Ethanol fuel is ethyl alcohol, the same type of alcohol found in alcoholic beverages, used as fuel. It is most often used as a motor fuel, mainly as a biofuel additive for gasoline. The first production car running entirely on ethanol was the Fiat 147, introduced in 1978 in Brazil by Fiat. Ethanol is commonly made from biomass such as corn or sugarcane. World ethanol production for transport fuel tripled between 2000 and 2007 from 17×109 liters (4.5×109 U.S. gal; 3.7×109 imp gal) to more than 52×109 liters (1.4×1010 U.S. gal; 1.1×1010 imp gal). From 2007 to 2008, the share of ethanol in global gasoline type fuel use increased from 3.7% to 5.4%. In 2011 worldwide ethanol fuel production reached 8.46×1010 liters (2.23×1010 U.S. gal; 1.86×1010 imp gal)
with the United States of America and Brazil being the top producers,
accounting for 62.2% and 25% of global production, respectively. US ethanol production reached 57.54×109 liters (1.520×1010 U.S. gal; 1.266×1010 imp gal) in 2017-04.
Ethanol fuel has a "gasoline gallon equivalency" (GGE) value of 1.5, i.e. to replace the energy of 1 volume of gasoline, 1.5 times the volume of ethanol is needed.
Ethanol-blended fuel is widely used in Brazil, the United States, and Europe. Most cars on the road today in the U.S. can run on blends of up to 10% ethanol, and ethanol represented 10% of the U.S. gasoline fuel supply derived from domestic sources in 2011. Furthermore, many used cars today are flexible-fuel vehicles able to use 100% ethanol fuel.
Since 1976 the Brazilian government has made it mandatory to
blend ethanol with gasoline, and since 2007 the legal blend is around 25% ethanol and 75% gasoline (E25). By December 2011 Brazil had a fleet of 14.8 million flex-fuel automobiles and light trucks and 1.5 million flex-fuel motorcycles that regularly use neat ethanol fuel (known as E100).
Bioethanol is a form of renewable energy that can be produced from agricultural feedstocks. It can be made from very common crops such as hemp, sugarcane, potato, cassava and corn.
There has been considerable debate about how useful bioethanol is in
replacing gasoline. Concerns about its production and use relate to increased food prices due to the large amount of arable land required for crops,
as well as the energy and pollution balance of the whole cycle of
ethanol production, especially from corn. Even though this debate has
the counterpart of animal agriculture being already a source of massive
arable land, therefore making ethanol a lower resource consumer in
constrast. Recent developments with cellulosic ethanol production and commercialization may allay some of these concerns.
Cellulosic ethanol offers promise because cellulose fibers, a major and universal component in plant cells walls, can be used to produce ethanol. According to the International Energy Agency, cellulosic ethanol could allow ethanol fuels to play a much bigger role in the future.
Chemistry
Structure of ethanol molecule. All bonds are single bonds
During ethanol fermentation, glucose and other sugars in the corn (or sugarcane or other crops) are converted into ethanol and carbon dioxide.
- C6H12O6 → 2 C2H5OH+ 2 CO2 + heat
Ethanol fermentation is not 100% selective with side products such as
acetic acid and glycols. They are mostly removed during ethanol
purification. Fermentation takes place in an aqueous solution. The
resulting solution has an ethanol content of around 15%. Ethanol is
subsequently isolated and purified by a combination of adsorption and
distillation.
During combustion, ethanol reacts with oxygen to produce carbon dioxide, water, and heat:
- C2H5OH + 3 O2 → 2 CO2 + 3 H2O + heat
Starch and cellulose
molecules are strings of glucose molecules. It is also possible to
generate ethanol out of cellulosic materials. That, however, requires a
pretreatment that splits the cellulose into glucose molecules and other
sugars that subsequently can be fermented. The resulting product is
called cellulosic ethanol, indicating its source.
Ethanol is also produced industrially from ethylene by hydration of the double bond in the presence of a catalyst and high temperature.
- C2H4 + H2O → C2H5OH
Most ethanol is produced by fermentation.
