From Wikipedia, the free encyclopedia

Indian summer by Józef Chełmoński (1875, National Museum in Warsaw) depicts a peasant woman with a thread of gossamer in her hand.
Spider silk is a protein fiber spun by spiders. Spiders use their silk to make webs or other structures, which function as nets to catch other animals, or as nests or cocoons to protect their offspring. They can also use their silk to suspend themselves.
Many small spiders use silk threads for ballooning, the popular, though technically inaccurate, scientific term for the dynamic kiting[1][2] spiderlings (mostly) use for dispersal. They extrude several threads into the air and let themselves be carried away by winds. Although most rides will end a few yards later, it seems to be a common way for spiders to invade islands. Many sailors have reported that spiders have been caught in their ship's sails, even when far from land. The extremely fine silk that spiders use for ballooning is known as gossamer.[3]
In some cases, spiders may even use silk as a source of food.[4]
Methods have been developed to collect silk from a spider by force.[5]
Biodiversity
Uses
All spiders produce silks, and a single spider can produce up to seven different types of silk for different uses.[6] This is in contrast to insect silks, where an individual usually only produces one type of silk.[7] Spider silks may be used in many different ecological ways, each with properties to match the silk's function(see Properties section). As spiders have evolved, so has their silks' complexity and diverse uses, for example from primitive tube webs 300–400 mya to complex orb webs 110 mya.[8]| Ecological use | Example | Reference |
|---|---|---|
| Prey capture | The orb webs produced by the Araneidae (typical orb-weavers); tube webs; tangle webs; sheet webs; lace webs, dome webs; single thread used by the Bolas spiders for "fishing". | [6][8] |
| Prey immobilization | Silk used as "swathing bands" to wrap up prey. Often combined with immobilising prey using a venom. In species of Scytodes the silk is combined with venom and squirted from the chelicerae. | [6] |
| Reproduction | Male spiders may produce sperm webs; spider eggs are covered in silk cocoons. | [6][9] |
| Dispersal | "Ballooning" or "kiting" used by many small spiders for dispersal. | [2] |
| Source of food | The kleptoparasitic Argyrodes eating the silk of host spider webs. Some daily weavers of temporary webs also eat their own unused silk daily, thus mitigating a heavy metabolic expense. | [10][4] |
| Nest lining and nest construction | Tube webs used by "primitive" spiders such as the European Tube Web Spider (Segestria florentina). Threads radiate out of nest to provide a sensory link to the outside. Silk is a component of the lids of Trapdoor spiders, and the "Water" or "Diving bell" spider Argyroneta aquatica builds its diving bell of silk. It is in fact difficult to think of any spider that does not use silk in constructing its abode. | [8] |
| Guide lines | Some spiders that venture from shelter will leave a trail of silk by which to find their way home again. | [10] |
| Drop lines and anchor lines | Many spiders, such as the Salticidae, that venture from shelter and leave a trail of silk, use that as an emergency line in case of falling from inverted or vertical surfaces. Many others, even web dwellers, will deliberately drop from a web when alarmed, using a silken thread as a drop line by which they can return in due course. Some, such as species of Paramystaria, also will hang from a drop line when feeding. | [10] |
| Alarm lines | Some spiders that do not spin actual trap webs do lay out alarm webs that the feet of their prey (such as ants) can disturb, cuing the spider to rush out and secure the meal if it is small enough, or to avoid contact if the intruder seems too formidable. | [10] |
| Pheromonal trails | Some wandering spiders will leave a largely continuous trail of silk impregnated with pheromones that the opposite sex can follow to find a mate. | [10] |
Types
Meeting the specification for all these ecological uses requires different types of silk suited to different broad properties, as either a fiber, a structure of fibers, or a silk-globule. These types include glues and fibers. Some types of fibers are used for structural support, others for constructing protective structures. Some can absorb energy effectively, whereas others transmit vibration efficiently. In a spider, these silk types are produced in different glands; so the silk from a particular gland can be linked to its use by the spider. See the later section for details on the mechanical properties of silk and how the structure of silk can achieve these different properties.| Gland | Silk Use |
|---|---|
| Ampullate (Major) | Dragline silk—used for the web’s outer rim and spokes and the lifeline. |
| Ampullate (Minor) | Used for temporary scaffolding during web construction. |
| Flagelliform | Capture-spiral silk—used for the capturing lines of the web. |
| Tubuliform | Egg cocoon silk—used for protective egg sacs. |
| Aciniform | Used to wrap and secure freshly captured prey; used in the male sperm webs; used in stabilimenta. |
| Aggregate | A silk glue of sticky globules. |
| Piriform | Used to form bonds between separate threads for attachment points. |
Properties
Mechanical properties
Each spider and each type of silk has a set of mechanical properties optimised for their biological function.Most silks, in particular dragline silk, have exceptional mechanical properties. They exhibit a unique combination of high tensile strength and extensibility (ductility). This enables a silk fiber to absorb a lot of energy before breaking (toughness, the area under a stress-strain curve).
