| Hematopoietic stem cell transplantation | |
|---|---|

Bone marrow transplant
| |
| ICD-9-CM | 41.0 |
| MeSH | D018380 |
| MedlinePlus | 003009 |
Hematopoietic stem cell transplantation (HSCT) is the transplantation of multipotent hematopoietic stem cells, usually derived from bone marrow, peripheral blood, or umbilical cord blood. It may be autologous (the patient's own stem cells are used), allogeneic (the stem cells come from a donor) or syngeneic (from an identical twin).
It is most often performed for patients with certain cancers of the blood or bone marrow, such as multiple myeloma or leukemia. In these cases, the recipient's immune system is usually destroyed with radiation or chemotherapy before the transplantation. Infection and graft-versus-host disease are major complications of allogeneic HSCT.
Hematopoietic stem cell transplantation remains a dangerous procedure with many possible complications; it is reserved for patients with life-threatening diseases. As survival following the procedure has increased, its use has expanded beyond cancer to autoimmune diseases and hereditary skeletal dysplasias; notably malignant infantile osteopetrosis and mucopolysaccharidosis.
Medical uses
Indications
Indications for stem cell transplantation are as follows:
Malignant (cancerous)
- Acute myeloid leukemia (AML)
- Chronic myeloid leukemia (CML)
- Acute lymphoblastic leukemia (ALL)
- Hodgkin lymphoma (HL) (relapsed, refractory)
- Non-Hodgkin lymphoma (NHL) (relapsed, refractory)
- Neuroblastoma
- Ewing sarcoma
- Multiple myeloma
- Myelodysplastic syndromes
- Gliomas, other solid tumors
Non-malignant (non-cancerous)
- Thalassemia
- Sickle cell anemia
- Aplastic anemia
- Fanconi anemia
- Malignant infantile osteopetrosis
- Mucopolysaccharidosis
- Immune deficiency syndromes
- Autoimmune diseases
Many recipients of HSCTs are multiple myeloma or leukemia patients who would not benefit from prolonged treatment with, or are already resistant to, chemotherapy. Candidates for HSCTs include pediatric cases where the patient has an inborn defect such as severe combined immunodeficiency or congenital neutropenia with defective stem cells, and also children or adults with aplastic anemia who have lost their stem cells after birth. Other conditions treated with stem cell transplants include sickle-cell disease, myelodysplastic syndrome, neuroblastoma, lymphoma, Ewing's sarcoma, desmoplastic small round cell tumor, chronic granulomatous disease, Hodgkin's disease and Wiskott–Aldrich syndrome. More recently non-myeloablative, ""mini transplant (microtransplantation),"
procedures have been developed that require smaller doses of
preparative chemo and radiation. This has allowed HSCT to be conducted
in the elderly and other patients who would otherwise be considered too
weak to withstand a conventional treatment regimen.
Number of procedures
In
2006 a total of 50,417 first hematopoietic stem cell transplants were
reported as taking place worldwide, according to a global survey of 1327
centers in 71 countries conducted by the Worldwide Network for Blood
and Marrow Transplantation. Of these, 28,901 (57 percent) were
autologous and 21,516 (43 percent) were allogeneic (11,928 from family
donors and 9,588 from unrelated donors). The main indications for
transplant were lymphoproliferative disorders (55 percent) and leukemias
(34 percent), and the majority took place in either Europe (48 percent)
or the Americas (36 percent).
The Worldwide Network for Blood and Marrow Transplantation
reported the millionth transplant to have been undertaken in December
2012.
In 2014, according to the World Marrow Donor Association
(WMDA), stem cell products provided for unrelated transplantation
worldwide had increased to 20,604 (4,149 bone marrow donations, 12,506
peripheral blood stem cell donations, and 3,949 cord blood units).
