischemic and other
injuries to the heart, brain, and other tissues; they may also act to
reduce inflammation, promote the growth and metastasis of certain
tumors, promote the growth of new blood vessels, in the central nervous
system regulate the release of neuropeptide hormones, and in the peripheral nervous system inhibit or reduce pain perception.
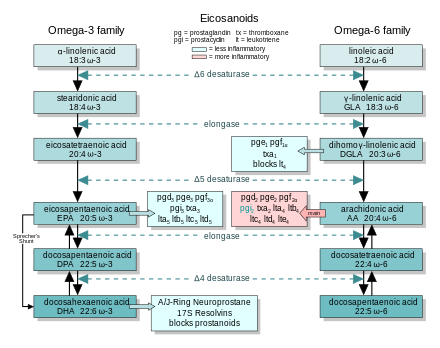
The ω-3 and ω-6 series
| “ | The reduction in AA-derived eicosanoids and the diminished activity of the alternative products generated from ω-3 fatty acids serve as the foundation for explaining some of the beneficial effects of greater ω-3 intake. | ” |
| — Kevin Fritsche, Fatty Acids as Modulators of the Immune Response | ||
Arachidonic acid (AA; 20:4 ω-6) sits at the head of the "arachidonic acid cascade" – more than twenty eicosanoid-mediated signaling paths controlling a wide array of cellular functions, especially those regulating inflammation, immunity, and the central nervous system.
In the inflammatory response, two other groups of dietary fatty
acids form cascades that parallel and compete with the arachidonic acid
cascade. EPA (20:5 ω-3) provides the most important competing cascade. DGLA (20:3 ω-6)
provides a third, less prominent cascade. These two parallel cascades
soften the inflammatory effects of AA and its products. Low dietary
intake of these less-inflammatory fatty acids, especially the ω-3s, has
been linked to several inflammation-related diseases, and perhaps some mental illnesses.
The U.S. National Institutes of Health and the National Library of Medicine state that there is 'A' level evidence that increased dietary ω-3 improves outcomes in hypertriglyceridemia, secondary cardiovascular disease prevention, and hypertension.
There is 'B' level evidence ('good scientific evidence') for increased dietary ω-3 in primary prevention of cardiovascular disease, rheumatoid arthritis, and protection from ciclosporin toxicity in organ transplant patients.
They also note more preliminary evidence showing that dietary ω-3 can ease symptoms in several psychiatric disorders.
Besides the influence on eicosanoids, dietary polyunsaturated
fats modulate immune response through three other molecular mechanisms.
They
(a) alter membrane composition and function, including the composition of lipid rafts;
(b) change cytokine biosynthesis; and (c) directly activate gene transcription. Of these, the action on eicosanoids is the best explored.
Mechanisms of ω-3 action
EFA
sources: Essential fatty acid production and metabolism to form
eicosanoids. At each step, the ω-3 and ω-6 cascades compete for the
enzymes.
In general, the eicosanoids derived from AA promote inflammation, and those from EPA and from GLA (via DGLA) are less inflammatory, or inactive, or even anti-inflammatory and pro-resolving.
The figure shows the ω-3 and -6 synthesis chains, along with the major eicosanoids from AA, EPA, and DGLA.
Dietary ω-3 and GLA counter the inflammatory effects of AA's eicosanoids in three ways, along the eicosanoid pathways:
- Displacement—Dietary ω-3 decreases tissue concentrations of AA, so there is less to form ω-6 eicosanoids.
- Competitive inhibition—DGLA and EPA compete with AA for access to the cyclooxygenase and lipoxygenase enzymes. So the presence of DGLA and EPA in tissues lowers the output of AA's eicosanoids.
- Counteraction—Some DGLA and EPA derived eicosanoids counteract their AA derived counterparts.
Role in inflammation
Since antiquity,
the cardinal signs of inflammation have been known as: calor (warmth),
dolor (pain), tumor (swelling), and rubor (redness). The eicosanoids are
involved with each of these signs.
Redness—An insect's sting will trigger the classic inflammatory response. Short acting vasoconstrictors — TXA2—are released quickly after the injury. The site may momentarily turn pale. Then TXA2 mediates the release of the vasodilators PGE2 and LTB4. The blood vessels engorge and the injury reddens.
Swelling—LTB4 makes the blood vessels more permeable. Plasma leaks out into the connective tissues, and they swell. The process also loses pro-inflammatory cytokines.
Heat—PGE2 is also a potent pyretic agent. Aspirin and NSAIDS—drugs that block the COX pathways and stop prostanoid synthesis—limit fever or the heat of localized inflammation.
History
In 1930, gynecologist Raphael Kurzrok and pharmacologist Charles Leib characterized prostaglandin
as a component of semen.
Between 1929 and 1932, Burr and Burr showed that restricting fat from
animal's diets led to a deficiency disease, and first described the essential fatty acids.
In 1935, von Euler identified prostaglandin.
In 1964, Bergström and Samuelsson
linked these observations when they showed that the "classical"
eicosanoids were derived from arachidonic acid, which had earlier been
considered to be one of the essential fatty acids.
In 1971, Vane showed that aspirin and similar drugs inhibit prostaglandin synthesis. Von Euler received the Nobel Prize in medicine in 1970, which
Samuelsson, Vane, and Bergström also received in 1982.
E. J. Corey received it in chemistry in 1990 largely for his synthesis of prostaglandins.