A frequent mistake made in the mainstream media is to confuse strength and toughness when comparing silk to other materials. As shown below in detail, weight for weight, silk is stronger than steel, but not as strong as Kevlar. Silk is, however, tougher than both.
Strength
In detail a dragline silk's tensile strength is comparable to that of high-grade alloy steel (450 - 1970 MPa),[11][12] and about half as strong as aramid filaments, such as Twaron or Kevlar (3000 MPa).[13]Density
Consisting of mainly protein, silks are about a sixth of the density of steel (1.31 g/cm3). As a result, a strand long enough to circle the Earth would weigh less than 500 grams (18 oz). (Spider dragline silk has a tensile strength of roughly 1.3 GPa. The tensile strength listed for steel might be slightly higher—e.g. 1.65 GPa,[14][15] but spider silk is a much less dense material, so that a given weight of spider silk is five times as strong as the same weight of steel.)Energy density
The energy density of dragline spider silk is 1.2x108J/m3.[16]Extensibility
Silks are also extremely ductile, with some able to stretch up to five times their relaxed length without breaking.Toughness
The combination of strength and ductility gives dragline silks a very high toughness (or work to fracture), which "equals that of commercial polyaramid (aromatic nylon) filaments, which themselves are benchmarks of modern polymer fiber technology".[17][18]Temperature
Whilst unlikely to be relevant in nature, dragline silks can hold their strength below −40°C (-40°F) and up to 220°C (428°F).[19]Supercontraction
When exposed to water, dragline silks undergo supercontraction, shrinking up to 50% in length and behaving like a weak rubber under tension.[20] Many hypotheses have been suggested as to its use in nature, with the most popular being to automatically tension webs built in the night using the morning dew.[citation needed]Highest-performance
The toughest known spider silk is produced by the species Darwin's bark spider (Caerostris darwini): "The toughness of forcibly silked fibers averages 350 MJ/m3, with some samples reaching 520 MJ/m3. Thus, C. darwini silk is more than twice as tough as any previously described silk, and over 10 times tougher than Kevlar".[21]Types of silk
Many species of spider have different glands to produce silk with different properties for different purposes, including housing, web construction, defense, capturing and detaining prey, egg protection, and mobility (gossamer for ballooning, or for a strand allowing the spider to drop down as silk is extruded). Different specialized silks have evolved with properties suitable for different uses. For example, Argiope argentata has five different types of silk, each used for a different purpose:[22][23]| Silk | Use |
|---|---|
| major-ampullate (dragline) silk | Used for the web's outer rim and spokes and the lifeline. Can be as strong per unit weight as steel, but much tougher. |
| capture-spiral (flagelliform) silk | Used for the capturing lines of the web. Sticky, extremely stretchy and tough. The capture spiral is sticky due to droplets of aggregate (a spider glue) that is placed on the spiral. The elasticity of flagelliform allows for enough time for the aggregate to adhere to the aerial prey flying into the web. |
| tubiliform (a.k.a. cylindriform) silk | Used for protective egg sacs. Stiffest silk. |
| aciniform silk | Used to wrap and secure freshly captured prey. Two to three times as tough as the other silks, including dragline. |
| minor-ampullate silk | Used for temporary scaffolding during web construction. |
| Piriform (pyriform) | Piriform serves as the attachment disk to dragline silk. Piriform is used in attaching spider silks together to construct a stable web. |
Structural
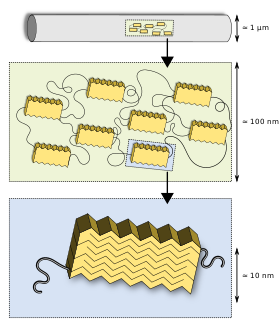
Macroscopic structure down to protein hierarchy
Silks, as well as many other biomaterials, have a hierarchical structure (e.g., cellulose, hair). The primary structure is its amino acid sequence, mainly consisting of highly repetitive glycine and alanine blocks,[24][25] which is why silks are often referred to as a block co-polymer. On a secondary structure level, the short side chained alanine is mainly found in the crystalline domains (beta sheets) of the nanofibril, glycine is mostly found in the so-called amorphous matrix consisting of helical and beta turn structures.[25][26] It is the interplay between the hard crystalline segments, and the strained elastic semi-amorphous regions, that gives spider silk its extraordinary properties.[27][28] Various compounds other than protein are used to enhance the fiber's properties. Pyrrolidine has hygroscopic properties which keeps the silk moist furthermore the additive wards off ant invasion. It occurs in especially high concentration in glue threads. Potassium hydrogen phosphate releases protons in aqueous solution, resulting in a pH of about 4, making the silk acidic and thus protecting it from fungi and bacteria that would otherwise digest the protein. Potassium nitrate is believed to prevent the protein from denaturing in the acidic milieu.[29]