Graft types
Autologous
Autologous HSCT requires the extraction (apheresis)
of hematopoietic stem cells (HSC) from the patient and storage of the
harvested cells in a freezer. The patient is then treated with high-dose
chemotherapy with or without radiotherapy with the intention of eradicating the patient's malignant cell population at the cost of partial or complete bone marrow
ablation (destruction of patient's bone marrow's ability to grow new
blood cells). The patient's own stored stem cells are then transfused
into his/her bloodstream, where they replace destroyed tissue and resume
the patient's normal blood cell production. Autologous transplants have
the advantage of lower risk of infection during the immune-compromised
portion of the treatment since the recovery of immune function is rapid.
Also, the incidence of patients experiencing rejection (and graft-versus-host disease
is impossible) is very rare due to the donor and recipient being the
same individual. These advantages have established autologous HSCT as
one of the standard second-line treatments for such diseases as lymphoma.
However, for other cancers such as acute myeloid leukemia,
the reduced mortality of the autogenous relative to allogeneic HSCT may
be outweighed by an increased likelihood of cancer relapse and related
mortality, and therefore the allogeneic treatment may be preferred for
those conditions.
Researchers have conducted small studies using non-myeloablative
hematopoietic stem cell transplantation as a possible treatment for type I (insulin dependent) diabetes in children and adults. Results have been promising; however, as of 2009 it was premature to speculate whether these experiments will lead to effective treatments for diabetes.
Allogeneic
Allogeneic HSCT involves two people: the (healthy) donor and the (patient) recipient. Allogeneic HSC donors must have a tissue (HLA) type that matches the recipient. Matching is performed on the basis of variability at three or more loci
of the HLA gene, and a perfect match at these loci is preferred. Even
if there is a good match at these critical alleles, the recipient will
require immunosuppressive medications to mitigate graft-versus-host disease. Allogeneic transplant donors may be related (usually a closely HLA matched sibling), syngeneic (a monozygotic
or 'identical' twin of the patient – necessarily extremely rare since
few patients have an identical twin, but offering a source of perfectly
HLA matched stem cells) or unrelated (donor who is not related
and found to have very close degree of HLA matching). Unrelated donors
may be found through a registry of bone marrow donors such as the National Marrow Donor Program.
People who would like to be tested for a specific family member or
friend without joining any of the bone marrow registry data banks may
contact a private HLA testing laboratory and be tested with a mouth swab
to see if they are a potential match. A "savior sibling" may be intentionally selected by preimplantation genetic diagnosis
in order to match a child both regarding HLA type and being free of any
obvious inheritable disorder. Allogeneic transplants are also performed
using umbilical cord blood
as the source of stem cells. In general, by transfusing healthy stem
cells to the recipient's bloodstream to reform a healthy immune system,
allogeneic HSCTs appear to improve chances for cure or long-term
remission once the immediate transplant-related complications are
resolved.
A compatible donor is found by doing additional HLA-testing from
the blood of potential donors. The HLA genes fall in two categories
(Type I and Type II). In general, mismatches of the Type-I genes (i.e.
HLA-A, HLA-B, or HLA-C) increase the risk of graft rejection. A
mismatch of an HLA Type II gene (i.e. HLA-DR, or HLA-DQB1) increases the risk of graft-versus-host disease. In addition, a genetic mismatch as small as a single DNA base pair
is significant so perfect matches require knowledge of the exact DNA
sequence of these genes for both donor and recipient. Leading
transplant centers currently perform testing for all five of these HLA
genes before declaring that a donor and recipient are HLA-identical.
Race and ethnicity
are known to play a major role in donor recruitment drives, as members
of the same ethnic group are more likely to have matching genes,
including the genes for HLA.
As of 2013, there were at least two commercialized allogeneic cell therapies, Prochymal and Cartistem.
Sources and storage of cells
To limit the risks of transplanted stem cell rejection or of severe graft-versus-host disease in allogeneic HSCT, the donor should preferably have the same human leukocyte antigens (HLA)
as the recipient. About 25 to 30 percent of allogeneic HSCT recipients
have an HLA-identical sibling. Even so-called "perfect matches" may have
mismatched minor alleles that contribute to graft-versus-host disease.
