Here
is a typical textbook question. Your car has run out of petrol. With
how much force do you need to push it to accelerate it to a given speed?
The answer comes from Newton’s second law of motion:
![\[ F=ma, \]](https://plus.maths.org/MI/30da62277d3b0842a5c4c577bb23a454/images/img-0001.png) |
where  is acceleration,
is acceleration, 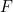 is force and
is force and 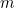 is mass. This wonderfully straightforward, yet subtle law allows you to
describe motion of all kinds and so it can, in theory at least, answer
pretty much any question a physicist might want to ask about the world.
is mass. This wonderfully straightforward, yet subtle law allows you to
describe motion of all kinds and so it can, in theory at least, answer
pretty much any question a physicist might want to ask about the world.
 is acceleration,
is acceleration, 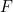 is force and
is force and 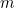 is mass. This wonderfully straightforward, yet subtle law allows you to
describe motion of all kinds and so it can, in theory at least, answer
pretty much any question a physicist might want to ask about the world.
is mass. This wonderfully straightforward, yet subtle law allows you to
describe motion of all kinds and so it can, in theory at least, answer
pretty much any question a physicist might want to ask about the world.
Or can it? When people first started considering the world at the
smallest scales, for example electrons orbiting the nucleus of an
atom, they realized that things get very weird indeed and that Newton's laws no longer
apply. To describe this tiny world you need quantum mechanics,
a theory developed at the beginning of the twentieth century. The
core equation of this theory, the analogue of Newton's second law, is called
Schrödinger's equation.
Waves and particles
"In classical mechanics we describe a state of a physical system
using position and momentum," explains Nazim Bouatta, a theoretical
physicist at the University of Cambridge. For example, if you’ve got a
table full of moving billiard balls and you know the position and the
momentum (that’s the mass times the velocity) of each ball at some time 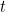 , then you know all there is to know about the system at that time
, then you know all there is to know about the system at that time 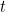 :
where everything is, where everything is going and how fast. "The kind
of question we then ask is: if we know the initial conditions of a
system, that is, we know the system at time
:
where everything is, where everything is going and how fast. "The kind
of question we then ask is: if we know the initial conditions of a
system, that is, we know the system at time 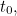 what is the dynamical evolution of this system? And we use Newton’s
second law for that. In quantum mechanics we ask the same question, but
the answer is tricky because position and momentum are no longer the
right variables to describe [the system]."
what is the dynamical evolution of this system? And we use Newton’s
second law for that. In quantum mechanics we ask the same question, but
the answer is tricky because position and momentum are no longer the
right variables to describe [the system]."
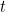 , then you know all there is to know about the system at that time
, then you know all there is to know about the system at that time 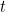 :
where everything is, where everything is going and how fast. "The kind
of question we then ask is: if we know the initial conditions of a
system, that is, we know the system at time
:
where everything is, where everything is going and how fast. "The kind
of question we then ask is: if we know the initial conditions of a
system, that is, we know the system at time 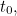 what is the dynamical evolution of this system? And we use Newton’s
second law for that. In quantum mechanics we ask the same question, but
the answer is tricky because position and momentum are no longer the
right variables to describe [the system]."
what is the dynamical evolution of this system? And we use Newton’s
second law for that. In quantum mechanics we ask the same question, but
the answer is tricky because position and momentum are no longer the
right variables to describe [the system]."
The problem is that the objects quantum mechanics tries to describe
don't always behave like tiny little billiard balls. Sometimes it is better to think of them as
waves. "Take the example of light. Newton, apart from
his work on gravity, was also interested in optics," says
Bouatta. "According to Newton, light was described by particles. But
then, after the work of many scientists, including the theoretical understanding provided by James Clerk Maxwell, we discovered that light was
described by waves."
But in 1905 Einstein realized that the
wave picture wasn't entirely correct either. To explain the
photoelectric effect (see the Plus article Light's identity crisis)
you need to think of a beam of light as a stream of particles, which
Einstein dubbed photons. The number of photons is proportional to the intensity of the light, and the energy E of each photon is proportional to its frequency f:
![\[ E=hf, \]](https://plus.maths.org/MI/74439a1276beaf8c24ebfd229e98407e/images/img-0001.png) |
Here  is Planck's constant, an incredibly small number named after the
physicist Max Planck who had already guessed this formula in 1900 in his work on black body radiation.