This first very basic model of silk was introduced by Termonia in 1994[30] suggested crystallites embedded in an amorphous matrix interlinked with hydrogen bonds. This model has refined over the years: Semi-crystalline regions were found[25] as well as a fibrillar skin core model suggested for spider silk,[31] later visualized by AFM and TEM.[32] Sizes of the nanofibrillar structure and the crystalline and semi-crystalline regions were revealed by neutron scattering.[33]
Non-protein composition
Various compounds other than protein are found in spider silks, such as sugars, lipids, ions, and pigments that might affect the aggregation behaviour and act as a protection layer in the final fiber.[16]Biosynthesis
The production of silks, including spider silk, differs in an important respect from the production of most other fibrous biological materials: rather than being continuously grown as keratin in hair, cellulose in the cell walls of plants, or even the fibers formed from the compacted faecal matter of beetles,[16] it is "spun" on demand from liquid silk precursor sometimes referred to as unspun silk dope, out of specialised glands.The spinning process occurs when a fiber is pulled away from the body of a spider, be that by the spider’s legs, by the spider's falling and using its own weight, or by any other method including being pulled by humans. The name "spinning" is misleading as no rotation of any component occurs, but the name comes from when it was thought that spiders produced their thread in a similar manner to the spinning wheels of old. In fact the process is a pultrusion[34]—similar to extrusion, with the subtlety that the force is induced by pulling at the finished fiber rather than being squeezed out of a reservoir of some kind.
The unspun silk dope is pulled through silk glands, of which there may be both numerous duplicates and also different types on any one spider species.
Silk gland
The gland's visible, or external, part is termed the spinneret. Depending on the complexity of the species, spiders will have two to eight sets of spinnerets, usually in pairs. There exist highly different specialised glands in different spiders, ranging from simply a sac with an opening at one end, to the complex, multiple-section Major Ampullate glands of the Nephila golden orb weaving spiders.[35]Behind each spinneret visible on the surface of the spider lies a gland, a generalised form of which is shown in the figure to the right, "Schematic of a generalised gland".

Schematic of a generalised gland of a Golden silk orb-weaver. Each differently coloured section highlights a discrete section of the gland[36][37][38]
The gland described here will be based upon the major ampullate gland from a golden orb weaving spiders as they are the most-studied and presumed to be the most complex.
- The first section of the gland labelled 1 on Figure 1 is the secretory or tail section of the gland. The walls of this section are lined with cells that secrete proteins Spidroin I and Spidroin II, the main components of this spider’s dragline. These proteins are found in the form of droplets that gradually elongate to form long channels along the length of the final fiber, hypothesized to assist in preventing crack formation or even self-healing of the fiber.[39]
- The second section is the storage sac. This stores and maintains the gel-like unspun silk dope until it is required by the spider. In addition to storing the unspun silk gel, it secretes proteins that coat the surface of the final fiber.[17]
- The funnel rapidly reduces the large diameter of the storage sac to the small diameter of the tapering duct.
- The final length is the tapering duct, the site of most of the fiber formation. This consists of a tapering tube with several tight about turns, a valve almost at the end (mentioned in detail at point No. 5 below) ending in a spigot from which the silk fiber emerges. The tube here tapers hyperbolically, therefore the unspun silk is under constant shear stress, which is an important factor in fiber formation. This section of the duct is lined with cells that exchange ions and remove water from the fiber. The spigot at the end has lips that clamp around the fiber, controlling fiber diameter and further retaining water.