Bone marrow
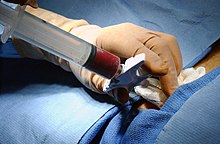
Bone marrow harvest.
In the case of a bone marrow transplant, the HSC are removed from a large bone of the donor, typically the pelvis, through a large needle that reaches the center of the bone. The technique is referred to as a bone marrow harvest and is performed under general anesthesia.
Peripheral blood stem cells
Peripheral blood stem cells
Peripheral blood stem cells are now the most common source of stem cells for HSCT. They are collected from the blood through a process known as apheresis. The donor's blood is withdrawn through a sterile needle in one arm and passed through a machine that removes white blood cells. The red blood cells are returned to the donor. The peripheral stem cell yield is boosted with daily subcutaneous injections of granulocyte-colony stimulating factor, serving to mobilize stem cells from the donor's bone marrow into the peripheral circulation.
Amniotic fluid
It is also possible to extract stem cells from amniotic fluid for both autologous or heterologous use at the time of childbirth.
Umbilical cord blood
Umbilical cord blood is obtained when a mother donates her infant's umbilical cord and placenta after birth. Cord blood
has a higher concentration of HSC than is normally found in adult
blood. However, the small quantity of blood obtained from an umbilical
cord (typically about 50 mL) makes it more suitable for transplantation
into small children than into adults. Newer techniques using ex-vivo
expansion of cord blood units or the use of two cord blood units from
different donors allow cord blood transplants to be used in adults.
Cord blood can be harvested from the umbilical cord of a child being born after preimplantation genetic diagnosis (PGD) for human leukocyte antigen (HLA) matching (see PGD for HLA matching) in order to donate to an ill sibling requiring HSCT.
Storage of HSC
Unlike
other organs, bone marrow cells can be frozen (cryopreserved) for
prolonged periods without damaging too many cells. This is a necessity
with autologous HSC because the cells must be harvested from the
recipient months in advance of the transplant treatment. In the case of
allogeneic transplants, fresh HSC are preferred in order to avoid cell
loss that might occur during the freezing and thawing process.
Allogeneic cord blood is stored frozen at a cord blood bank because it is only obtainable at the time of childbirth. To cryopreserve HSC, a preservative, DMSO, must be added, and the cells must be cooled very slowly in a controlled-rate freezer to prevent osmotic cellular injury during ice crystal formation. HSC may be stored for years in a cryofreezer, which typically uses liquid nitrogen.
Conditioning regimens
Myeloablative
The chemotherapy or irradiation given immediately prior to a transplant is called the conditioning regimen,
the purpose of which is to help eradicate the patient's disease prior
to the infusion of HSC and to suppress immune reactions. The bone marrow
can be ablated (destroyed) with dose-levels that cause minimal injury to other tissues. In allogeneic transplants a combination of cyclophosphamide with total body irradiation is conventionally employed. This treatment also has an immunosuppressive effect that prevents rejection of the HSC by the recipient's immune system. The post-transplant prognosis often includes acute and chronic graft-versus-host disease
that may be life-threatening. However, in certain leukemias this can
coincide with protection against cancer relapse owing to the graft-versus-tumor effect. Autologous
transplants may also use similar conditioning regimens, but many other
chemotherapy combinations can be used depending on the type of disease.
Non-myeloablative
A
newer treatment approach, non-myeloablative allogeneic transplantation,
also termed reduced-intensity conditioning (RIC), uses doses of chemotherapy and radiation too low to eradicate all the bone marrow cells of the recipient.
Instead, non-myeloablative transplants run lower risks of serious
infections and transplant-related mortality while relying upon the graft versus tumor effect to resist the inherent increased risk of cancer relapse. Also significantly, while requiring high doses of immunosuppressive agents in the early stages of treatment, these doses are less than for conventional transplants. This leads to a state of mixed chimerism early after transplant where both recipient and donor HSC coexist in the bone marrow space.