"So we were facing
the situation that sometimes the correct way of describing
light was as waves and sometimes it was as particles," says
Bouatta.
is Planck's constant, an incredibly small number named after the
physicist Max Planck who had already guessed this formula in 1900 in his work on black body radiation.
"So we were facing
the situation that sometimes the correct way of describing
light was as waves and sometimes it was as particles," says
Bouatta.
 is Planck's constant, an incredibly small number named after the
physicist Max Planck who had already guessed this formula in 1900 in his work on black body radiation.
"So we were facing
the situation that sometimes the correct way of describing
light was as waves and sometimes it was as particles," says
Bouatta.
is Planck's constant, an incredibly small number named after the
physicist Max Planck who had already guessed this formula in 1900 in his work on black body radiation.
"So we were facing
the situation that sometimes the correct way of describing
light was as waves and sometimes it was as particles," says
Bouatta.
The double slit experiment: The top picture shows the interference
pattern created by waves passing though the slits, the middle picture
shows what you'd expect to see when particles are fired through the
slits, and the bottom picture shows what actually happens when you fire
particles such as electrons through the slits: you get the interference
pattern you expect from waves, but the electrons are registered as
arriving as particles.
Einstein's result linked in with the age-old endeavour, started in the 17th century by Christiaan Huygens and explored again in the 19th century by William Hamilton:
to unify the physics of optics (which was all about waves) and mechanics
(which was all about particles). Inspired by the schizophrenic
behaviour of light the
young French physicist
Louis de
Broglie took a dramatic step in this journey: he postulated that not only light, but also matter
suffered from the so-called wave-particle duality. The tiny
building blocks of matter, such as electrons, also behave like particles
in some situations and like waves in others.
De Broglie's idea, which he announced in the 1920s, wasn't based on experimental evidence, rather it
sprung from theoretical considerations inspired by Einstein's theory of relativity. But experimental evidence
was soon to follow. In the late 1920s experiments involving particles
scattering off a crystal confirmed the wave-like nature of electrons.
One of the most famous demonstrations of wave-particle duality
is the double slit experiment. In it electrons (or other particles like photons or neutrons) are fired one
at a time all over a screen containing two slits. Behind the screen
there's a second one which can detect where the electrons that made it
through the slits end up. If the electrons behaved like particles,
then you would expect them to pile up around two straight lines behind the
two slits. But what you actually see on the detector screen is an
interference pattern: the pattern you would get if the
electrons were waves,
each wave passing through both slits at once and then interfering
with itself as it spreads out again on the other side. Yet on the
detector screen, the electrons are registered as arriving just as you
would expect: as particles. It's a very weird result indeed but one that
has been replicated many
times — we simply have to accept that this is the way the world
works.
Schrödinger's equation
The radical new picture proposed by de Broglie required new
physics. What does a wave associated to a particle look like
mathematically? Einstein had already related the energy 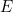 of a photon to the frequency
of a photon to the frequency 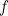 of light, which in turn is related to the wavelength
of light, which in turn is related to the wavelength 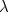 by the formula
by the formula 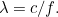 Here
Here  is the speed of light. Using results from relativity theory it is also
possible to relate the energy of a photon to its momentum. Putting all
this together gives the relationship
is the speed of light. Using results from relativity theory it is also
possible to relate the energy of a photon to its momentum. Putting all
this together gives the relationship
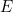 of a photon to the frequency
of a photon to the frequency 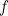 of light, which in turn is related to the wavelength
of light, which in turn is related to the wavelength 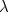 by the formula
by the formula 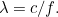 Here
Here  is the speed of light. Using results from relativity theory it is also
possible to relate the energy of a photon to its momentum. Putting all
this together gives the relationship
is the speed of light. Using results from relativity theory it is also
possible to relate the energy of a photon to its momentum. Putting all
this together gives the relationship 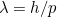
between the photon’s wavelength 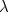 and momentum
and momentum 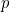 (
(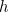 again is Planck’s constant).
again is Planck’s constant).
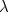 and momentum
and momentum 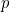 (
(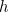 again is Planck’s constant).
again is Planck’s constant).
Following on from this,
de Broglie postulated that the same relationship between wavelength and momentum should hold for any particle.
At this point it's best to suspend your intuition about what it
really means to say that a particle behaves like a wave
and just follow through with the mathematics.
In classical mechanics the evolution over time of a wave, for example a sound wave or a water wave, is described by a wave equation: a differential equation whose solution is a wave function, which gives you the shape of the wave at any time 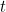 (subject to suitable boundary conditions).
(subject to suitable boundary conditions).