- Almost at the end of the tapering duct is a valve, approximate position marked "5" on figure 1. Though discovered some time ago, the precise purpose of this valve is still under discussion. It is believed to assist in restarting and rejoining broken fibers[40] acting much in the way of a helical pump, regulating the thickness of the fiber,[34] and/ or clamping the fiber as a spider falls upon it.[40][41] There is some discussion on the similarity of the silk worm’s silk press and the roles each of these valves play in the production of silk in these two organisms.
As an example of a complex spinning field, the spinneret apparatus of an adult Araneus diadematus (garden cross spider) consists of the following glands:[29]
- 500 Glandulae piriformes for attachment points
- 4 Glandulae ampullaceae for the web frame
- about 300 Glandulae aciniformes for the outer lining of egg sacs, and for ensnaring prey
- 4 Glandulae tubuliformes for egg sac silk
- 4 Glandulae aggregatae for glue
- 2 Glandulae coronatae for the thread of glue lines.
Artificial synthesis
In order to artificially synthesize spider silk into fibers, there are two broad areas that must be covered. These are synthesis of the feedstock (the unspun silk dope in spiders), and synthesis of the spinning conditions (the funnel, valve, tapering duct, and spigot). There have been a number of different approaches discussed below.Feedstock
As discussed in the Structural section of the article, the molecular structure of unspun silk is both complex and extremely long. Though this endows the silk fibers with their desirable properties, it also makes replication of the fiber somewhat of a challenge. Various organisms have been used as a basis for attempts to replicate some components or all of some or all of the proteins involved. These proteins must then be extracted, purified and then spun before their properties can be tested. The table below shows the results including the true gold standard- actual stress and strain of the fibers as compared to the best spider dragline.| Organism | Details | Average Maximum breaking stress (MPa) | Average Strain (%) | Reference |
|---|---|---|---|---|
| Gold Standard: Darwin’s bark spider (Caerostris darwini) | Malagasy spider famed for making webs with strands up to 25 m long across rivers. "...C. darwini silk is more than twice as tough as any previously described silk" | 1850 ±350 | 33 ±0.08 | [21] |
| Gold Standard: Nephila clavipes | Typical golden orb weaving spider | 710–1200 | 18–27 | [43][44] |
| Bombyx mori Silkworms | Silkworms were genetically altered to express spider proteins and fibers measured.[45] | 660 | 18.5 | [46] |
| E. coli | Synthesizing such a large and repetitive molecule (250–320 kDa) is complex. Yet, if this is not achieved, the properties will not match those of actual spiders. Here a 285 kDa protein was produced and spun. | 508 ±108 | 15 ±5 | [47] |
| Goats | Goats were genetically modified to secrete silk proteins in their milk, which could then be purified. | 285–250 | 30–40 | [48] |
| Tobacco & potato plants | Spider proteins were inserted into tobacco and potato plants, the rationale being that should this be successful, scaled-up harvesting would be much facilitated. Patents have been granted in this area,[49] but no fibers have yet been described in the literature. | n/a | n/a | [50] |
Geometry
As was shown in the biosynthesis section, spider silks with comparatively simple molecular structure need complex ducts to be able to spin an effective fiber. There have been a number of methods used to produce fibers, of which the main types are briefly discussed below.Syringe and needle
Feedstock is simply forced through a hollow needle using a syringe. This method has been shown to make fibers successfully on multiple occasions.[51][52]Although very cheap and easy to assemble, the shape and conditions of the gland are very loosely approximated. Fibers created using this method may need encouragement to change from liquid to solid by removing the water from the fiber with such chemicals as the environmentally undesirable methanol[53] or acetone,[52] and also may require post-stretching of the fiber to attain fibers with desirable properties.[47][51]
Microfluidics
As the field of microfluidics matures, it is likely that more attempts to spin fibers will be made using microfluidics. These have the advantage of being very controllable and able to test spin very small volumes of unspun fiber[54][55] but setup and development costs are likely to be high. A patent has been granted in this area for spinning fibers in a method mimicking the process found in nature, and fibers are successfully being continuously spun by a commercial company.[56]Electrospinning