Decreasing doses of immunosuppressive therapy then allow donor T-cells to eradicate the remaining recipient HSC and to induce the graft-versus-tumor effect. This effect is often accompanied by mild graft-versus-host disease,
the appearance of which is often a surrogate marker for the emergence
of the desirable graft versus tumor effect, and also serves as a signal
to establish an appropriate dosage level for sustained treatment with
low levels of immunosuppressive agents.
Because of their gentler conditioning regimens, these transplants
are associated with a lower risk of transplant-related mortality and
therefore allow patients who are considered too high-risk for
conventional allogeneic HSCT to undergo potentially curative therapy for
their disease. The optimal conditioning strategy for each disease and
recipient has not been fully established, but RIC can be used in elderly
patients unfit for myeloablative regimens, for whom a higher risk of
cancer relapse may be acceptable.
Engraftment
After
several weeks of growth in the bone marrow, expansion of HSC and their
progeny is sufficient to normalize the blood cell counts and re-initiate
the immune system. The offspring of donor-derived hematopoietic stem cells have been documented to populate many different organs of the recipient, including the heart, liver, and muscle,
and these cells had been suggested to have the abilities of
regenerating injured tissue in these organs. However, recent research
has shown that such lineage infidelity does not occur as a normal
phenomenon
.
Chimerism monitoring is a method to monitor the balance between
the patient's own stem cells and the new stem cells from a donor. In
case the patient's own stem cells are increasing in number
post-treatment, this might be a sign the treatment did not work as
intended.
Complications
HSCT
is associated with a high treatment-related mortality in the recipient,
which limits its use to conditions that are themselves
life-threatening. (The one-year survival rate has been estimated to be
roughly 60%, although this figure includes deaths from the underlying
disease as well as from the transplant procedure.) Major complications are veno-occlusive disease, mucositis, infections (sepsis), graft-versus-host disease and the development of new malignancies.
Infection
Bone
marrow transplantation usually requires that the recipient's own bone
marrow be destroyed (myeloablation). Prior to the administration of new
cells (engraftment) patients may go for several weeks without
appreciable numbers of white blood cells to help fight infection. This puts a patient at high risk of infections, sepsis and septic shock, despite prophylactic antibiotics. However, antiviral medications, such as acyclovir and valacyclovir, are quite effective in prevention of HSCT-related outbreak of herpetic infection in seropositive patients. The immunosuppressive agents employed in allogeneic transplants for the prevention or treatment of graft-versus-host disease further increase the risk of opportunistic infection.
Immunosuppressive drugs are given for a minimum of 6-months after a
transplantation, or much longer if required for the treatment of
graft-versus-host disease. Transplant patients lose their acquired immunity, for example immunity to childhood diseases such as measles or polio. For this reason transplant patients must be re-vaccinated with childhood vaccines once they are off immunosuppressive medications.
Veno-occlusive disease
Severe liver injury can result from hepatic veno-occlusive disease (VOD). Elevated levels of bilirubin, hepatomegaly
and fluid retention are clinical hallmarks of this condition. There is
now a greater appreciation of the generalized cellular injury and
obstruction in hepatic vein sinuses, and hepatic VOD has lately been
referred to as sinusoidal obstruction syndrome (SOS). Severe cases of
SOS are associated with a high mortality rate. Anticoagulants or defibrotide may be effective in reducing the severity of VOD but may also increase bleeding complications. Ursodiol has been shown to help prevent VOD, presumably by facilitating the flow of bile.
Mucositis
The
injury of the mucosal lining of the mouth and throat is a common
regimen-related toxicity following ablative HSCT regimens. It is usually
not life-threatening but is very painful, and prevents eating and
drinking. Mucositis is treated with pain medications plus intravenous
infusions to prevent dehydration and malnutrition.