Sources
Sugar cane harvest
Cornfield in South Africa
Switchgrass
About 5% of the ethanol produced in the world in 2003 was actually a petroleum product. It is made by the catalytic hydration of ethylene with sulfuric acid as the catalyst. It can also be obtained via ethylene or acetylene, from calcium carbide, coal,
oil gas, and other sources. Two million short tons (1,786,000 long
tons; 1,814,000 t) of petroleum-derived ethanol are produced annually.
The principal suppliers are plants in the United States, Europe, and
South Africa.
Petroleum derived ethanol (synthetic ethanol) is chemically identical
to bio-ethanol and can be differentiated only by radiocarbon dating.
Bio-ethanol is usually obtained from the conversion of carbon-based feedstock.
Agricultural feedstocks are considered renewable because they get
energy from the sun using photosynthesis, provided that all minerals
required for growth (such as nitrogen and phosphorus) are returned to
the land. Ethanol can be produced from a variety of feedstocks such as sugar cane, bagasse, miscanthus, sugar beet, sorghum, grain, switchgrass, barley, hemp, kenaf, potatoes, sweet potatoes, cassava, sunflower, fruit, molasses, corn, stover, grain, wheat, straw, cotton, other biomass, as well as many types of cellulose waste and harvesting, whichever has the best well-to-wheel assessment.
An alternative process to produce bio-ethanol from algae is being developed by the company Algenol. Rather than grow algae
and then harvest and ferment it, the algae grow in sunlight and produce
ethanol directly, which is removed without killing the algae. It is
claimed the process can produce 6,000 U.S. gallons per acre (5,000
imperial gallons per acre; 56,000 liters per hectare) per year compared
with 400 US gallons per acre (330 imp gal/acre; 3,700 L/ha) for corn
production.
Currently, the first generation processes for the production of
ethanol from corn use only a small part of the corn plant: the corn
kernels are taken from the corn plant and only the starch, which
represents about 50% of the dry kernel mass, is transformed into
ethanol. Two types of second generation processes are under development.
The first type uses enzymes and yeast fermentation to convert the plant cellulose into ethanol while the second type uses pyrolysis to convert the whole plant to either a liquid bio-oil or a syngas. Second generation processes can also be used with plants such as grasses, wood or agricultural waste material such as straw.
Production
Although there are various ways ethanol fuel can be produced, the most common way is via fermentation.
The basic steps for large-scale production of ethanol are: microbial (yeast) fermentation of sugars, distillation, dehydration (requirements vary, see Ethanol fuel mixtures, below), and denaturing (optional). Prior to fermentation, some crops require saccharification or hydrolysis of carbohydrates such as cellulose and starch into sugars. Saccharification of cellulose is called cellulolysis (see cellulosic ethanol). Enzymes are used to convert starch into sugar.
Fermentation
Ethanol is produced by microbial fermentation of the sugar. Microbial fermentation currently only works directly with sugars. Two major components of plants, starch
and cellulose, are both made of sugars—and can, in principle, be
converted to sugars for fermentation. Currently, only the sugar (e.g.,
sugar cane) and starch (e.g., corn) portions can be economically
converted. There is much activity in the area of cellulosic ethanol,
where the cellulose part of a plant is broken down to sugars and
subsequently converted to ethanol.
Distillation
Ethanol plant in West Burlington, Iowa
Ethanol plant in Sertãozinho, Brazil.
For the ethanol to be usable as a fuel, the yeast solids and the
majority of the water must be removed. After fermentation, the mash is heated so that the ethanol evaporates. This process, known as distillation, separates the ethanol, but its purity is limited to 95–96% due to the formation of a low-boiling water-ethanol azeotrope
with maximum (95.6% m/m (96.5% v/v) ethanol and 4.4% m/m (3.5% v/v)
water). This mixture is called hydrous ethanol and can be used as a
fuel alone, but unlike anhydrous
ethanol, hydrous ethanol is not miscible in all ratios with gasoline,
so the water fraction is typically removed in further treatment to burn
in combination with gasoline in gasoline engines.
Dehydration
There are three dehydration processes to remove the water from an azeotropic ethanol/water mixture. The first process, used in many early fuel ethanol plants, is called azeotropic distillation and consists of adding benzene or cyclohexane to the mixture. When these components are added to the mixture, it forms a heterogeneous azeotropic mixture in vapor–liquid-liquid equilibrium,
which when distilled produces anhydrous ethanol in the column bottom,
and a vapor mixture of water, ethanol, and cyclohexane/benzene.