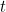 (subject to suitable boundary conditions).
(subject to suitable boundary conditions).
For example, suppose you have waves traveling through a string that is stretched out along the  -axis and vibrates in the
-axis and vibrates in the 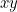 -plane. In order to describe the wave completely, you need to find the displacement
-plane. In order to describe the wave completely, you need to find the displacement 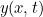 of the string in the
of the string in the 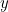 -direction at every point
-direction at every point  and every time
and every time 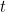 . Using Newton’s second law of motion it is possible to show that
. Using Newton’s second law of motion it is possible to show that 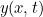 obeys the following wave equation
obeys the following wave equation
 -axis and vibrates in the
-axis and vibrates in the 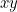 -plane. In order to describe the wave completely, you need to find the displacement
-plane. In order to describe the wave completely, you need to find the displacement 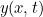 of the string in the
of the string in the 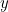 -direction at every point
-direction at every point  and every time
and every time 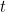 . Using Newton’s second law of motion it is possible to show that
. Using Newton’s second law of motion it is possible to show that 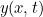 obeys the following wave equation
obeys the following wave equation
:
![\[ \frac{\partial ^2y}{\partial x^2} = \frac{1}{v^2} \frac{\partial ^2 y}{\partial t^2}, \]](https://plus.maths.org/MI/16029de402170a8a48a856dc39d21fab/images/img-0006.png) |
where  is the speed of the waves.
is the speed of the waves.
 is the speed of the waves.
is the speed of the waves. A snapshot in time of a string vibrating in the xy-plane. The wave shown here is described by the cosine function.
A snapshot in time of a string vibrating in the xy-plane. The wave shown here is described by the cosine function.
A general solution 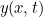 to this equation is quite complicated, reflecting the fact that the
string can be wiggling around in all sorts of ways, and that you need
more information (initial conditions and boundary conditions) to find
out exactly what kind of motion it is. But as an example, the function
to this equation is quite complicated, reflecting the fact that the
string can be wiggling around in all sorts of ways, and that you need
more information (initial conditions and boundary conditions) to find
out exactly what kind of motion it is. But as an example, the function
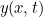 to this equation is quite complicated, reflecting the fact that the
string can be wiggling around in all sorts of ways, and that you need
more information (initial conditions and boundary conditions) to find
out exactly what kind of motion it is. But as an example, the function
to this equation is quite complicated, reflecting the fact that the
string can be wiggling around in all sorts of ways, and that you need
more information (initial conditions and boundary conditions) to find
out exactly what kind of motion it is. But as an example, the function![\[ y(x,t)=A \cos {\omega (t-\frac{x}{v})} \]](https://plus.maths.org/MI/b3644d0d4a0314bc4ed8b5dac3941c77/images/img-0002.png) |
describes a wave traveling in the positive  -direction with an angular frequency
-direction with an angular frequency 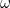 , so as you would expect, it is a possible solution to the wave equation.
, so as you would expect, it is a possible solution to the wave equation.
 -direction with an angular frequency
-direction with an angular frequency 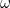 , so as you would expect, it is a possible solution to the wave equation.
, so as you would expect, it is a possible solution to the wave equation.
By analogy, there should be a wave equation governing the evolution
of the mysterious "matter waves", whatever they may be, over time. Its
solution would be a wave function 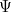 (but resist thinking of it as describing an actual wave) which tells
you all there is to know about your quantum system — for example a
single particle moving around in a box — at any time
(but resist thinking of it as describing an actual wave) which tells
you all there is to know about your quantum system — for example a
single particle moving around in a box — at any time 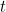 .
It was the Austrian physicist Erwin Schrödinger who came up with this
equation in 1926. For a single particle moving around in three
dimensions the equation can be written as
.
It was the Austrian physicist Erwin Schrödinger who came up with this
equation in 1926. For a single particle moving around in three
dimensions the equation can be written as
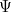 (but resist thinking of it as describing an actual wave) which tells
you all there is to know about your quantum system — for example a
single particle moving around in a box — at any time
(but resist thinking of it as describing an actual wave) which tells
you all there is to know about your quantum system — for example a
single particle moving around in a box — at any time 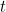 .
It was the Austrian physicist Erwin Schrödinger who came up with this
equation in 1926. For a single particle moving around in three
dimensions the equation can be written as
.