Hemorrhagic cystitis
The
mucosal lining of the bladder could also be involved in approximately 5
percent of the children undergoing hematopoietic stem cell
transplantation. This causes hematuria, frequency, abdominal pain and
thrombocytopnea.
Graft-versus-host disease
Graft-versus-host disease (GVHD) is an inflammatory disease that is
unique to allogeneic transplantation. It is an attack by the "new" bone
marrow's immune cells against the recipient's tissues. This can occur
even if the donor and recipient are HLA-identical because the immune
system can still recognize other differences between their tissues. It
is aptly named graft-versus-host disease because bone marrow
transplantation is the only transplant procedure in which the
transplanted cells must accept the body rather than the body accepting
the new cells.
Acute graft-versus-host disease typically occurs in the first 3 months after transplantation and may involve the skin, intestine, or the liver. High-dose corticosteroids such as prednisone are a standard treatment; however this immuno-suppressive treatment often leads to deadly infections.
Chronic graft-versus-host disease may also develop after
allogeneic transplant. It is the major source of late treatment-related
complications, although it less often results in death. In addition to
inflammation, chronic graft-versus-host disease may lead to the
development of fibrosis, or scar tissue, similar to scleroderma; it may cause functional disability and require prolonged immunosuppressive therapy.
Graft-versus-host disease is usually mediated by T cells, which react to foreign peptides presented on the MHC of the host.
Graft-versus-tumor effect
Graft-versus-tumor effect
(GVT) or "graft versus leukemia" effect is the beneficial aspect of the
Graft-versus-Host phenomenon. For example, HSCT patients with either
acute, or in particular chronic, graft-versus-host disease after an
allogeneic transplant tend to have a lower risk of cancer relapse. This is due to a therapeutic immune reaction of the grafted donor T lymphocytes against the diseased bone marrow
of the recipient. This lower rate of relapse accounts for the increased
success rate of allogeneic transplants, compared to transplants from
identical twins, and indicates that allogeneic HSCT is a form of
immunotherapy. GVT is the major benefit of transplants that do not
employ the highest immuno-suppressive regimens.
Graft versus tumor is mainly beneficial in diseases with slow
progress, e.g. chronic leukemia, low-grade lymphoma, and in some cases
multiple myeloma. However, it is less effective in rapidly growing acute
leukemias.
If cancer relapses after HSCT, another transplant can be
performed, infusing the patient with a greater quantity of donor white
blood cells (Donor lymphocyte infusion).
Oral carcinoma
Patients after HSCT are at a higher risk for oral carcinoma. Post-HSCT oral cancer may have more aggressive behavior with poorer prognosis, when compared to oral cancer in non-HSCT patients.
Prognosis
Prognosis
in HSCT varies widely dependent upon disease type, stage, stem cell
source, HLA-matched status (for allogeneic HSCT) and conditioning
regimen. A transplant offers a chance for cure or long-term remission if
the inherent complications of graft versus host disease,
immuno-suppressive treatments and the spectrum of opportunistic
infections can be survived.
In recent years, survival rates have been gradually improving across
almost all populations and sub-populations receiving transplants.
Mortality for allogeneic stem cell transplantation can be estimated using the prediction model created by Sorror et al.,
using the Hematopoietic Cell Transplantation-Specific Comorbidity Index
(HCT-CI). The HCT-CI was derived and validated by investigators at the
Fred Hutchinson Cancer Research Center (Seattle, WA). The HCT-CI
modifies and adds to a well-validated comorbidity index, the Charlson
Comorbidity Index (CCI) (Charlson et al.)
The CCI was previously applied to patients undergoing allogeneic HCT
but appears to provide less survival prediction and discrimination than
the HCT-CI scoring system.
Risks to donor
The risks of a complication depend on patient characteristics, health care providers and the apheresis procedure, and the colony-stimulating factor used (G-CSF). G-CSF drugs include filgrastim (Neupogen, Neulasta), and lenograstim (Graslopin).