When condensed, this becomes a two-phase liquid mixture. The
heavier phase, poor in the entrainer (benzene or cyclohexane), is
stripped of the entrainer and recycled to the feed—while the lighter
phase, with condensate from the stripping, is recycled to the second
column. Another early method, called extractive distillation,
consists of adding a ternary component that increases ethanol's
relative volatility. When the ternary mixture is distilled, it produces
anhydrous ethanol on the top stream of the column.
With increasing attention being paid to saving energy, many
methods have been proposed that avoid distillation altogether for
dehydration. Of these methods, a third method has emerged and has been
adopted by the majority of modern ethanol plants. This new process uses molecular sieves
to remove water from fuel ethanol. In this process, ethanol vapor under
pressure passes through a bed of molecular sieve beads. The bead's
pores are sized to allow adsorption
of water while excluding ethanol. After a period of time, the bed is
regenerated under vacuum or in the flow of inert atmosphere (e.g. N2)
to remove the adsorbed water. Two beds are often used so that one is
available to adsorb water while the other is being regenerated. This
dehydration technology can account for energy saving of 3,000
btus/gallon (840 kJ/L) compared to earlier azeotropic distillation.
Recent research has demonstrated that complete dehydration prior
to blending with gasoline is not always necessary. Instead, the
azeotropic mixture can be blended directly with gasoline so that
liquid-liquid phase equilibrium can assist in the elimination of water. A
two-stage counter-current setup of mixer-settler tanks can achieve
complete recovery of ethanol into the fuel phase, with minimal energy
consumption.
Post-production water issues
Ethanol is hygroscopic,
meaning it absorbs water vapor directly from the atmosphere. Because
absorbed water dilutes the fuel value of the ethanol and may cause phase
separation of ethanol-gasoline blends (which causes engine stall),
containers of ethanol fuels must be kept tightly sealed. This high miscibility with water means that ethanol cannot be efficiently shipped through modern pipelines, like liquid hydrocarbons, over long distances.
The fraction of water that an ethanol-gasoline fuel can contain
without phase separation increases with the percentage of ethanol.
For example, E30 can have up to about 2% water. If there is more than
about 71% ethanol, the remainder can be any proportion of water or
gasoline and phase separation does not occur. The fuel mileage declines
with increased water content. The increased solubility of water with
higher ethanol content permits E30 and hydrated ethanol to be put in the
same tank since any combination of them always results in a single
phase. Somewhat less water is tolerated at lower temperatures. For E10
it is about 0.5% v/v at 21° C and decreases to about 0.23% v/v at −34° C
.
Consumer production systems
While biodiesel
production systems have been marketed to home and business users for
many years, commercialized ethanol production systems designed for
end-consumer use have lagged in the marketplace. In 2008, two different
companies announced home-scale ethanol production systems. The AFS125
Advanced Fuel System
from Allard Research and Development is capable of producing both
ethanol and biodiesel in one machine, while the E-100 MicroFueler from E-Fuel Corporation is dedicated to ethanol only.
Engines
Fuel economy
Ethanol
contains approx. 34% less energy per unit volume than gasoline, and
therefore in theory, burning pure ethanol in a vehicle reduces miles
per US gallon 34%, given the same fuel economy, compared to burning pure gasoline. However, since ethanol has a higher octane rating, the engine can be made more efficient by raising its compression ratio. Using a variable geometry or twin scroll turbocharger, the compression ratio can be optimized for the fuel, making fuel economy almost constant for any blend.
For E10 (10% ethanol and 90% gasoline), the effect is small (~3%) when compared to conventional gasoline, and even smaller (1–2%) when compared to oxygenated and reformulated blends.
For E85 (85% ethanol), the effect becomes significant. E85 produces
lower mileage than gasoline, and requires more frequent refueling.
Actual performance may vary depending on the vehicle. Based on EPA tests
for all 2006 E85 models, the average fuel economy for E85 vehicles was
25.56% lower than unleaded gasoline. The EPA-rated mileage of current United States flex-fuel vehicles
should be considered when making price comparisons, but E85 is a high
performance fuel, with an octane rating of about 94–96, and should be
compared to premium.