It was the Austrian physicist Erwin Schrödinger who came up with this
equation in 1926. For a single particle moving around in three
dimensions the equation can be written as ![\[ \frac{ih}{2\pi } \frac{\partial \Psi }{\partial t} = -\frac{h^2}{8 \pi ^2 m} \left(\frac{\partial ^2 \Psi }{\partial x^2} + \frac{\partial ^2 \Psi }{\partial y^2} + \frac{\partial ^2 \Psi }{\partial z^2}\right) + V\Psi . \]](https://plus.maths.org/MI/8af6d8caf2764a1f6b7f4ec8b06ec4b4/images/img-0003.png) |
Here 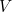 is the potential energy of the particle (a function of
is the potential energy of the particle (a function of  ,
, 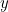 ,
,  and
and 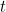 ),
), 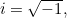
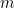 is the mass of the particle and
is the mass of the particle and 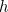 is Planck’s constant. The solution to this equation is the wave function
is Planck’s constant. The solution to this equation is the wave function 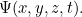
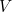 is the potential energy of the particle (a function of
is the potential energy of the particle (a function of  ,
, 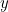 ,
,  and
and 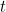 ),
), 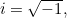
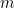 is the mass of the particle and
is the mass of the particle and 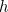 is Planck’s constant. The solution to this equation is the wave function
is Planck’s constant. The solution to this equation is the wave function 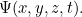
In some situations the potential energy does not depend on time 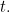 In this case we can often solve the problem by considering the simpler time-independent version of the Schrödinger equation for a function
In this case we can often solve the problem by considering the simpler time-independent version of the Schrödinger equation for a function 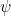 depending only on space, i.e.
depending only on space, i.e. 
 In this case we can often solve the problem by considering the simpler time-independent version of the Schrödinger equation for a function
In this case we can often solve the problem by considering the simpler time-independent version of the Schrödinger equation for a function  depending only on space, i.e.
depending only on space, i.e. 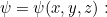
![\[ \frac{\partial ^2 \psi }{\partial x^2} + \frac{\partial ^2 \psi }{\partial y^2} + \frac{\partial ^2 \psi }{\partial z^2} + \frac{8 \pi ^2 m}{h^2}(E-V)\psi = 0, \]](https://plus.maths.org/MI/f9c35b1f5c4d678cbdfbe13153a2bc36/images/img-0004.png) |
where 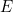 is the total energy of the particle. The solution
is the total energy of the particle. The solution 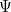 to the full equation is then
to the full equation is then
 is the total energy of the particle. The solution
is the total energy of the particle. The solution 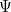 to the full equation is then
to the full equation is then![\[ \Psi = \psi e^{-(2 \pi i E/h)t}. \]](https://plus.maths.org/MI/f9c35b1f5c4d678cbdfbe13153a2bc36/images/img-0007.png) |
These equations apply to one particle moving in three
dimensions, but they have counterparts describing a system with any
number of particles. And rather than formulating the wave function as a
function of position and time, you can also formulate it as a function
of momentum and time.
Enter uncertainty
We'll see how to solve Schrödinger's equation for a simple example
in the second article, and also that its solution is indeed similar to the mathematical
equation that describes a wave.
But what does this solution actually mean? It doesn't give you a precise location for your particle at a given time 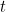 , so it doesn't give you the trajectory of a particle over time. Rather it's a function which, at a given time
, so it doesn't give you the trajectory of a particle over time. Rather it's a function which, at a given time 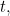 gives you a value
gives you a value 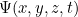 for all possible locations
for all possible locations 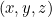 . What does this value mean? In 1926 the physicist Max Born came up with a probabilistic interpretation. He postulated that the square of the absolute value of the wave function,
. What does this value mean? In 1926 the physicist Max Born came up with a probabilistic interpretation. He postulated that the square of the absolute value of the wave function,
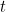 , so it doesn't give you the trajectory of a particle over time. Rather it's a function which, at a given time
, so it doesn't give you the trajectory of a particle over time. Rather it's a function which, at a given time 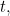 gives you a value
gives you a value 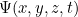 for all possible locations
for all possible locations 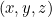 . What does this value mean? In 1926 the physicist Max Born came up with a probabilistic interpretation. He postulated that the square of the absolute value of the wave function,
. What does this value mean? In 1926 the physicist Max Born came up with a probabilistic interpretation. He postulated that the square of the absolute value of the wave function,![\[ |\Psi (x,y,z,t)|^2 \]](https://plus.maths.org/MI/130aca3ec0a6c79df9d213e46d8c3fa7/images/img-0005.png) |
gives you the probability density for finding the particle at position 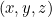 at time
at time 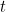 . In other words, the probability that the particle will be found in a region
. In other words, the probability that the particle will be found in a region 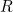 at time
at time 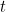 is given by the integral
is given by the integral
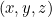 at time
at time 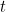 . In other words, the probability that the particle will be found in a region
. In other words, the probability that the particle will be found in a region 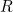 at time
at time 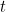 is given by the integral
is given by the integral ![\[ \int _{R} |\Psi (x,y,z,t)|^2 dxdydz. \]](https://plus.maths.org/MI/130aca3ec0a6c79df9d213e46d8c3fa7/images/img-0007.png) |
 Werner Heisenberg, 1901-1976.