Electrospinning is a very old technique whereby a fluid is held in a container in a manner such that it is able to flow out through capillary action. A conducting substrate is positioned below, and a large difference in electrical potential is applied between the fluid and the substrate. The fluid is attracted to the substrate, and tiny fibers jump almost instantly from their point of emission, the Taylor cone, to the substrate, drying as they travel. This method has been shown to create nano-scale fibers from both silk dissected from organisms and regenerated silk fibroin.Other artificial shapes formed from silk
Silk can be formed into other shapes and sizes such as spherical capsules for drug delivery, cell scaffolds and wound healing, textiles, cosmetics, coatings, and many others.[57]Research milestones
Due to spider silk being a scientific research field with a long and rich history, there can be unfortunate occurrences of researchers independently rediscovering previously published findings. What follows is a table of the discoveries made in each of the constituent areas, acknowledged by the scientific community as being relevant and significant by using the metric of scientific acceptance, citations. Thus, only papers with 50 or more citations are included.| Area of contribution | Year | Main researchers [Ref] | Title of paper | Contribution to the field |
|---|---|---|---|---|
| Chemical Basis | 1960 | Fischer, F. & Brander, J.[58] | "Eine Analyse der Gespinste der Kreuzspinne" (Amino acid composition analysis of spider silk) | |
| 1960 | Lucas, F. & et al.[59][60] | "The Composition of Arthropod Silk Fibrons; Comparative studies of fibroins" | ||
| Gene Sequence | 1990 | Xu, M. & Lewis, R. V.[61] | "Structure of a Protein Superfiber - Spider Dragline Silk" | |
| Mechanical Properties | 1964 | Lucas, F.[62] | "Spiders and their silks" | First time compared mechanical properties of spider silk with other materials in a scientific paper. |
| 1989 | Vollrath, F. & Edmonds, D. T.[63] | "Modulation of the Mechanical Properties of Spider Silk by Coating with Water" | First important paper suggesting the water interplay with spider silk fibroin modulating the properties of silk. | |
| 2001 | Vollrath, F. & Shao, Z.Z.[64] | "The effect of spinning conditions on the mechanics of a spider's dragline silk" | ||
| Structural Characterization | 1992 | Hinman, M.B. & Lewis, R. V[24] | "Isolation of a clone encoding a second dragline silk fibroin. Nephila clavipes dragline silk is a two-protein fiber" | |
| 1994 | Simmons, A. & et al.[65] | "Solid-State C-13 Nmr of Nephila-Clavipes Dragline Silk Establishes Structure and Identity of Crystalline Regions" | First NMR study of spider silk. | |
| 1999 | Shao, Z., Vollrath, F. & et al.[66] | "Analysis of spider silk in native and supercontracted states using Raman spectroscopy" | First Raman study of spider silk. | |
| 1999 | Riekel, C., Muller, M.& et al.[67] | "Aspects of X-ray diffraction on single spider fibers" | First X-ray on single spider silk fibers. | |
| 2000 | Knight, D.P., Vollrath, F. & et al.[68] | "Beta transition and stress-induced phase separation in the spinning of spider dragline silk" | Secondary structural transition confirmation during spinning. | |
| 2001 | Riekel, C. & Vollrath, F.[69] | "Spider silk fibre extrusion: combined wide- and small-angle X- ray microdiffraction experiments" | First X-ray on spider silk dope. | |
| 2002 | Van Beek, J. D. & et al.[26] | "The molecular structure of spider dragline silk: Folding and orientation of the protein backbone" | ||
| Structure-Property Relationship | 1986 | Gosline, G.M. & et al.[70] | "The structure and properties of spider silk" | First attempt to link structure with properties of spider silk |
| 1994 | Termonia, Y[30] | "Molecular Modeling of Spider Silk Elasticity" | X-ray evidence presented in this paper; simple model of crystallites embedded in amorphous regions. | |
| 1996 | Simmons, A. & et al.[25] | "Molecular orientation and two-component nature of the crystalline fraction of spider dragline silk" | Two types of alanine-rich crystalline regions were defined. | |
| 2006 | Vollrath, F. & Porter, D.[71] | "Spider silk as an archetypal protein elastomer" | New insight and model to spider silk based on Group Interaction Modelling. | |
| Native Spinning | 1991 | Kerkam, K., Kaplan, D. & et al.[72] | "Liquid Crystallinity of Natural Silk Secretions" | |
| 1999 | Knight, D.P. & Vollrath, F.[73] | "Liquid crystals and flow elongation in a spider's silk production line" | ||
| 2001 | Vollrath, F. & Knight, D.P.[16] | "Liquid crystalline spinning of spider silk" | The most cited paper on spider silk | |