Cold start during the winter
The Brazilian 2008 Honda Civic
flex-fuel has outside direct access to the secondary reservoir gasoline
tank in the front right side, the corresponding fuel filler door is
shown by the arrow.
High ethanol blends present a problem to achieve enough vapor pressure for the fuel to evaporate and spark the ignition during cold weather (since ethanol tends to increase fuel enthalpy of vaporization). When vapor pressure is below 45 kPa starting a cold engine becomes difficult. To avoid this problem at temperatures below 11 °C (52 °F),
and to reduce ethanol higher emissions during cold weather, both the US
and the European markets adopted E85 as the maximum blend to be used in
their flexible fuel vehicles, and they are optimized to run at such a
blend. At places with harsh cold weather, the ethanol blend in the US
has a seasonal reduction to E70 for these very cold regions, though it is still sold as E85. At places where temperatures fall below −12 °C (10 °F)
during the winter, it is recommended to install an engine heater
system, both for gasoline and E85 vehicles. Sweden has a similar
seasonal reduction, but the ethanol content in the blend is reduced to E75 during the winter months.
Brazilian flex fuel vehicles can operate with ethanol mixtures up to E100, which is hydrous
ethanol (with up to 4% water), which causes vapor pressure to drop
faster as compared to E85 vehicles. As a result, Brazilian flex vehicles
are built with a small secondary gasoline reservoir located near the
engine. During a cold start pure gasoline is injected to avoid starting
problems at low temperatures. This provision is particularly necessary
for users of Brazil's southern and central regions, where temperatures
normally drop below 15 °C (59 °F)
during the winter. An improved flex engine generation was launched in
2009 that eliminates the need for the secondary gas storage tank. In March 2009 Volkswagen do Brasil launched the Polo E-Flex, the first Brazilian flex fuel model without an auxiliary tank for cold start.
Fuel mixtures
Hydrated ethanol × gasoline type C price table for use in Brazil
EPA's E15 label required to be displayed in all E15 fuel dispensers in the U.S.
In many countries cars are mandated to run on mixtures of ethanol.
All Brazilian light-duty vehicles are built to operate for an ethanol
blend of up to 25% (E25), and since 1993 a federal law requires mixtures between 22% and 25% ethanol, with 25% required as of mid July 2011. In the United States all light-duty vehicles are built to operate normally with an ethanol blend of 10% (E10). At the end of 2010 over 90 percent of all gasoline sold in the U.S. was blended with ethanol. In January 2011 the U.S. Environmental Protection Agency (EPA) issued a waiver to authorize up to 15% of ethanol blended with gasoline (E15) to be sold only for cars and light pickup trucks with a model year of 2001 or newer.
Beginning with the model year 1999, an increasing number of
vehicles in the world are manufactured with engines that can run on any
fuel from 0% ethanol up to 100% ethanol without modification. Many cars
and light trucks (a class containing minivans, SUVs and pickup trucks) are designed to be flexible-fuel vehicles using ethanol blends up to 85% (E85)
in North America and Europe, and up to 100% (E100) in Brazil. In older
model years, their engine systems contained alcohol sensors in the fuel
and/or oxygen sensors in the exhaust that provide input to the engine
control computer to adjust the fuel injection to achieve stochiometric
(no residual fuel or free oxygen in the exhaust) air-to-fuel ratio for
any fuel mix. In newer models, the alcohol sensors have been removed,
with the computer using only oxygen and airflow sensor feedback to
estimate alcohol content. The engine control computer can also adjust
(advance) the ignition timing to achieve a higher output without
pre-ignition when it predicts that higher alcohol percentages are
present in the fuel being burned. This method is backed up by advanced
knock sensors – used in most high performance gasoline engines
regardless of whether they are designed to use ethanol or not – that
detect pre-ignition and detonation.
Other engine configurations
- ED95 engines
Since 1989 there have also been ethanol engines based on the diesel principle operating in Sweden. They are used primarily in city buses, but also in distribution trucks and waste collectors. The engines, made by Scania,
have a modified compression ratio, and the fuel (known as ED95) used is
a mix of 93.6% ethanol and 3.6% ignition improver, and 2.8% denaturants.