Drug risks
Filgrastim
is typically dosed in the 10 microgram/kg level for 4–5 days during the
harvesting of stem cells. The documented adverse effects of filgrastim
include splenic rupture
(indicated by left upper abdominal or shoulder pain, risk 1 in 40000),
Acute respiratory distress syndrome (ARDS), alveolar hemorrhage, and
allergic reactions (usually expressed in first 30 minutes, risk 1 in
300). In addition, platelet and hemoglobin levels dip post-procedure, not returning to normal until after one month.
The question of whether geriatrics (patients over 65) react the
same as patients under 65 has not been sufficiently examined.
Coagulation issues and inflammation of atherosclerotic plaques are known
to occur as a result of G-CSF injection. G-CSF has also been described
to induce genetic changes in mononuclear cells of normal donors.
There is no statistically significant evidence either for or against
the hypothesis that myelodysplasia (MDS) or acute myeloid leukaemia
(AML) can be induced by GCSF in susceptible individuals.
Access risks
Blood
was drawn peripherally in a majority of patients, but a central line to
jugular/subclavian/femoral veins may be used in 16 percent of women and
4 percent of men. Adverse reactions during apheresis
were experienced in 20 percent of women and 8 percent of men, these
adverse events primarily consisted of numbness/tingling, multiple line
attempts, and nausea.
Clinical observations
A
study involving 2408 donors (18–60 years) indicated that bone pain
(primarily back and hips) as a result of filgrastim treatment is
observed in 80 percent of donors by day 4 post-injection.
This pain responded to acetaminophen or ibuprofen in 65 percent of
donors and was characterized as mild to moderate in 80 percent of donors
and severe in 10 percent.
Bone pain receded post-donation to 26 percent of patients 2 days
post-donation, 6 percent of patients one week post-donation, and less than 2
percent 1 year post-donation. Donation is not recommended for those with
a history of back pain. Other symptoms observed in more than 40 percent of donors include myalgia, headache, fatigue, and insomnia.
These symptoms all returned to baseline 1 month post-donation, except
for some cases of persistent fatigue in 3 percent of donors.
In one metastudy that incorporated data from 377 donors, 44
percent of patients reported having adverse side effects after
peripheral blood HSCT.
Side effects included pain prior to the collection procedure as a
result of GCSF injections, post-procedural generalized skeletal pain,
fatigue and reduced energy.
Severe reactions
A
study that surveyed 2408 donors found that serious adverse events
(requiring prolonged hospitalization) occurred in 15 donors (at a rate
of 0.6 percent), although none of these events were fatal. Donors were not observed to have higher than normal rates of cancer with up to 4–8 years of follow up.
One study based on a survey of medical teams covered approximately
24,000 peripheral blood HSCT cases between 1993 and 2005, and found a
serious cardiovascular adverse reaction rate of about 1 in 1500.
This study reported a cardiovascular-related fatality risk within the
first 30 days HSCT of about 2 in 10000. For this same group, severe
cardiovascular events were observed with a rate of about 1 in 1500. The
most common severe adverse reactions were pulmonary edema/deep vein
thrombosis, splenic rupture, and myocardial infarction. Haematological
malignancy induction was comparable to that observed in the general
population, with only 15 reported cases within 4 years.
History
Georges Mathé,
a French oncologist, performed the first European bone marrow
transplant in November 1958 on five Yugoslavian nuclear workers whose
own marrow had been damaged by irradiation caused by a criticality accident at the Vinča Nuclear Institute,
but all of these transplants were rejected. Fortunately, the five
treated were able to ultimately recover, perhaps in part due to the
transplants. Mathé later pioneered the use of bone marrow transplants in the treatment of leukemia.
Stem cell transplantation was pioneered using bone-marrow-derived stem cells by a team at the Fred Hutchinson Cancer Research Center from the 1950s through the 1970s led by E. Donnall Thomas, whose work was later recognized with a Nobel Prize in Physiology or Medicine. Thomas' work showed that bone marrow cells infused intravenously could repopulate the bone marrow and produce new blood cells. His work also reduced the likelihood of developing a life-threatening complication called graft-versus-host disease. Collaborating with University of Washington Professor Eloise Giblett, he discovered genetic markers that could confirm donor matches.