| Reconstituted /Synthetic Spider Silk and Artificial Spinning | 1995 | Prince, J. T., Kaplan, D. L. & et al.[74] | "Construction, Cloning, and Expression of Synthetic Genes Encoding Spider Dragline Silk" | First successful synthesis of Spider silk by E. coli. |
| 1998 | Arcidiacono, S., Kaplan, D.L. & et al.[75] | "Purification and characterization of recombinant spider silk expressed in Escherichia coli" | ||
| 1998 | Seidel, A., Jelinski, L.W. & et al.[76] | "Artificial Spinning of Spider Silk" | First controlled wet-spinning of reconstituted spider silk. |
Human uses
Peasants in the southern Carpathian Mountains used to cut up tubes built by Atypus and cover wounds with the inner lining. It reportedly facilitated healing, and even connected with the skin. This is believed to be due to antiseptic properties of spider silk[78] and because the silk is rich in vitamin K, which can be effective in clotting blood.[79]
Some fishermen in the Indo-Pacific ocean use the web of Nephila to catch small fish.[29]
The silk of Nephila clavipes has recently been used to help in mammalian neuronal regeneration.[80]
At one time, it was common to use spider silk as a thread for crosshairs in optical instruments such as telescopes, microscopes,[81] and telescopic rifle sights.[82]
Due to the difficulties in extracting and processing substantial amounts of spider silk, the largest known piece of cloth made of spider silk is an 11-by-4-foot (3.4 by 1.2 m) textile with a golden tint made in Madagascar in 2009.[83] Eighty-two people worked for four years to collect over one million golden orb spiders and extract silk from them.[84]
In 2011, spider silk fibers were used in the field of optics to generate very fine diffraction patterns over N-slit interferometric signals utilized in optical communications.[85] In 2012, spider silk fibers were used to create a set of violin strings.[86]
Spider silk is used to suspend inertial confinement fusion targets during laser ignition, as it remains considerably elastic and has a high energy to break at temperatures as low as 10-20K. In addition, it is made from "light" atomic number elements that won't emit x-rays during irradiation that could preheat the target so that the pressure differential required for fusion is not achieved.[87]
Attempts at producing synthetic spider silk
Replicating the complex conditions required to produce fibers that are comparable to spider silk has proven difficult to accomplish in a laboratory environment. What follows is a miscellaneous list of attempts on this problem.However, in the absence of hard data accepted by the relevant scientific community, it is difficult to judge whether these attempts have been successful or constructive.
- One approach that does not involve farming spiders is to extract the spider silk gene and use other organisms to produce the spider silk. In 2000, Canadian biotechnology company Nexia successfully produced spider silk protein in transgenic goats that carried the gene for it; the milk produced by the goats contained significant quantities of the protein, 1–2 grams of silk proteins per liter of milk. Attempts to spin the protein into a fiber similar to natural spider silk resulted in fibers with tenacities of 2–3 grams per denier (see BioSteel).[88][89] Nexia used wet spinning and squeezed the silk protein solution through small extrusion holes in order to simulate the behavior of the spinneret, but this procedure has so far not been sufficient to replicate the properties of native spider silk.[90]
- Extrusion of protein fibers in an aqueous environment is known as "wet-spinning". This process has so far produced silk fibers of diameters ranging from 10 to 60 μm, compared to diameters of 2.5–4 μm for natural spider silk.
- In March 2010, researchers from the Korea Advanced Institute of Science & Technology (KAIST) succeeded in making spider silk directly using the bacteria E.coli, modified with certain genes of the spider Nephila clavipes. This approach eliminates the need to milk spiders and allows the manufacture the spider silk in a more cost-effective manner.[91]
- The company Kraig Biocraft Laboratories has used research from the Universities of Wyoming and Notre Dame in a collaborative effort to create a silkworm that has been genetically altered to produce spider silk. In September 2010 it was announced at a press conference at the University of Notre Dame that the effort had been successful.[92][93]
- The company AMSilk has succeeded in making spidroin using bacteria, and making it into spider silk. They are now focusing on increasing production rate of the spider silk.[94]