The ignition improver makes it possible for the fuel to ignite in the
diesel combustion cycle. It is then also possible to use the energy
efficiency of the diesel principle with ethanol. These engines have been
used in the United Kingdom by Reading Buses but the use of bioethanol fuel is now being phased out.
- Dual-fuel direct-injection
A 2004 MIT
study and an earlier paper published by the Society of Automotive
Engineers identified a method to exploit the characteristics of fuel
ethanol substantially more efficiently than mixing it with gasoline. The
method presents the possibility of leveraging the use of alcohol to
achieve definite improvement over the cost-effectiveness of hybrid
electric. The improvement consists of using dual-fuel direct-injection
of pure alcohol (or the azeotrope or E85) and gasoline, in any ratio up
to 100% of either, in a turbocharged, high compression-ratio,
small-displacement engine having performance similar to an engine having
twice the displacement. Each fuel is carried separately, with a much
smaller tank for alcohol. The high-compression (for higher efficiency)
engine runs on ordinary gasoline under low-power cruise conditions.
Alcohol is directly injected into the cylinders (and the gasoline
injection simultaneously reduced) only when necessary to suppress
‘knock’ such as when significantly accelerating. Direct cylinder
injection raises the already high octane rating of ethanol up to an
effective 130. The calculated over-all reduction of gasoline use and CO2
emission is 30%. The consumer cost payback time shows a 4:1 improvement
over turbo-diesel and a 5:1 improvement over hybrid. The problems of
water absorption into pre-mixed gasoline (causing phase separation),
supply issues of multiple mix ratios and cold-weather starting are also
avoided.
- Increased thermal efficiency
In a 2008 study, complex engine controls and increased exhaust gas recirculation
allowed a compression ratio of 19.5 with fuels ranging from neat
ethanol to E50. Thermal efficiency up to approximately that for a diesel
was achieved. This would result in the fuel economy of a neat ethanol vehicle to be about the same as one burning gasoline.
- Fuel cells powered by an ethanol reformer
In June 2016, Nissan announced plans to develop fuel cell vehicles powered by ethanol rather than hydrogen, the fuel of choice by the other car manufacturers that have developed and commercialized fuel cell vehicles, such as the Hyundai Tucson FCEV, Toyota Mirai, and Honda FCX Clarity.
The main advantage of this technical approach is that it would be
cheaper and easier to deploy the fueling infrastructure than setting up
the one required to deliver hydrogen at high pressures, as each hydrogen
fueling station cost US$1 million to US$2 million to build.
Nissan plans to create a technology that uses liquid ethanol fuel
as a source to generate hydrogen within the vehicle itself. The
technology uses heat to reform ethanol into hydrogen to feed what is
known as a solid oxide fuel cell (SOFC). The fuel cell generates
electricity to supply power to the electric motor driving the wheels,
through a battery that handles peak power demands and stores regenerated
energy. The vehicle would include a tank for a blend of water and
ethanol, which is fed into an onboard reformer that splits it into pure
hydrogen and carbon dioxide. According to Nissan, the liquid fuel could
be an ethanol-water blend at a 55:45 ratio. Nissan expects to
commercialize its technology by 2020.
Experience by country
The world's top ethanol fuel producers in 2011 were the United States with 13.9×109 U.S. gallons (5.3×1010 liters; 1.16×1010 imperial gallons) and Brazil with 5.6×109 U.S. gallons (2.1×1010 liters; 4.7×109 imperial gallons), accounting together for 87.1% of world production of 22.36×109 U.S. gallons (8.46×1010 liters; 1.862×1010 imperial gallons).