The first physician to perform a successful human bone marrow transplant on a disease other than cancer was Robert A. Good at the University of Minnesota in 1968.
In 1975, John Kersey, M.D., also of the University of Minnesota,
performed the first successful bone marrow transplant to cure lymphoma.
His patient, a 16-year-old-boy, is today the longest-living lymphoma
transplant survivor.
Donor registration and recruitment
At
the end of 2012, 20.2 million people had registered their willingness
to be a bone marrow donor with one of the 67 registries from 49
countries participating in Bone Marrow Donors Worldwide.
17.9 million of these registered donors had been ABDR typed, allowing
easy matching. A further 561,000 cord blood units had been received by
one of 46 cord blood banks from 30 countries participating. The highest
total number of bone marrow donors registered were those from the US
(8.0 million), and the highest number per capita were those from Cyprus
(15.4 percent of the population).
Within the United States, racial minority groups are the least
likely to be registered and therefore the least likely to find a
potentially life-saving match. In 1990, only six African-Americans were
able to find a bone marrow match, and all six had common European
genetic signatures.
Africans are more genetically diverse than people of European
descent, which means that more registrations are needed to find a match.
Bone marrow and cord blood banks exist in South Africa, and a new
program is beginning in Nigeria.
Many people belonging to different races are requested to donate as
there is a shortage of donors in African, Mixed race, Latino,
Aboriginal, and many other communities.
Two registries in the United States recruit unrelated allogeneic donors: NMDP/Be the Match and the Gift of Life Marrow Registry.
Research
HIV
In 2007, a team of doctors in Berlin, Germany, including Gero Hütter, performed a stem cell transplant for leukemia patient Timothy Ray Brown, who was also HIV-positive. From 60 matching donors, they selected a [CCR5]-Δ32 homozygous individual with two genetic copies
of a rare variant of a cell surface receptor. This genetic trait
confers resistance to HIV infection by blocking attachment of HIV to the
cell. Roughly one in 1000 people of European ancestry have this
inherited mutation, but it is rarer in other populations.
The transplant was repeated a year later after a leukemia relapse. Over
three years after the initial transplant, and despite discontinuing antiretroviral therapy, researchers cannot detect HIV in the transplant recipient's blood or in various biopsies of his tissues.
Levels of HIV-specific antibodies have also declined, leading to
speculation that the patient may have been functionally cured of HIV.
However, scientists emphasise that this is an unusual case. Potentially fatal transplant complications (the "Berlin patient" suffered from graft-versus-host disease and leukoencephalopathy) mean that the procedure could not be performed in others with HIV, even if sufficient numbers of suitable donors were found.
In 2012, Daniel Kuritzkes reported results of two stem cell
transplants in patients with HIV. They did not, however, use donors
with the Δ32 deletion. After their transplant procedures, both were put
on antiretroviral therapies, during which neither showed traces of HIV
in their blood plasma and purified CD4 T cells using a sensitive culture
method (less than 3 copies/mL). However, the virus was once again
detected in both patients some time after the discontinuation of
therapy.
In 2019, a British man became the second to be cleared of HIV
after receiving a bone marrow transplant from a virus-resistant (Δ32)
donor. This patient is being called "the London patient" (a reference to
the famous Berlin patient).
Multiple sclerosis
Since McAllister's 1997 report on a patient with multiple sclerosis (MS) who received a bone marrow transplant for CML, over 600 reports have been published describing HSCTs performed primarily for MS.
These have been shown to "reduce or eliminate ongoing clinical
relapses, halt further progression, and reduce the burden of disability
in some patients" that have aggressive highly active MS, "in the absence
of chronic treatment with disease-modifying agents".