Werner Heisenberg, 1901-1976.
This probabilistic picture links in with
a rather shocking consequence of de Broglie's formula for the wavelength and momentum of a particle, discovered by Werner Heisenberg in 1927.
Heisenberg found that there is a fundamental limit to the
precision to which you can measure the position and the momentum of a
moving particle. The more precise you want to be about the one, the
less you can say about the other. And this is not down to the quality
of your measuring instrument, it is a fundamental uncertainty of
nature. This result is now known as Heisenberg's uncertainty
principle and it's one of the results that's often quoted
to illustrate the weirdness of quantum mechanics. It means that in
quantum mechanics we simply cannot talk about the location or the
trajectory of a particle.
"If we believe in this uncertainty picture, then we have to accept a
probabilistic account [of what is happening] because we don’t have exact
answers to questions like ’where is the electron at time 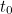 ?’,"
says Bouatta. In other words, all you can expect from the mathematical
representation of a quantum state, from the wave function, is that it
gives you a probability.
?’,"
says Bouatta. In other words, all you can expect from the mathematical
representation of a quantum state, from the wave function, is that it
gives you a probability.
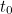 ?’,"
says Bouatta. In other words, all you can expect from the mathematical
representation of a quantum state, from the wave function, is that it
gives you a probability.
?’,"
says Bouatta. In other words, all you can expect from the mathematical
representation of a quantum state, from the wave function, is that it
gives you a probability.
Whether or not the wave function has any physical interpretation was
and still is a touchy question. "The question was, we have this wave
function, but are we really thinking that there are waves
propagating in space and time?" says Bouatta. "De Broglie, Schrödinger
and Einstein were
trying to provide a realistic account, that it's like a light wave,
for example, propagating in a vacuum. But [the physicists], Wolfgang Pauli,
Werner Heisenberg and Niels Bohr
were against this realistic picture. For them the
wave function was only a tool for computing probabilities." We'll have a
closer look at the interpretation of the wave function in the third article of this series.
Does it work?
 Louis de Broglie, 1892-1987.
Louis de Broglie, 1892-1987.
Why should we believe this rather fantastical set-up?
In this article we have presented Schrödinger's equation as if it were plucked out of
thin air, but where does it actually come from? How did Schrödinger
derive it? The famous physicist Richard Feynman considered this a
futile question: "Where did we get that [equation] from? It's
not possible to derive it from anything you know. It came out of the
mind of Schrödinger."
Yet, the equation has held its own in every experiment so far. "It's the most
fundamental equation in quantum mechanics," says Bouatta. "It's the starting
point for every quantum mechanical system we want to describe:
electrons, protons, neutrons, whatever." The equation's
earliest success, which was also one of
Schrödinger's motivations, was to describe a phenomenon that had
helped to give birth to quantum mechanics in the first place: the discrete energy
spectrum of the hydrogen atom. According to Ernest Rutherford's
atomic model, the frequency of radiation emitted by atoms such as
hydrogen should vary
continuously. Experiments showed, however, that it doesn't: the
hydrogen atom only emits radiation at certain frequencies, there is a
jump when the frequency changes. This discovery flew in the face of
conventional wisdom, which endorsed a maxim set out by the 17th
century philosopher and mathematician Gottfried Leibniz: "nature does not make jumps".
In 1913 Niels Bohr came up with a new
atomic model in which electrons are restricted to certain energy
levels. Schrödinger applied his equation to the hydrogen
atom and found that his solutions exactly reproduced the energy levels
stipulated by Bohr. "This was an amazing result — and one of the first major
achievement of Schrödinger's equation." says Bouatta.
With countless experimental successes under its belt, Schrödinger's
equation has become the established analogue of Newton's second law of
motion for quantum mechanics. Now let's see Schrödinger's equation in
action, using the simple example of a particle moving around in a box.
We will also explore another weird consequence of the equation called quantum tunneling.