Strong incentives, coupled with other industry development initiatives,
are giving rise to fledgling ethanol industries in countries such as
Germany, Spain, France, Sweden, China, Thailand, Canada, Colombia,
India, Australia, and some Central American countries.
| Annual fuel ethanol production by country (2007–2011) Top 10 countries/regional blocks (Millions of U.S. liquid gallons per year) | ||||||
|---|---|---|---|---|---|---|
| World rank |
Country/Region | 2011 | 2010 | 2009 | 2008 | 2007 |
| 1 | 13,900.00 | 13,231.00 | 10,938.00 | 9,235.00 | 6,485.00 | |
| 2 | 5,573.24 | 6,921.54 | 6,577.89 | 6,472.20 | 5,019.20 | |
| 3 | 1,199.31 | 1,176.88 | 1,039.52 | 733.60 | 570.30 | |
| 4 | 554.76 | 541.55 | 541.55 | 501.90 | 486.00 | |
| 5 | 435.20 | 89.80 | 79.20 | |||
| 6 | 462.30 | 356.63 | 290.59 | 237.70 | 211.30 | |
| 7 | 91.67 | 66.00 | 52.80 | |||
| 8 | 83.21 | 79.30 | 74.90 | |||
| 9 | 87.20 | 66.04 | 56.80 | 26.40 | 26.40 | |
| 10 | Other | 247.27 | ||||
| World Total | 22,356.09 | 22,946.87 | 19,534.99 | 17,335.20 | 13,101.70 | |
Environment
Energy balance
All biomass goes through at least some of these steps: it needs to be
grown, collected, dried, fermented, distilled, and burned. All of these
steps require resources and an infrastructure. The total amount of
energy input into the process compared to the energy released by burning
the resulting ethanol fuel is known as the energy balance (or "energy returned on energy invested"). Figures compiled in a 2007 report by National Geographic Magazine point to modest results for corn ethanol
produced in the US: one unit of fossil-fuel energy is required to
create 1.3 energy units from the resulting ethanol. The energy balance
for sugarcane ethanol produced in Brazil is more favorable, with one
unit of fossil-fuel energy required to create 8 from the ethanol. Energy
balance estimates are not easily produced, thus numerous such reports
have been generated that are contradictory. For instance, a separate
survey reports that production of ethanol from sugarcane, which requires
a tropical climate to grow productively, returns from 8 to 9 units of
energy for each unit expended, as compared to corn, which only returns
about 1.34 units of fuel energy for each unit of energy expended.
A 2006 University of California Berkeley study, after analyzing six
separate studies, concluded that producing ethanol from corn uses much
less petroleum than producing gasoline.
Carbon dioxide, a greenhouse gas,
is emitted during fermentation and combustion. This is canceled out by
the greater uptake of carbon dioxide by the plants as they grow to
produce the biomass.
When compared to gasoline, depending on the production method, ethanol releases less greenhouse gases.
Air pollution
Compared with conventional unleaded gasoline,
ethanol is a particulate-free burning fuel source that combusts with
oxygen to form carbon dioxide, carbon monoxide, water and aldehydes. The Clean Air Act requires the addition of oxygenates to reduce carbon monoxide emissions in the United States. The additive MTBE
is currently being phased out due to ground water contamination, hence
ethanol becomes an attractive alternative additive. Current production
methods include air pollution from the manufacturer of macronutrient fertilizers such as ammonia.
A study by atmospheric scientists at Stanford University found
that E85 fuel would increase the risk of air pollution deaths relative
to gasoline by 9% in Los Angeles, US: a very large, urban, car-based
metropolis that is a worst-case scenario. Ozone levels are significantly increased, thereby increasing photochemical smog and aggravating medical problems such as asthma.
Brazil burns significant amounts of ethanol biofuel. Gas chromatograph
studies were performed of ambient air in São Paulo, Brazil, and
compared to Osaka, Japan, which does not burn ethanol fuel. Atmospheric Formaldehyde was 160% higher in Brazil, and Acetaldehyde was 260% higher.
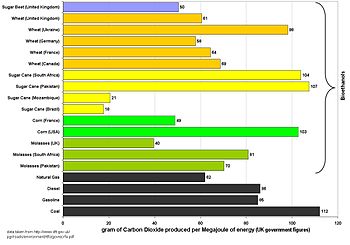
Carbon dioxide
UK government calculation of carbon intensity of corn bioethanol grown in the US and burnt in the UK.
Graph of UK figures for the carbon intensity of bioethanol and fossil fuels.
This graph assumes that all bioethanols are burnt in their country of
origin and that previously existing cropland is used to grow the
feedstock.
The calculation of exactly how much
carbon dioxide is produced in the manufacture of bioethanol is a complex
and inexact process, and is highly dependent on the method by which the
ethanol is produced and the assumptions made in the calculation. A
calculation should include:
- The cost of growing the feedstock
- The cost of transporting the feedstock to the factory
- The cost of processing the feedstock into bioethanol
Such a calculation may or may not consider the following effects:
- The cost of the change in land use of the area where the fuel feedstock is grown.
- The cost of transportation of the bioethanol from the factory to its point of use
- The efficiency of the bioethanol compared with standard gasoline
- The amount of carbon dioxide produced at the tail pipe.
- The benefits due to the production of useful bi-products, such as cattle feed or electricity.
The graph on the right shows figures calculated by the UK government for the purposes of the Renewable transport fuel obligation.
The January 2006 Science article from UC Berkeley's ERG,
estimated reduction from corn ethanol in GHG to be 13% after reviewing a
large number of studies. In a correction to that article released
shortly after publication, they reduce the estimated value to 7.4%. A National Geographic Magazine overview article (2007) puts the figures at 22% less CO2
emissions in production and use for corn ethanol compared to gasoline
and a 56% reduction for cane ethanol. Carmaker Ford reports a 70%
reduction in CO2 emissions with bioethanol compared to petrol for one of their flexible-fuel vehicles.
An additional complication is that production requires tilling new soil
which produces a one-off release of GHG that it can take decades or
centuries of production reductions in GHG emissions to equalize.
As an example, converting grass lands to corn production for ethanol
takes about a century of annual savings to make up for the GHG released
from the initial tilling.
Change in land use
Large-scale farming is necessary to produce agricultural alcohol and
this requires substantial amounts of cultivated land. University of
Minnesota researchers report that if all corn grown in the U.S. were
used to make ethanol it would displace 12% of current U.S. gasoline
consumption.
There are claims that land for ethanol production is acquired through
deforestation, while others have observed that areas currently
supporting forests are usually not suitable for growing crops. In any case, farming may involve a decline in soil fertility due to reduction of organic matter,
a decrease in water availability and quality, an increase in the use of
pesticides and fertilizers, and potential dislocation of local
communities. New technology enables farmers and processors to increasingly produce the same output using less inputs.
Cellulosic ethanol production is a new approach that may
alleviate land use and related concerns. Cellulosic ethanol can be
produced from any plant material, potentially doubling yields, in an
effort to minimize conflict between food needs vs. fuel needs. Instead
of utilizing only the starch by-products from grinding wheat and other
crops, cellulosic ethanol production maximizes the use of all plant
materials, including gluten. This approach would have a smaller carbon footprint
because the amount of energy-intensive fertilisers and fungicides
remain the same for higher output of usable material. The technology for
producing cellulosic ethanol is currently in the commercialization stage.
Using biomass for electricity instead of ethanol
Converting
biomass to electricity for charging electric vehicles may be a more
"climate-friendly" transportation option than using biomass to produce
ethanol fuel, according to an analysis published in Science in May 2009 Researchers continue to search for more cost-effective developments in both cellulosic ethanol and advanced vehicle batteries.
Health costs of ethanol emissions
For
each billion ethanol-equivalent gallons of fuel produced and combusted
in the US, the combined climate-change and health costs are $469 million
for gasoline, $472–952 million for corn ethanol depending on
biorefinery heat source (natural gas, corn stover, or coal) and
technology, but only $123–208 million for cellulosic ethanol depending
on feedstock (prairie biomass, Miscanthus, corn stover, or switchgrass).
Efficiency of common crops
As
ethanol yields improve or different feedstocks are introduced, ethanol
production may become more economically feasible in the US. Currently,
research on improving ethanol yields from each unit of corn is underway
using biotechnology. Also, as long as oil prices remain high, the
economical use of other feedstocks, such as cellulose, become viable. By-products such as straw or wood chips can be converted to ethanol. Fast growing species like switchgrass can be grown on land not suitable for other cash crops and yield high levels of ethanol per unit area.
| Crop | Annual yield (Liters/hectare, US gal/acre) | Greenhouse-gas savings vs. petrol[a] |
Comments | |
|---|---|---|---|---|
| Sugar cane | 6800–8000 L/ha, 727–870 g/acre |
87%–96% | Long-season annual grass. Used as feedstock for most bioethanol produced in Brazil. Newer processing plants burn residues not used for ethanol to generate electricity. Grows only in tropical and subtropical climates. | |
| Miscanthus | 7300 L/ha, 780 g/acre |
37%–73% | Low-input perennial grass. Ethanol production depends on development of cellulosic technology. | |
| Switchgrass | 3100–7600 L/ha, 330–810 g/acre |
37%–73% | Low-input perennial grass. Ethanol production depends on development of cellulosic technology. Breeding efforts underway to increase yields. Higher biomass production possible with mixed species of perennial grasses. | |
| Poplar | 3700–6000 L/ha, 400–640 g/acre |
51%–100% | Fast-growing tree. Ethanol production depends on development of cellulosic technology. Completion of genomic sequencing project will aid breeding efforts to increase yields. | |
| Sweet sorghum | 2500–7000 L/ha, 270–750 g/acre |
No data | Low-input annual grass. Ethanol production possible using existing technology. Grows in tropical and temperate climates, but highest ethanol yield estimates assume multiple crops per year (possible only in tropical climates). Does not store well. | |
| Corn | 3100–4000 L/ha, 330–424 g/acre |
10%–20% | High-input annual grass. Used as feedstock for most bioethanol produced in USA. Only kernels can be processed using available technology; development of commercial cellulosic technology would allow stover to be used and increase ethanol yield by 1,100 – 2,000 litres/ha. | |
| Source (except those indicated): Nature 444 (7 December 2006): 673–676. [a] – Savings of GHG emissions assuming no land use change (using existing crop lands). | ||||
Reduced petroleum imports and costs
One rationale given for extensive ethanol production in the U.S. is its benefit to energy security, by shifting the need for some foreign-produced oil to domestically produced energy sources.
Production of ethanol requires significant energy, but current U.S.
production derives most of that energy from coal, natural gas and other
sources, rather than oil.
Because 66% of oil consumed in the U.S. is imported, compared to a net
surplus of coal and just 16% of natural gas (figures from 2006), the displacement of oil-based fuels to ethanol produces a net shift from foreign to domestic U.S. energy sources.
According to a 2008 analysis by Iowa State University, the growth
in US ethanol production has caused retail gasoline prices to be US
$0.29 to US $0.40 per gallon lower than would otherwise have been the
case.
Motorsport
Leon Duray qualified third for the 1927 Indianapolis 500 auto race with an ethanol-fueled car. The IndyCar Series adopted a 10% ethanol blend for the 2006 season, and a 98% blend in 2007.
The American Le Mans Series
sports car championship introduced E10 in the 2007 season to replace
pure gasoline. In the 2008 season, E85 was allowed in the GT class and
teams began switching to it.
In 2011, the three national NASCAR stock car series mandated a switch from gasoline to E15, a blend of Sunoco GTX unleaded racing fuel and 15% ethanol.
Australia's V8 Supercar championship uses Shell E85 for its racing fuel.
Stock Car Brasil Championship runs on neat ethanol, E100.
Ethanol fuel may also be utilized as a rocket fuel. As of 2010, small quantities of ethanol are used in lightweight rocket-racing aircraft.
Replacement cooking fuel
Project Gaia is a U.S. non-governmental, non-profit
organization involved in the creation of a commercially viable
household market for alcohol-based fuels in Ethiopia and other countries
in the developing world.
The project considers alcohol fuels to be a solution to fuel shortages,
environmental damage, and public health issues caused by traditional
cooking in the developing world. Targeting poor and marginalized
communities that face health issues from cooking over polluting fires,
Gaia currently works in Ethiopia, Nigeria, Brazil, Haiti, and Madagascar, and is in the planning stage of projects in several other countries.
Research
Ethanol plant in Turner County, South Dakota
Ethanol research focuses on alternative sources, novel catalysts and production processes. INEOS produced ethanol from vegetative material and wood waste. The bacterium E.coli when genetically engineered with cow rumen genes and enzymes can produce ethanol from corn stover. Other potential feedstocks are municipal waste, recycled products, rice hulls, sugarcane bagasse, wood chips, switchgrass and carbon dioxide.